Introduction
Materials and Methods
Plant material and sample collection
Analysis of volatile compounds
Data analysis
Results and Discussion
Analysis of volatile compounds by cultivar and maturity stage
Analysis of volatile compounds in F. vesca
Conclusion
Introduction
Strawberries are one of the most popular fruits worldwide, with global production of 23.5 tons ha-1 in 2021 (FAOSTAT, 2021). They are rich in vitamin C and have a unique balance of sour and sweet flavors, along with a distinct aroma (Schieberle et al., 1997; Ulrich et al., 1997; Lee et al., 2003). The flesh of strawberries consumed by humans consists of an enlarged receptacle (Hancock, 2020). During strawberry fruit ripening, auxins promote the accumulation of fructose derivatives and secondary products (Fait et al., 2008; Vallarino et al., 2018), with sucrose, glucose, and fructose being the most abundant soluble sugars (Halford et al., 2011). Sucrose, amino acids, phenolic compounds, and volatile compounds are major indicators of fruit ripening and play an important role in determining fruit quality (Li et al., 2019; Fan et al., 2021). Furthermore, volatile compounds are basic aromatic components that not only act as attracting substances for moisture mediators but also as antibacterial agents against pathogenic fungi and bacteria (Arroyo et al., 2007; Xu et al., 2019).
Strawberries develop under the influence of auxins and ripen through three non-climacteric stages: an increase in the fruit size, an increased weight, and a color change, which occur approximately 40 days after flowering (Zhang et al., 2011; Karki et al., 2024). Strawberry fruit ripening is a complex process that involves genetic and environmental factors leading to biochemical and physiological changes in the fruit (Giovannoni, 2001; Giovannoni, 2004). Changes in color, texture, flavor, aroma, and nutrients occur during ripening, and maturation is influenced by external and internal factors (Moya-León et al., 2019). As strawberries ripen, several sugars and organic acids accumulate, and volatile compounds are released (Menager et al., 2004), significantly impacting the fruit quality, post-harvest quality, and consumer preferences (Alvarez-Suarez et al., 2014).
Flavor is a perceptual response that combines taste, smell, and touch (Prescott, 2004). The flavors of various fruits, including strawberries, are influenced by factors such as sugars, acids, fruit color, textures, and volatile compounds (Hall, 1968; Christensen, 1983). The flavor of strawberry is produced by a complex mixture of numerous volatile and functional compounds, with more than 280 volatile compounds having been identified in mature cultivated strawberries (Ulrich et al., 2018). Low-molecular-weight volatile compounds produced during ripening provide a fruity flavor, with methyl and ethyl esters accounting for 25 to 90% (Fan et al., 2021). The fruity flavor of strawberries is affected by the genotype and environment (Forney et al., 2000), and wild strawberries tend to have a stronger flavor than cultivated strawberries (Ulrich et al., 2007). The biosynthetic pathways, enzymes, and regulatory mechanisms underlying the accumulation of volatile compounds in Fragaria have been partially elucidated, and it is known that the composition of volatile compounds varies with the cultivar, developmental stage, and with certain post-harvest factors and the analytical techniques used (Urrutia et al., 2017).
In Korea, strawberries are cultivated over an area of 5,692 ha, with a production volume of 157,784 tons per year (KOSIS, 2022). Presently, the domestic cultivar penetration rate is 96.3%, with the ‘Sulhyang’ cultivar ranking first (84.5%) in terms of cultivar share, followed by ‘Keumsil’, ‘Jukhyang’, and ‘Maehyang’ (RDA, 2022). ‘Sulhyang’ is a forced culture cultivar developed by crossing ‘Yukbo’ and ‘Janghee’ at the Nonsan Strawberry Experiment Station of the Chungnam Agricultural Research and Extension Services in 2005. It is resistant to powdery mildew, juicy, and has a soft texture (Kim et al., 2006). ‘Keumsil’, bred in 2007 by crossing ‘Maehyang’, which has high flesh hardness, and ‘Sulhyang’, which offers high yields, is a cultivar suitable for forcing culture. It has an upright growth habit, elliptical leaves, strong vigorous growth, and cone-shaped, light red fruits (Yoon et al., 2020). In 2012, the Damyang Agricultural Technology Center developed a new strawberry cultivar, ‘Jukhyang’, by crossing ‘Red Pearl’ and ‘Maehyang’. It is a cultivar suitable for forcing culture due to its low dormancy, high yield, and good fruit quality (Lee et al., 2014). ‘Maehyang’ is a new strawberry cultivar released in 2002 at the ARES Nonsan Strawberry Experiment Station in South Chungcheong Province, having originated as a cross between ‘Tochinomine’ and ‘Akihime’. It has several excellent characteristics, including weak dormancy, vigorous growth, a high yield and good fruit quality, making it a suitable variety for forcing culture (Kim et al., 2004).
A number of studies have sought to analyze the variation and contents of the bioactive compounds in domestic strawberry cultivars (Kim et al., 2013; Kim et al., 2015). However, although strawberry is an important horticultural crop in Korea, studies of volatile compounds in strawberry fruit are limited. Therefore, four domestic strawberry cultivars and two European wild cultivars were used to determine differences in the qualitative characteristics of the volatile compounds in strawberries according to the maturation stage and cultivar.
Materials and Methods
Plant material and sample collection
Six cultivars of strawberry, specifically ‘Keumsil’, ‘Maehyang’, ‘Sulhyang’, ‘Arihyang’, Fragaria vesca ‘Yellow Wonder’, and F. vesca ‘Baron Solemacher’, were cultivated in this study. Strawberry seedlings were planted at 18-cm intervals in two rows in a tunnel-type greenhouse at Kyungpook National University (35°53'N 128°36'E). Seedlings of the ‘Keumsil’, ‘Maehyang’, ‘Sulhyang’, and ‘Arihyang’ cultivars were obtained from the Kyungnam Agriculture Research Station (Jinju, Korea), while seeds of F. vesca ‘Yellow Wonder’ and F. vesca ‘Baron Solemacher’ were purchased from a commercial seed company (123Seeds, Oost-Souburg, Netherlands). The experiment conducted here lasted from September 14, 2020 to March 29, 2021, with ‘Keumsil’ transplanted on September 14, 2020 and ‘Maehyang’, ‘Sulhyang’, and ‘Arihyang’ transplanted on September 21, 2020. F. vesca ‘Yellow Wonder’ and F. vesca ‘Baron Solemacher’ were sown on September 19, 2019 and April 2, 2020, respectively, and were transplanted on September 21, 2020. The soil type on which strawberries were grown was well-drained, silty soil mulched with black polyethylene film, with a base fertilizer applied at 2000 kg per 1000 m2 of commercial organic matter according to local recommendations. Irrigation was performed at regular intervals using a nutrient solution (N‑P‑K‑Ca‑Mg‑S = 16‑4‑8‑4-mEq·L-1). The experimental design used was a randomized complete block design, with five replications in the strawberry cultivars ‘Keumsil’, ‘Maehyang’, ‘Sulhyang’, and ‘Arihyang’ (20 strawberry transplants for each replication).
Fruit samples from the ‘Keumsil’, ‘Maehyang’, ‘Sulhyang’, and ‘Arihyang’ cultivars were collected at four maturity stages, as described by Wang et al. (2021), and only mature fruits were collected from the F. vesca ‘Yellow Wonder’ and F. vesca ‘Baron Solemacher’ cultivars (Fig. 1). Three fruits per cultivar, per replicate, per stage (n = 3) were sampled in the field (Table 1). Strawberry fruits in the white, green, pink, and red stages of ‘Keumsil’, ‘Maehyang’, ‘Sulhyang’, and ‘Arihyang’ were collected on February 3, 4, 5, and 9, 2021, respectively. The fruits from F. vesca ‘Yellow Wonder’ and F. vesca ‘Baron Solemacher’ were collected on February 8, 2021. All collected samples were frozen at ‒50 degrees C using a freezer and then analyzed.
Table 1.
Sampling stages, dates, replicates, and number of fruits for strawberry cultivars
Analysis of volatile compounds
The analysis of the volatile compounds was performed as previously described (Rambla et al., 2017). Approximately 3 g of frozen fruit in each case was sampled using a cork borer, with the sample placed in a 10 mL headspace glass vial and incubated in a 37°C water bath for 10 min. Then, 6.6 g of CaCl2·2H2O and 3 mL of 100 mM EDTA (pH 7.5) were added and sonicated for 5 min. Samples in the headspace glass vials were preheated at 50°C for 10 min. A 65 µm polydimethylsiloxane–divinylbenzene fiber (Supelco, Inc., Bellefonte, PA, USA) was exposed to the vial headspace at 50°C for 10 min to extract volatile compounds. The volatile compound analysis was conducted using a gas chromatograph–mass spectrometer (7890B-5977B GC/MSD, Agilent, Santa Clara, CA, USA) at the Instrumental Analysis Center of Kyungpook National University (Daegu, Korea). This analysis was performed within 12 hours of extraction. Volatile compounds were desorbed from the injection port of the gas chromatograph at 250°C for one minute in the splitless mode. Separations were performed on a DB-5 ms column (60 m × 0.25 mm, 1 µm film thickness, J&W Scientific, Folsom, CA, USA), and helium gas was used at a flow rate of 1.2 mL·min-1. The temperature program started at 35°C for 2 min, then increased at 5°C·min-1 to 250°C, and was held at 250°C for 5 min. The mass spectra were obtained with ionization energy of 70 eV, a scan rate of 7 scans·s-1, and a mass-to-charge ratio scan range of 35 to 220. Volatile compounds were tentatively identified through a comparison (concordance > 70%) of the experimental MS spectra and spectra from the mass spectral library of the Wiley Registry (11th edition/NIST 2017). Chromatograms were analyzed using MSD ChemStation data analysis software (Agilent, Santa Clara, CA, USA).
Data analysis
The experimental plots were laid out into five blocks based on a randomized complete block design for each strawberry cultivar. Three strawberry fruits at each stage were randomly collected from the cultivar and analyzed (n = 3). The mean and standard deviation of all data were calculated using the MS Excel program (Microsoft, Redmond, WA, USA). All figures were created using Sigmaplot 14.0 (Systat Software, Inc.).
Results and Discussion
Analysis of volatile compounds by cultivar and maturity stage
The volatile compounds of the four strawberry cultivars (‘Sulhyang’, ‘Maehyang’, ‘Keumsil’, and ‘Arihyang’) were detected by means of GC–MS at different maturation stages (Fig. 2). Consequently, 12 to 59 volatile compounds were qualitatively detected in all cultivars and at all stages (Suppl. Fig. S1-S4). More than 90 volatile compounds have been identified in strawberries, of which 44 contribute to the taste and aroma of strawberries (Du et al., 2011). Strawberry volatile compounds are divided into three categories: sweetness, fruity, and liking (Du et al., 2011; Schwieterman et al., 2014; Fan et al., 2021). The strongest flavor category is fruity, followed by sweetness (Du et al., 2011; Schwieterman et al., 2014; Fan et al., 2021). Fifty-nine volatile compounds were analyzed in four cultivars, among which methyl hexanoate, hexyl acetate, and 4-methoxy-2,5-dimethyl-3(2H)-furanone are related to sweetness, while 1-hexanol and ethyl hexanoate were reported to be related to liking (Du et al., 2011; Schwieterman et al., 2014; Fan et al., 2021).
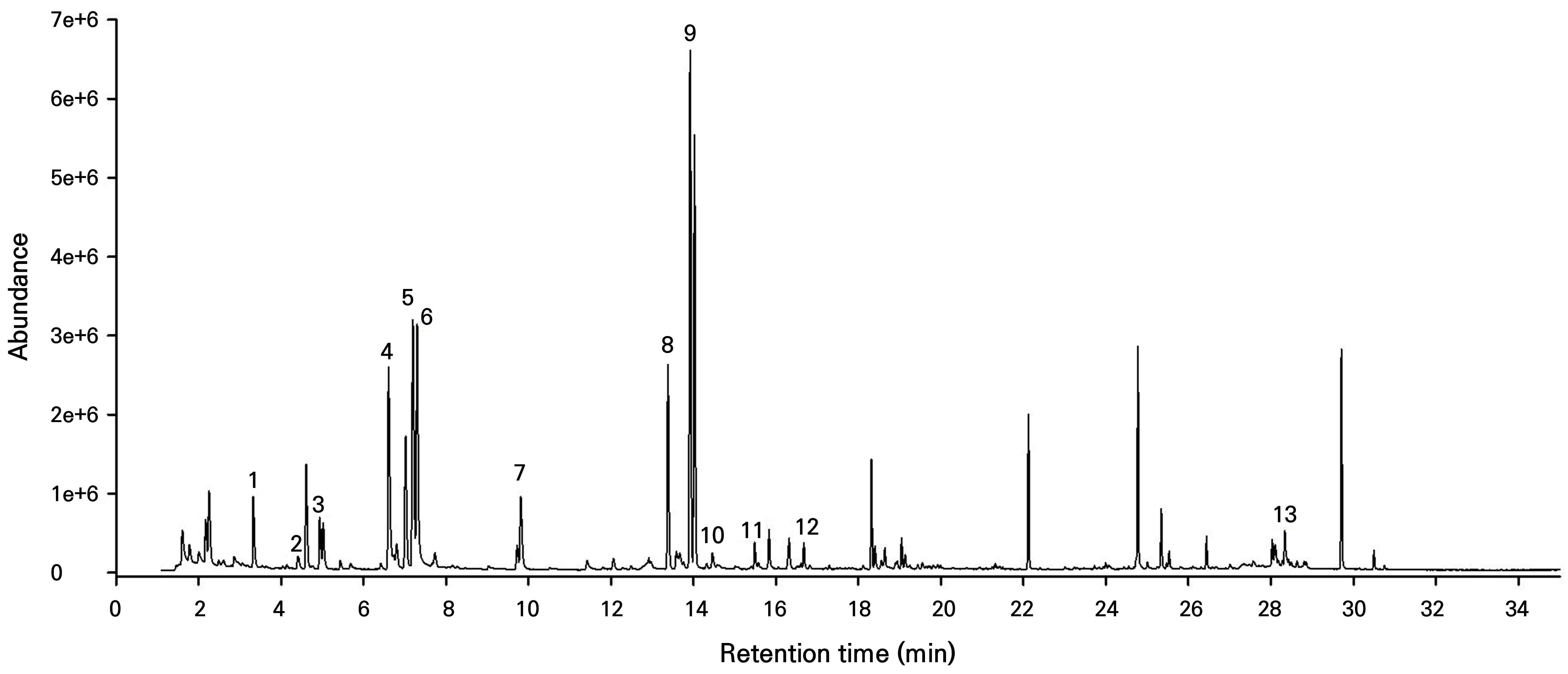
Fig. 2.
GC-MS chromatogram of volatile compounds in the red stage sample extract of 'Sulhyang'. 1: methyl butanoate, 2: methyl isovalerate, 3: hexanal, 4: 2-hexenal, 5: 2-hexen,-1-ol, (e)-, 6: 1-hexanol, 7: methyl hexanoate, 8: ethyl hexanoate, 9: hexyl acetate, 10: 2-ethyl-1-hexanol, 11: 4-methoxy-2,5-dimethyl-3(2h)-furanone, 12: linalool, 13: γ-dodecalactone.
In the ‘Sulhyang’ cultivar, 41, 47, 31, and 53 volatile compounds were detected in the green, white, pink, and red stages, respectively, while methyl isovalerate was detected only in ‘Sulhyang’ (Table 2 and Suppl. Fig. S1). Among these, four, five, seven, and 13 volatile compounds contributing to the taste and aroma of strawberries were identified in the green, white, pink, and red stages, respectively (Table 2 and Suppl. Figs. S1-S4). Methyl isovalerate, closely related to apple and pineapple flavor (Du et al., 2011) and which has been reported to contribute to fruit quality significantly (Campbell et al., 2020), was detected in ‘Sulhyang’.
In the ‘Maehyang’ cultivar, 15, 12, 42, and 55 volatile compounds were detected in the green, white, pink, and red stages, respectively, with the highest relative level of hexyl acetate being found in ‘Maehyang’ (Table 2 and Suppl. Fig. S2). In the ‘Keumsil’ cultivar, 22, 23, 29, and 55 volatile compounds were detected in the green, white, pink, and red stages, respectively (Table 2 and Suppl. Fig. S3). In the ‘Arihyang’ cultivar, 15, 20, 35, and 59 volatile compounds were detected in the green, white, pink, and red stages, respectively, and hexyl butanoate was detected only in ‘Arihyang’ (Table 2 and Suppl. Fig. S4). Hexyl acetate, reportedly associated with fruitiness and sweetness (Schwieterman et al., 2014; Fan et al., 2021), was detected in ‘Maehyang’ (Table 2), whereas hexyl butanoate, which has been reported to be associated with sweetness and overall liking (Schwieterman et al., 2014), was detected in ‘Arihyang’ (Table 2).
In all cultivars, more volatile compounds were detected in the pink and red stages than in the green and white stages (Table 3 and Supplementary row data). Additionally, ten volatile compounds (methyl butanoate; methyl isovalerate; ethyl butyrate; butyl acetate; methyl hexanoate; methyl hexanoate; ethyl hexanoate; 4-methoxy-2,5-dimethyl-3(2H)-furanone; hexyl butyrate; γ-dodecalactone) were detected only in the red stage (Table 2). At the green stage, 15–41 volatile compounds were detected for each cultivar, with ‘Sulhyang’ highest at 41. Among them, there were a total of five major volatile compounds (hexanal; 2-hexenal; 2-Hexen,-1-ol, (E)-; 1-hexanol; 1-hexanol, 2-ethyl-), with ‘Keumsil’ containing the most at five and ‘Arihyang’ containing the least at two (Suppl. Fig. S1-S4). The green stage showed a relatively high level of 1-hexanol compared to the other stages. In the white stage, 12 to 47 volatile compounds were detected for each cultivar, and like the green stage, ‘Sulhyang’ contained the most at 47. There were six major volatile compounds (hexanal; 2-hexenal; 2-hexen,-1-ol, (E)-; 1-hexanol; 1-hexanol, 2-ethyl-), and ‘Arihyang’ was detected least, in only two (Suppl. Fig. S1-S4). In the white stage, 2-hexen,-1-ol, (E)- was increased in the remaining cultivars, except in ‘Sulhyang’; it then tended to decrease in the pink and red stages. At the pink stage, 29 to 42 volatile compounds were detected for each cultivar, with ‘Maehyang’ having the most with 42 detected. There were nine major volatile compounds (methyl butanoate; hexanal; 2-hexenal; 2-hexen,-1-ol, (E)-; 1-hexanol; ethyl hexanoate; hexyl acetate; 1-hexanol,2-ethyl-; linalool), and all nine were detected in ‘Arihyang’ (Suppl. Fig. S1-S4). Specifically, the relative level of hexyl acetate was highest in the pink stage of all cultivars but tended to decrease in the red stage. In the red stage, 53-59 volatile compounds were detected for each cultivar, with ‘Arihyang’ being detected most at 59. There were 17 major volatile compounds (methyl butanoate; methyl isovalerate; hexanal; ethyl butyrate; butyl acetate; 2-hexenal; 2-hexen,-1-ol, (E)-; 1-hexanol; methyl hexanoate; ethyl hexanoate; hexyl acetate; 1-hexanol, 2-ethyl-; 4-methoxy-2,5-dimethyl-3(2H)-furanone; linalool; hexyl butyrate; 1,6,10-dodecatrien-3-ol, 3,7,11-trimethyl-, (E)-; γ-dodecalactone), and 15 were detected in ‘Arihyang’ (Table 2 and Suppl. Fig. S1-S4). When comparing all stages, the red stage exhibited the highest number of volatile compounds. Additionally, methyl butanoate was specifically detected only in ‘Sulhyang’, butyl acetate only in’ Maehyang’, and hexyl butyrate only in ‘Arihyang’. It was previously reported that ten volatile compounds (methyl butanoate; methyl isovalerate; ethyl butyrate; butyl acetate; methyl hexanoate; methyl hexanoate; ethyl hexanoate; 4-methoxy-2,5-dimethyl-3(2H)-furanone; hexyl butyrate; γ-dodecalactone) were detected only in the red stage of all strawberry cultivars, and most of them were related to sweetness (Du et al., 2011; Schwieterman et al., 2014; Fan et al., 2021). Looking at ‘Sulhyang’, seven volatile compounds were detected only in the red stage; accordingly, it is believed that these compounds may be involved in sensory preference (Table 3). However, for more accurate results, a quantitative volatile compound analysis and a sensory evaluation will be necessary.
Table 2.
Odor thresholds and relative levels of volatile compounds contributing to strawberry taste and aroma analyzed in the red stage of four strawberry cultivars
|
Retention time | Compound |
Odor threshold values (µg/kg)z |
Odor descriptiony | Relative level | |||||||
| ‘Sulhyang’ | ‘Maehyang’ | ‘Keumsil’ | ‘Arihyang’ | ||||||||
| 3.326 |
Methyl butanoate | 0.01 |
Sour, cheesy | 28.20 ± 2.83y | 5.21 ± 1.72 | 0.18w | NDv | NAu | 21.31 ± 5.99 | 0.76 | |
| 4.406 |
Methyl isovalerate | - |
Fruity, apple, pineapple | 6.01 ± 0.13 | ND | NAv | ND | NA | ND | NA | |
| 4.936 | Hexanal | - |
Fresh, fruity, green | 15.18 ± 2.18 | 22.00 ± 8.75 | 1.45 | 8.92 ± 3.32 | 0.59 | 16.15 ± 6.50 | 1.06 | |
| 5.018 |
Ethyl butanoate | 0.00001 | Pineapple | ND | ND | NA | 15.17 ± 3.21 | NA | 13.11 ± 6.72 | NA | |
| 5.425 |
Butyl acetate | 0.1 | Fruity | ND | 2.47 ± 0.32 | NA | ND | NA | ND | NA | |
| 6.661 | 2-Hexenal | 0.1 |
Green, grassy | 73.05 ± 11.08 | 36.95 ± 11.08 | 0.51 | 46.64 ± 5.23 | 0.64 | 52.08 ± 11.47 | 0.70 | |
| 7.197 |
2-Hexen, -1-ol, (E)- | 0.1 | Fruity | 82.88 ± 13.18 | 92.46 ± 18.31 | 1.12 | 75.75 ± 5.03 | 0.91 | 64.54 ± 3.15 | 0.78 | |
| 7.304 | 1-Hexanol | 0.1 |
Green, grassy | 91.86 ± 14.42 | 143.68 ± 33.32 | 1.56 | 51.63 ± 8.39 | 0.56 | 60.51 ± 13.54 | 0.66 | |
| 9.809 |
Methyl hexanoate | 0.1 |
Fruity, pineapple, sweaty, cheesy | 43.22 ± 6.47 | 3.48 ± 1.12 | 0.08 | 24.66 ± 3.76 | 0.57 | 40.41 ± 18.54 | 0.93 | |
| 13.381 |
Ethyl hexanoate | 0.0001 | Fruity | 39.63 ± 19.15 | 3.59 ± 1.12 | 0.09 | 49.00 ± 25.68 | 1.24 | 20.01 ± 11.85 | 0.50 | |
| 13.916 |
Hexyl acetate | 0.1 |
Fruity, green, apple, banana | 188.68 ± 39.31 | 242.45 ± 37.23 | 1.28 | 100.67 ± 36.65 | 0.53 | 143.41 ± 48.67 | 0.76 | |
| 14.455 |
2-ethyl- 1-Hexanol | - | Floral | 6.78 ± 0.11 | 5.94 ± 0.93 | 0.88 | 6.17 ± 0.80 | 0.91 | 25.60 ± 28.20 | 3.78 | |
| 15.479 |
4-Methoxy- 2,5-dimethyl- 3(2H)-furanone | 1.0 |
Sweet, caramel | 9.68 ± 1.00 | 2.86 ± 1.30 | 0.30 | 7.15 ± 4.03 | 0.74 | 22.27 ± 7.39 | 2.30 | |
| 16.678 | Linalool | 0.001 |
Citrus, fruity, floral | 6.01 ± 1.84 | 30.30 ± 3.44 | 5.04 | 28.38 ± 5.56 | 4.72 | 26.30 ± 2.99 | 4.38 | |
| 19.123 |
Hexyl butanoate | - |
Fruity, apricot | ND | ND | NA | ND | NA | 4.55 ± 1.60 | NA | |
| 26.441 |
1,6,10- Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)- | - | Woody | ND | ND | NA | 29.62 ± 10.09 | NA | 20.32 ± 24.24 | NA | |
| 28.338 | γ-Dodecalactone | 0.01 |
Peach, sweet | 11.68 ± 2.69 | ND | NA | 9.43 ± 0.44 | 0.81 | 13.40 ± 3.62 | 1.15 | |
Table 3.
Odor thresholds and relative levels of volatile compounds contributing to strawberry taste and aroma analyzed in different maturity stages of ‘Sulhyang’
|
Retention time | Compound |
Odor threshold values (µg/kg)z |
Odor descriptiony | Relative level | |||
| Green | White | Pink | Red | ||||
| 3.326 |
Methyl butanoate | 0.01 |
Sour, cheesy | NDx | ND | ND | 28.20 ± 2.83w |
| 4.406 |
Methyl isovalerate | - |
Fruity, apple, pineapple | ND | ND | ND | 6.01 ± 0.13 |
| 4.936 | Hexanal | - |
Fresh, fruity, green | 7.50 ± 1.28 | 8.69 ± 1.77 | 5.84 ± 2.03 | 15.18 ± 2.18 |
| 5.018 |
Ethyl butanoate | 0.00001 | Pineapple | 25.84 ± 5.41 | 24.70 ± 3.76 | 33.99 ± 8.19 | 73.05 ± 11.08 |
| 5.425 |
Butyl acetate | 0.1 | Fruity | ND | ND | 81.66 ± 12.83 | 82.88 ± 13.18 |
| 6.661 | 2-Hexenal | 0.1 |
Green, grassy | 234.21 ± 5.65 | 293.90 ± 21.32 | 132.45 ± 16.67 | 91.86 ± 14.42 |
| 7.197 |
2-Hexen, -1-ol, (E)- | 0.1 | Fruity | ND | ND | ND | 43.22 ± 6.47 |
| 7.304 | 1-Hexanol | 0.1 |
Green, grassy | ND | ND | 4.31 ± 4.20 | 39.63 ± 19.15 |
| 9.809 |
Methyl hexanoate | 0.1 |
Fruity, pineapple, sweaty, cheesy | ND | 5.09 ± 1.48 | 284.41 ± 47.12 | 188.68 ± 39.31 |
| 13.381 |
Ethyl hexanoate | 0.0001 | Fruity | 6.64 ± 0.80 | 15.64 ± 0.93 | 11.03 ± 0.44 | 6.78 ± 0.11 |
| 13.916 |
Hexyl acetate | 0.1 |
Fruity, green, apple, banana | ND | ND | ND | 9.68 ± 1.00 |
| 14.455 |
2-ethyl- 1-hexanol | - | Floral | ND | ND | ND | 6.01 ± 1.84 |
| 15.479 |
4-Methoxy-2, 5-dimethyl- 3(2H)-furanone | 1.0 |
Sweet, caramel | ND | ND | ND | 28.20 ± 2.83 |
| 16.678 | Linalool | 0.001 |
Citrus, fruity, floral | ND | ND | ND | 6.01 ± 0.13 |
| 19.123 |
Hexyl butanoate | - |
Fruity, apricot | 7.50 ± 1.28 | 8.69 ± 1.77 | 5.84 ± 2.03 | 15.18 ± 2.18 |
| 26.441 |
1,6,10- Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)- | - | Woody | 25.84 ± 5.41 | 24.70 ± 3.76 | 33.99 ± 8.19 | 73.05 ± 11.08 |
| 28.338 | γ-Dodecalactone | 0.01 |
Peach, sweet | ND | ND | 81.66 ± 12.83 | 82.88 ± 13.18 |
As reported by Fan et al., (2021), 2-hexenol was tested in 158 cultivars at the pink and red stages and found to be negatively correlated with sweetness and liking. In this study, 1-hexanol and 2-ethyl-1-hexanol, which show fruity and liking flavors (Schwieterman et al., 2014), showed higher relative levels in the green, white, and pink than in the red stage (Suppl. Fig. S1-S4). These compounds were identified in ‘Sulhyang’, ‘Maehyang’, and ‘Keumsil’ (Table 2). Because the relative levels of 1-hexanol and 2-ethyl-1-hexanol were higher in the immature residual stage than in the mature red stage, they are expected to show a negative correlation with sweetness (Schwieterman et al., 2014). The specific volatile compounds that contribute to the organoleptic taste and aroma of strawberries have not yet been fully characterized, and their compositions vary according to the cultivar and growth stage. Therefore, additional research on the functional aspects of aromatic compounds detected in strawberries of different cultivars and stages is required.
Larsen et al. (1992) reported odor threshold values (OTVs) of 24 strawberry volatile compounds categorized as esters, alcohols, carbonyls, and acids (Table 2). OTV refers to the minimum concentration of volatile compounds that the human nose can detect, and the lower the OTV, the better the human nose can detect it. Ethyl butanoate had the lowest OTV of 0.00001 (Larsen et al., 1992) and was detected in the red stage of the ‘Keumsil’ and ‘Arihyang’ cultivars (Table 2). Ethyl butanoate exhibits flavors of pineapple, passionfruit, and strawberry and is used to flavor various beverages (Rodriguez-Nogales et al., 2005; Grosso et al., 2013). Ethyl hexanoate has the second lowest OTV of 0.0001 and is the main aromatic substance of pineapple (Wei et al., 2011). Linalool had the third lowest OTV of 0.001, and similar to ethyl hexanoate, linalool was detected in the red stage of all cultivars (Table 2). Ethyl butanoate and ethyl hexanoate are positively correlated with fruity, sweet, and liking flavors (Fan et al., 2021), whereas linalool is associated with citrus, fruity, and floral flavors (Du et al., 2011). These findings suggest that the three low-OTV compounds discussed above are the major volatile compounds of strawberries and have a significant impact on their flavor.
Nine volatile compounds contributing to strawberry taste and aroma were detected in the red stage of all cultivars in this study (Table 2). In addition, three, two, three, and five unique volatile compounds were detected in ‘Sulhyang’, ‘Maehyang’, ‘Keumsil’, and ‘Arihyang’, respectively. Using ‘Sulhyang’ as a reference cultivar, the relative levels of ethyl butanoate, ethyl hexanoate, and linalool were compared (Table 2). Ethyl butanoate was only detected in the ‘Keumsil’ and ‘Arihyang’ cultivars, and the relative level of ethyl hexanoate was highest in ‘Keumsil’ and lowest in ‘Maehyang’ (Table 2). Linalool was four times more abundant than in ‘Sulhyang’ in ‘Keumsil’, ‘Maehyang’, and ‘Arihyang’ cultivars. These results suggest that the ‘Keumsil’ and ‘Arihyang’ cultivars, in which volatile compounds with low OTVs were detected, can be expected to have a stronger aroma compared to other cultivars. A quantitative analysis of the volatile compounds in strawberries would enable more accurate profiling of their flavor and aroma characteristics.
Analysis of volatile compounds in F. vesca
F. vesca cultivars are derived from wild species in Europe, West Asia, and North America (Dyduch-Siemińska et al., 2015). F. vesca has been widely cultivated in European gardens and has high genetic diversity due to its extensive cultivation range and long cultivation history (Dyduch-Siemińska et al., 2015). F. vesca was evaluated using the ‘Yellow Wonder’ (YW) cultivar with yellow fruits and ‘Baron Solemacher’ (BS) with red fruits, with 53 and 44 volatile compounds detected in YW and BS, respectively (Table 4). Among these, eleven volatile compounds (methyl butanoate; hexanal; ethyl butanoate; butyl acetate; 2-hexanal; 1-hexanol; methyl hexanoate; ethyl hexanoate; hexyl acetate; 2-ethyl-1-hexanol, hexyl butanoate) were associated with strawberry sensory taste and flavor (Du et al., 2011; Schwieterman et al., 2014; Fan et al., 2021). Eleven from VY and eight from BS were detected (Table 3). However, other studies found more than 100 volatile compounds in F. vesca (Ulrich et al., 2007; Urrutia et al., 2017). Differences in the relative levels of aromatic compounds identified in this study can be attributed to environmental factors that arise during cultivation. The relative level of ethyl butanoate was found to be approximately 1.14 times higher in BS than in YW. However, the relative level of ethyl hexanoate was found to be approximately 1.13 times higher in YW than in BS. Compared with domestic strawberry cultivars, ethyl butanoate from the BS cultivar had a relative level that was 8.29–9.60 times higher and ethyl hexanoate had a relative level that was 0.93–10.28 times higher (Fig. 3). Specifically, five volatile compounds (2-hexen,-1-ol, (E)-; 4-methoxy-2,5-dimethyl-3(2H)-furanone; linalool; 1, 6,10-dodecatrien-3-ol, 3,7,11-trimethyl-,(E)-; γ-dodecalactone) detected in four domestic strawberry cultivars were not detected in F. vesca. These results suggest that wild strawberry cultivars are more aromatic than domestic strawberry cultivars.
Table 4.
Odor thresholds and relative levels of volatile compounds contributing to strawberry taste and aroma analyzed in two wild strawberry cultivars
|
Retention time | Compound |
Odor threshold values (µg/kg)z | Odor descriptiony | Relative level | |
| ‘Yellow Wonder’ | ‘Baron Solemacher’ | ||||
| 3.331 | Methyl butanoate | 0.01 | Sour, cheesy | 16.17 ± 2.80x | 7.35 ± 2.18 |
| 4.941 | Hexanal | - | Fresh, fruity, green | 12.40 ± 4.56 | NDz |
| 5.018 | Ethyl butanoate | 0.00001 | Pineapple | 110.58 ± 45.10 | 125.80 ± 59.00 |
| 5.420 | Butyl acetate | 0.1 | Fruity | 19.56 ± 1.57 | 14.27 ± 3.54 |
| 6.619 | 2-Hexanal | 0.1 | Green, grassy | 38.20 ± 13.32 | 27.15 ± 26.77 |
| 7.313 | 1-Hexanol | 0.1 | Green, grassy | 113.58 ± 34.41 | 70.39 ± 54.48 |
| 9.840 | Methyl hexanoate | 0.1 |
Fruity, pineapple, sweaty, cheesy | 10.97 ± 5.47 | 12.39 ± 8.44 |
| 13.398 | Ethyl hexanoate | 0.0001 | Fruity | 41.86 ± 30.77 | 118.86 ± 109.21 |
| 13.925 | Hexyl acetate | 0.1 | Fruity, green, apple, banana | 155.23 ± 17.62 | 247.33 ± 160.63 |
| 14.468 | 2-Ethyl-1-hexanol | - | Floral | 3.97 ± 0.61 | ND |
| 19.119 | Hexyl butanoate | - | Fruity, apricot | 14.82 ± 4.77 | ND |
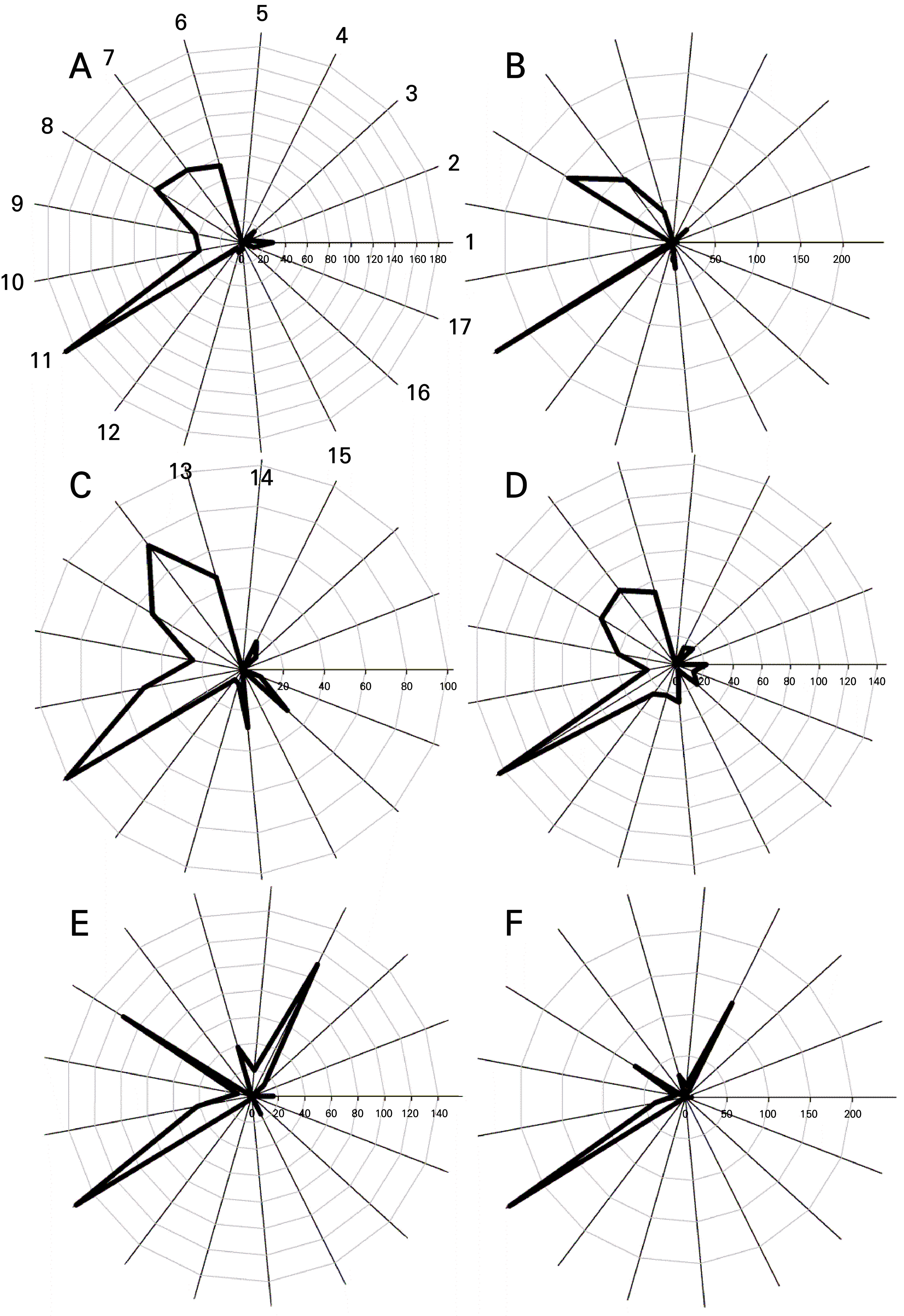
Fig. 3.
Comparison of relative levels of volatile compounds contributing to strawberry taste and aroma in six strawberry cultivar mature stages: (A) ‘Sulhyang’, (B) ‘Maehyang’, (C) ‘Keumsil’, (D) ‘Arihyang’, (E) ‘Yellow Wonder’, and (F) ‘Baron Solemacher’. 1: methyl butanoate, 2: methyl isovalerate, 3: hexanal, 4: ethyl butyrate, 5: butyl acetate, 6: 2-hexenal, 7: 2-hexen,-1-ol, (e)-, 8: 1-hexanol, 9: methyl hexanoate, 10: ethyl hexanoate, 11: hexyl acetate, 12: 2-ethyl-1-hexanol, 12: 4-methoxy-2,5-dimethyl-3(2h)-furanone, 14: linalool, 15: hexyl butyrate, 16: 1,6,10-dodecatrien-3-ol, 3,7,11-trimethyl-,(e)-, 17: γ-dodecalactone.
Conclusion
Most strawberry cultivars have been developed to produce large, firm fruits with high yields, but fruit aroma is one of the most important characteristics for consumers. If we detect aromatic compounds that exist or that are highly expressed only in cultivars that are strongly preferred by consumers and apply those compounds to breeding, it will then be possible to breed varieties that reflect consumer preferences. Moreover, if the breeding direction is determined by selecting target volatile compounds (methyl butanoate; methyl isovalerate; ethyl butyrate; butyl acetate; methyl hexanoate; methyl hexanoate; ethyl hexanoate; 4-methoxy-2,5-dimethyl-3(2H)-furanone; hexyl butyrate; γ-dodecalactone) based on OTV, breeding will be more efficient. Here, we suggest that ten volatile compounds reported to be related to sweet flavor at the red stage can be used for breeding. However, due to the diversity of strawberry cultivation facilities in Korea, future research should concentrate on the composition of aromatic compounds according to the cultivation environment. Additionally, research on volatile compounds in global domestic strawberry varieties is lacking. Therefore, the qualitative analysis results of the volatile compounds detected in this study can serve as a basis for the future breeding of strawberry fruits with different flavors.
Supplementary Material
Supplementary materials are available at Horticultural Science and Technology website (https://www.hst-j.org).
- HORT_20240028_Fig_S1.pdf
Supplementary Fig. S1. Comparison of peak areas of volatile compounds contributing to strawberry taste and aroma according to fruit development and ripening in ‘Sulhyang’. Each vertical bar represents mean ± SD of three replicates. 1: Methyl butanoate, 2: Methyl isovalerate, 3: Hexanal, 4: Ethyl butyrate, 5: Butyl acetate, 6: 2-Hexenal, 7: 2-Hexen,-1-ol, (E)-, 8: 1-Hexanol, 9: Methyl hexanoate, 10: Ethyl hexanoate, 11: Hexyl acetate, 12: 2-Ethyl-1-Hexanol, 12: 4-Methoxy-2,5-dimethyl-3(2H)-furanone, 14: Linalool, 15: Hexyl butyrate, 16: 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)-, 17: γ-Dodecalactone.
- HORT_20240028_Fig_S2.pdf
Supplementary Fig. S2. Comparison of peak areas of volatile compounds contributing to strawberry taste and aroma according to fruit development and ripening in ‘Maehyang’. Each vertical bar represents mean ± SD of three replicates. 1: Methyl butanoate, 2: Methyl isovalerate, 3: Hexanal, 4: Ethyl butyrate, 5: Butyl acetate, 6: 2-Hexenal, 7: 2-Hexen,-1-ol, (E)-, 8: 1-Hexanol, 9: Methyl hexanoate, 10: Ethyl hexanoate, 11: Hexyl acetate, 12: 2-Ethyl-1-Hexanol, 12: 4-Methoxy-2,5-dimethyl-3(2H)-furanone, 14: Linalool, 15: Hexyl butyrate, 16: 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)-, 17: γ-Dodecalactone.
- HORT_20240028_Fig_S3.pdf
Supplementary Fig. S3. Comparison of peak areas of volatile compounds contributing to strawberry taste and aroma according to fruit development and ripening in ‘Keumsil’. Each vertical bar represents mean ± SD of three replicates. 1: Methyl butanoate, 2: Methyl isovalerate, 3: Hexanal, 4: Ethyl butyrate, 5: Butyl acetate, 6: 2-Hexenal, 7: 2-Hexen,-1-ol, (E)-, 8: 1-Hexanol, 9: Methyl hexanoate, 10: Ethyl hexanoate, 11: Hexyl acetate, 12: 2-Ethyl-1-Hexanol, 12: 4-Methoxy-2,5-dimethyl-3(2H)-furanone, 14: Linalool, 15: Hexyl butyrate, 16: 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)-, 17: γ-Dodecalactone.
- HORT_20240028_Fig_S4.pdf
Supplementary Fig. S4. Comparison of peak areas of volatile compounds contributing to strawberry taste and aroma according to fruit development and ripening in ‘Arihyang’. Each vertical bar represents mean ± SD of three replicates. 1: Methyl butanoate, 2: Methyl isovalerate, 3: Hexanal, 4: Ethyl butyrate, 5: Butyl acetate, 6: 2-Hexenal, 7: 2-Hexen,-1-ol, (E)-, 8: 1-Hexanol, 9: Methyl hexanoate, 10: Ethyl hexanoate, 11: Hexyl acetate, 12: 2-Ethyl-1-Hexanol, 12: 4-Methoxy-2,5-dimethyl-3(2H)-furanone, 14: Linalool, 15: Hexyl butyrate, 16: 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)-, 17: γ-Dodecalactone.



