Introduction
Materials and Methods
Cultivation of Peanut Seed in Oak Sawdust
Seedling Harvesting and Measurements of the Length and Weight
Measurement of the Vigor Index
Morphological Analysis
PCR Amplification and Illumina Sequencing
Statistical Method
MiSeq Pipeline Method
Results
Peanut Seed Germination under Different Oak Sawdust Fermentation Periods
Effect of the Sawdust Fermentation Period on Seedling Weights
Lengths of the True Leaf with the Epicotyl, Hypocotyl, and the Roots of Seedlings under Different Sawdust Fermentation Periods
Vigor Index of Peanut Seedlings under Different Sawdust Fermentation Periods
Morphological Characteristics of Seedlings under Different Sawdust Fermentation Periods
Fungal Communities in Fermented Oak Sawdust with Different Periods
Discussion
Introduction
Peanut sprouts have high levels of resveratrol (2,551.8 mg/100 g), which possesses an antioxidant potential with 89.1% of the lipid peroxidation of linoleic acid emulsion (Gülçin, 2010; Kang et al., 2010). In addition, peanut sprouts have less fat than peanut seeds (Li et al., 2014). Sprouts grown under optimized environmental conditions are intended for commercial production using diverse growth systems (Rizzardi et al., 2009; Song et al., 2020; Choi et al., 2022; Lee et al., 2022; Kim et al., 2022). Oak sawdust has been used as a growing medium to germinate horticulture plant seeds and grow seedlings that require specific soil conditions for germination (Jung et al., 2017; Cho and Lee, 2018; Kim et al., 2019; Ahn et al., 2021). Oak sawdust has been used for biofuel production, packaging, and insulation (Rominiyi et al., 2017). In general, oak sawdust waste obtained during wood processing is an environmental concern. Sawdust is mainly composed lignin, hemicellulose, and cellulose (Alexander, 1978; Eriksson et al., 2012). Lignin is an organic substrate that does not readily break down, requiring decomposition by specialized enzymes from specific microorganisms (Bonnarme and Jeffries, 1990; Lennox et al., 2010; Lennox et al., 2019).
Previous studies have tested oak sawdust as a growing medium for peanuts. The peanut seed sowing orientation was optimized in oak sawdust, increasing germination rates, sprout vigor, and the sprout morphology quality (Ahn et al., 2017). Another study reported that peanut germination and sprout biomass increased along with in the fermentation period of oak sawdust (Ahn et al., 2021). In addition, a microbiome analysis revealed that bacterial communities change with the fermentation period (Ahn et al., 2021). However, only the bacterial community was analyzed in this study. In microbial communities, fungi also significantly affect seed germination and sprout growth (Kirkpatrick and Bazzaz, 1979; Crist and Friese, 1993).
We studied the peanut seed germination and sprout growth rates in oak sawdust fermented over four different periods of time and observed the variation of the fungal community as the fermentation period increased.
Materials and Methods
Cultivation of Peanut Seed in Oak Sawdust
The oak sawdust was provided by Farmsko (Ochang, Korea). Oak trees were cut and crunched to create 1–5 mm sawdust particles using a sawdust making machine (RM Corp, Pocheon, Korea). During this process, water was added to the sawdust, resulting in a moisture content of 55% or more. The sawdust was then fermented for 30, 45, or 60 days as described in Korea patent #KR101235913B1. From this, twenty peanuts were sown in non-fermented (0), 30-day (30), 45-day (45), and 60-day (60) fermented sawdust in a 17 × 17 × 10.5 cm tray (SW Company, Seoul, Korea). The sowing spaces between seeds and the sowing depth were 3 and 2 cm, respectively, and the seeds were vertically sown in a hypocotyl-end-down orientation. The depth of the oak sawdust was 6 cm, and the seeds were covered with up to 2 cm of sawdust. The trays were covered with black plastic lids (17 × 17 × 5 cm) and placed in a growth chamber in the dark at 30°C and 85% relative humidity. The seeds were watered with 500 mL of water every day in the morning.
Seedling Harvesting and Measurements of the Length and Weight
Each seedling was uprooted from the sawdust ten days past sowing (dps) and cleaned with water. The washed seedlings were blotted on a paper towel to dry. The seed germination rates were calculated, and the seedling weight and length (epicotyl with true leaf, hypocotyl, and root) were measured.
Measurement of the Vigor Index
To determine the optimal fermentation period of oak sawdust for the growth of peanut seedlings, the vigor index (VI) was calculated and compared using the germination rate of peanuts and each tissue length: VI =% of seed germination × the length of epicotyl with true leaf, hypocotyl, and root. The TVI was obtained from the sum of these three individual tissue vigor indexes.
Morphological Analysis
The morphologies of the peanut seedlings in each fermentation treatment group (0, 30, 45, and 60 days) were observed. Representative peanut seedlings were placed on black cotton flannel, and the shapes of the seedlings were photographed using a camera (iPhone 12 Pro, Apple, CA).
PCR Amplification and Illumina Sequencing
When harvesting the seedlings, oak sawdust samples were also collected for a microbial analysis. PCR was conducted on DNA extracted from the fermented oak sawdust of each treatment using primers targeting the fungal ITS2 region of the ribosomal DNA using a protocol modified from that used in previous studies (Bellemain et al., 2010; Edgar, 2010; Bolger et al., 2014). The forward primer (FP) ITS2-Mi (5'-TCGTC GGCAGCGTCAGATGTGTATAAGAGACAGGCATCGATGAAGAACGCAGC-3') and the reverse primer (RP) ITS4-Mi (5'-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGTCCTCCGCTTATTGATA TG-3') were applied. The PCR conditions were as follows: denaturation (95°C for 180 s), 25 cycles of denaturation (95°C for 30 s), annealing at 55°C for 30 s with an extension at 72°C for 30 s, and a final extension at 72°C for 300 s. To tag the Illumina NexTera barcode, secondary PCR was conducted using the universal FP I5 and RP I7 (Al-Bulushi et al., 2017). This PCR assessment used identical conditions except with only eight amplification cycles. Amplification was confirmed using electrophoresis in 2% agarose to separate the PCR products. A purification kit (QIAquick, Qiagen, Valencia, CA) was applied to purify the PCR-amplified products. The purified samples were combined with the same concentrations and short fragments were eliminated using an AMPure XP system (Beckman Coulter Life Sciences, Indianapolis, IN). The DNA 7500 chip was applied to evaluate the product quality using an Agilent 2100 bioanalyzer (Santa Clara, CA). A MiSeq system was utilized to sequence the mixed amplicons (Illumina, San Diego, CA).
Statistical Method
The numerical data are the mean values ± SD. The differences between the means among the four fermentation groups (0, 30, 45, and 60 days) were statistically determined, and graphs were generated using Prism 5.0 (Graphpad, San Diego, CA).
MiSeq Pipeline Method
First, the raw sequence data were quality-filtered using Trimmomatic 0.321, and the quality was checked (<Q25) according to previous studies (Chao and Lee, 1992; Masella et al., 2012). The paired-end sequences were then merged by PandaSeq2. ChunLab’s in-house program was applied to trim the primer sequences using a similarity cut-off of 0.8. HMMER’s hmmsearch program and DUDE-Seq were used to detect non-specific amplicons not encoding 16s rRNA and to denoise the sequences, respectively. UCLUST-clustering was applied to extract non-redundant reads. BLAST 2.2.224 was applied for taxonomic assignments, and pairwise alignment 5 was used to run similarity calculations with the EzTaxon database. After clustering the sequence data using the CD-Hit7 program and the UCLUST 8 algorithm, an alpha diversity analysis was conducted to analyze and define the average diversity of fungi in each sawdust treatment.
Results
Peanut Seed Germination under Different Oak Sawdust Fermentation Periods
The peanut seed germination rates (3.9, 0, 30.8, and 65.4%) increased along with the fermentation period (0, 30, 40, and 60 days, respectively) of oak sawdust (Fig. 1). Seeds sown in the 60-day treatment germinated more rapidly (5 dps) than those sown in the 0, 30, and 45-day treatments (7 dps). The seeds sown in the 45-day- and 60-day-fermented sawdust showed corresponding germination rates of 30.8 and 65.4% (Fig. 1), while those sown in unfermented and in the 30-day-fermented sawdust showed germination rates of 3.9 and 0%, respectively (Fig. 1). In general, the number of days between seed sowing and shoot emergence varied between the different fermentation period treatments.
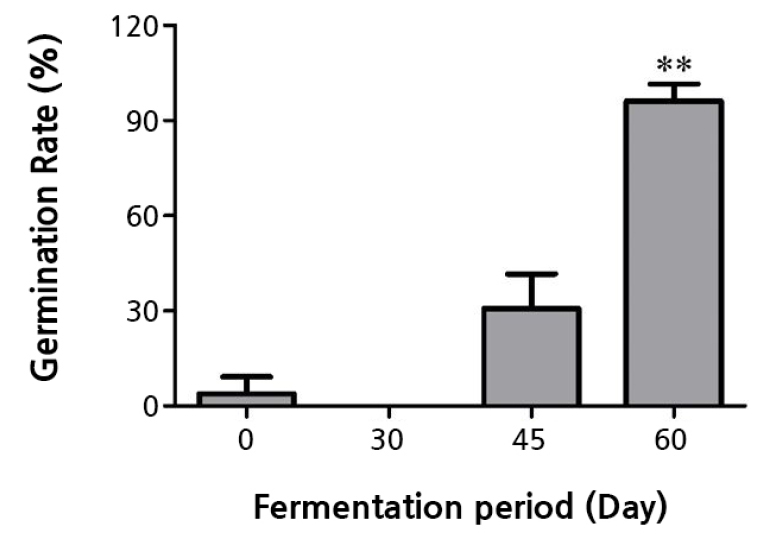
Fig. 1.
Seed germination rates of peanut seeds sown in oak sawdust with 0, 30, 45, and 60 days of fermentation. 0: no fermentation, 30: 30 days of fermentation, 45: 45 days of fermentation, and 60: 60 days of fermentation. The seed germination rates of the fermentation treatment groups were statistically compared to that of the 0-day fermentation treatment group. The asterisk denotes statistical significance (** p < 0.01).
Effect of the Sawdust Fermentation Period on Seedling Weights
At 10 dps, the peanut sprouts were uprooted, washed with water and blotted, and their weights were then measured. The average weight of the peanut sprouts grown in the sawdust fermented for 60 days was significantly higher (2.8 g) compared to the unfermented case (1.1 g) (Fig. 2). The peanut sprouts grown under fermentation for 30 and 45 days had average weights of 1.2 g and 1.6 g, respectively.
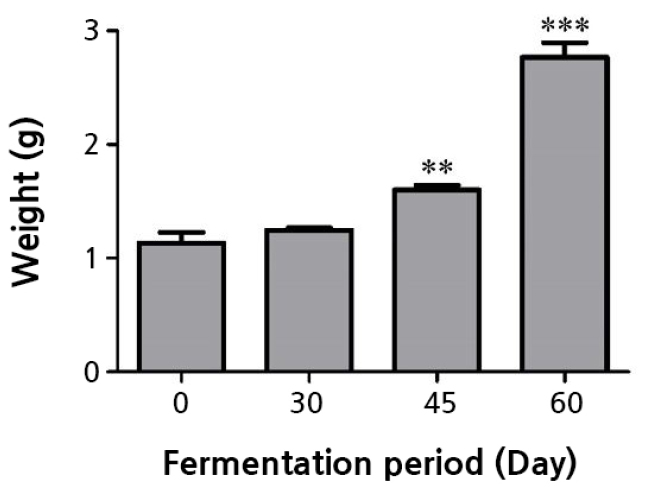
Fig. 2.
Mean weights of seedlings grown in oak sawdust with 0, 30, 45, and 60 days of fermentation. 0: no fermentation, 30: 30 days of fermentation, 45: 45 days of fermentation, and 60: 60 days of fermentation. The mean seedling weights of the fermentation treatment groups were statistically compared to that of the 0-day fermentation treatment group. Asterisks denote different levels of statistical significance (** p < 0.01 and *** p < 0.001).
Lengths of the True Leaf with the Epicotyl, Hypocotyl, and the Roots of Seedlings under Different Sawdust Fermentation Periods
The true leaves of the peanut sprouts were initially observed at 6 dps on sprouts grown in the 60-day fermentation condition. The average length of the true leaf with epicotyls on the sprouts in this condition (2.5 cm) was longer than that from the unfermented condition (0.0 cm) (Fig. 3A), where the single germinated seed failed to produce an epicotyl, and longer than that from the 45-day treatment (0.6 cm). The sprouts in the 60-day fermentation condition (2.4 cm) had a significantly longer average length of the hypocotyl compared to those sprouts that went unfermented (0.0 cm) and to those under 45-day fermentation (1.2 cm) (Fig. 3B). Lastly, the sprouts fermented for 60 days (6.6 cm) showed a longer average length of the root compared to the unfermented case (0.4 cm) and to those in the 45-day fermentation condition (1.3 cm) (Fig. 3C). Given that the seeds of the 30-day fermentation treatment failed to germinate, the lengths referred to above were all considered as 0 cm.
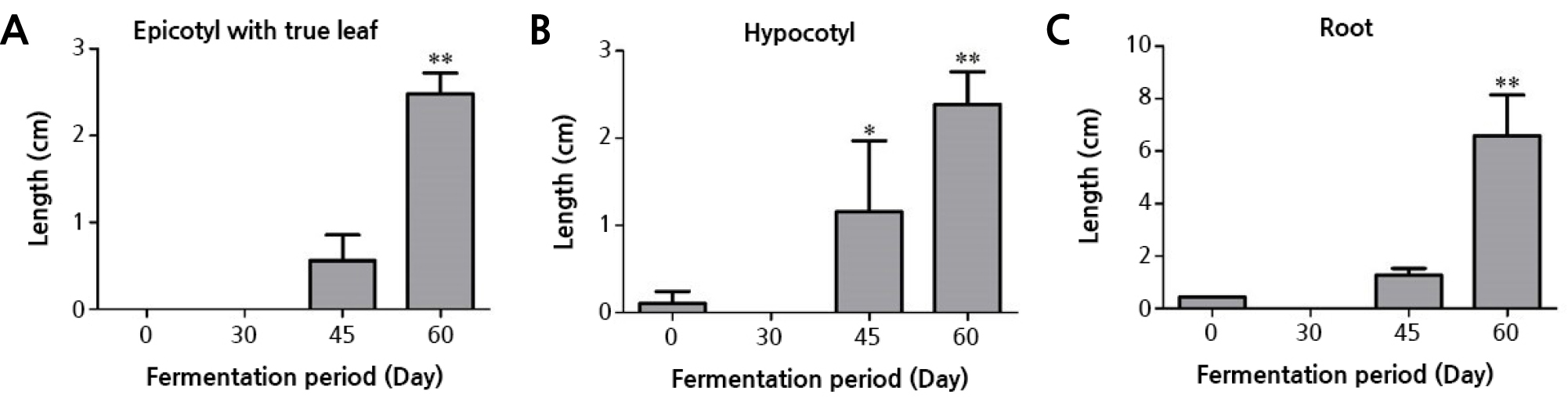
Fig. 3.
Mean lengths of the epicotyls with true leaves, hypocotyls, and the roots of seedlings grown in oak sawdust with 0, 30, 45, and 60 days of fermentation. (A) Epicotyls with true leaves, (B) Hypocotyls, and (C) Roots. 0: no fermentation, 30: 30 days of fermentation, 45: 45 days of fermentation, and 60: 60 days of fermentation. The mean lengths of the fermentation treatment groups were statistically compared to that of the 0-day fermentation treatment group for each seedling part. Asterisks denote different levels of statistical significance (* p < 0.05 and ** p < 0.01).
Vigor Index of Peanut Seedlings under Different Sawdust Fermentation Periods
The seedling total vigor index was compared to investigate the effect of the sawdust fermentation period on the growth of the peanut seedlings (Table 1 and Fig. 4). The vigor index of the seedlings grown in the sawdust fermented for 60 days was highest (1,145.8) among the treatments. The lowest index (0) was found in the seedlings grown in the sawdust fermented for 30 days, and these failed to germinate. The vigor index of the seedlings grown in 45-day-fermented sawdust was 298.1, and in unfermented sawdust, it was 34.5. In the seedlings of all treatments in general, the vigor index value of the root (germination rate × root length) was higher than the values for both the epicotyl with the true leaf width (germination rate × epicotyl with the true leaf length) and the hypocotyl (germination rate × hypocotyl length, Fig. 4).
Table 1.
Germination Rate, Length Measurements, Vigor Indices (VIs) and Total VI of Peanut Seedlings Grown in Oak Tree Sawdust with 0, 30, 45, and 60 Days of Fermentation
|
Fermentation Periodz |
Germination Rate (%) | Length (cm) | Vigor Index (VI)y | Totalx | ||||
|
True Leaf with Epicotyl | Hypocotyl | Root |
True Leaf with Epicotyl | Hypocotyl | Root |
VI (Epicotyl with True Leaf) + VI (Hypocotyl) + VI (Root) | ||
| 0 | 3.85 ± 0.48 | 0 ± 0 | 0.1 ± 0.01 | 0 ± 0 | 0 ± 0 | 0.77 ± 0.10 | 0 ± 0 | 0.87 ± 0.11 |
| 30 | 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 45 | 30.77 ± 0.96 | 0.56 ± 0.03 | 1.15 ± 0.07 | 1.27 ± 0.02 | 15.53 ± 0.28 | 39.94 ± 3.33 | 40.53 ± 1.96 | 96.01 ± 5.57 |
| 60 | 96.15 ± 0.48 | 2.48 ± 0.02 | 2.38 ± 0.03 | 6.59 ± 0.14 | 237.91 ± 0.89 | 230.36 ± 4.38 | 638.2 ± 16.38 | 1106.47 ± 21.65 |
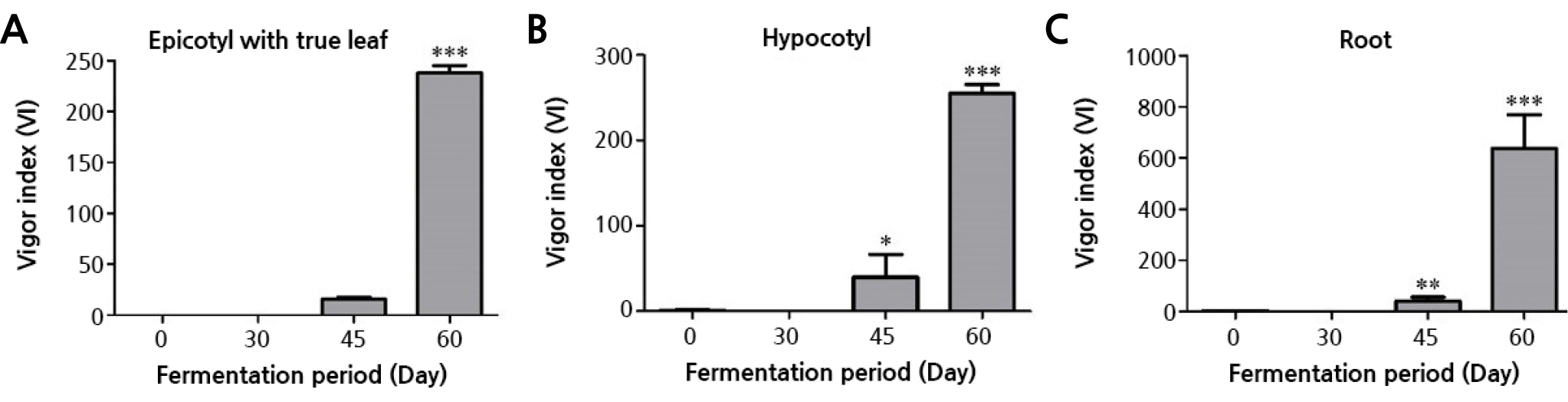
Fig. 4.
Vigor indexes of seedlings grown in oak sawdust with 0, 30, 45, and 60 days of fermentation. 0: no fermentation, 30: 30 days of fermentation, 45: 45 days of fermentation, and 60: 60 days of fermentation. (A) Epicotyls with true leaves, (B) Hypocotyls, and (C) Roots. The mean vigor indexes of the fermentation treatment groups were statistically compared to that of the 0-day fermentation treatment group. Asterisks denote different levels of statistical significance (* p < 0.05, ** p < 0.01 and *** p < 0.001).
Morphological Characteristics of Seedlings under Different Sawdust Fermentation Periods
The morphologies of seedlings grown in each sawdust fermentation treatment (0, 30, 45, 60 days) were observed and photographed (Fig. 5). Only a single seed germinated in the unfermented sawdust (0 days) group (Fig. 5A), and in this case, a neither true leaf nor an epicotyl could be observed. No seed germinated in the sawdust fermented for 30 days (Fig. 5A and 5B). In both the 45- and 60-day fermentation conditions, at least some germinated seedlings had true leaves, hypocotyls, and roots (Fig. 5C and 5D). In particular, most seedlings of the 60-day-fermented sawdust group produced true leaves, epicotyls, hypocotyls, and roots (Fig. 5D). Moreover, the average lengths of the true leaf and epicotyl were longer in this group than those in the 30-day-fermented sawdust group (Fig. 5C and 5D). In addition, the root lengths of seedlings in the 60-day-fermented sawdust group were significantly longer, and the density of the root hairs on the primary root was greater than those of the 45-days-fermented sawdust group (Fig. 5C and 5D).
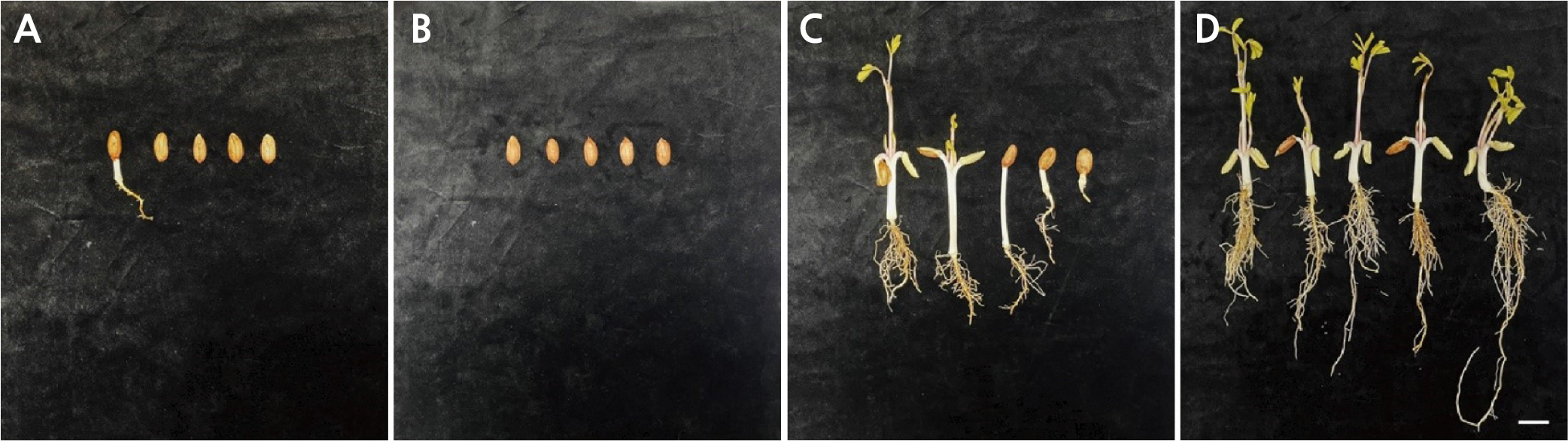
Fig. 5.
Phenotypes of seedlings grown in oak sawdust with 0, 30, 45, and 60 days of fermentation. (A) No fermentation, (B) 30 days of fermentation, (C) 45 days of fermentation, and (D) 60 days of fermentation. The non-fermented and 30-day fermentation treatment groups had only one germinated seed and no germination, respectively. Scale bar = 2 cm.
Fungal Communities in Fermented Oak Sawdust with Different Periods
The members of the fungal community at both the phylum and species levels were classified using the MiSeq pipeline (Fig. 6). In unfermented sawdust, the fungal microbiota communities were dominated by species of the genus Polycephalomyces (43.2%), the families Trichocomaceae (22.7%) and Aspergillaceae (8.63%), and the genus Blastobotrys (8.63%). In the sawdust fermented for 30 days, species of the families Corticiaceae (38.53%), Trechisporaceae (17.74%), Ophiostomataceae (11.17%), and Entolomataceae (8.2%) became the relatively most abundant taxa. In the sawdust fermented for 45 days, species of the families Ophiostomataceae (31.59%), Corticiaceae (21.55%), Trichocomaceae (17.31%), and Cephalothecaceae (9.99%) became the most abundant. Lastly, in the 60-day case, the fungal microbiota communities contained dominant species from the Ophiostomataceae (31.59%), Thermoascaceae (24.06%), Cephalothecaceae (14.34%), and Trichocomaceae (7.19%) families. The fungal communities changed as the fermentation period of the oak sawdust was increased.
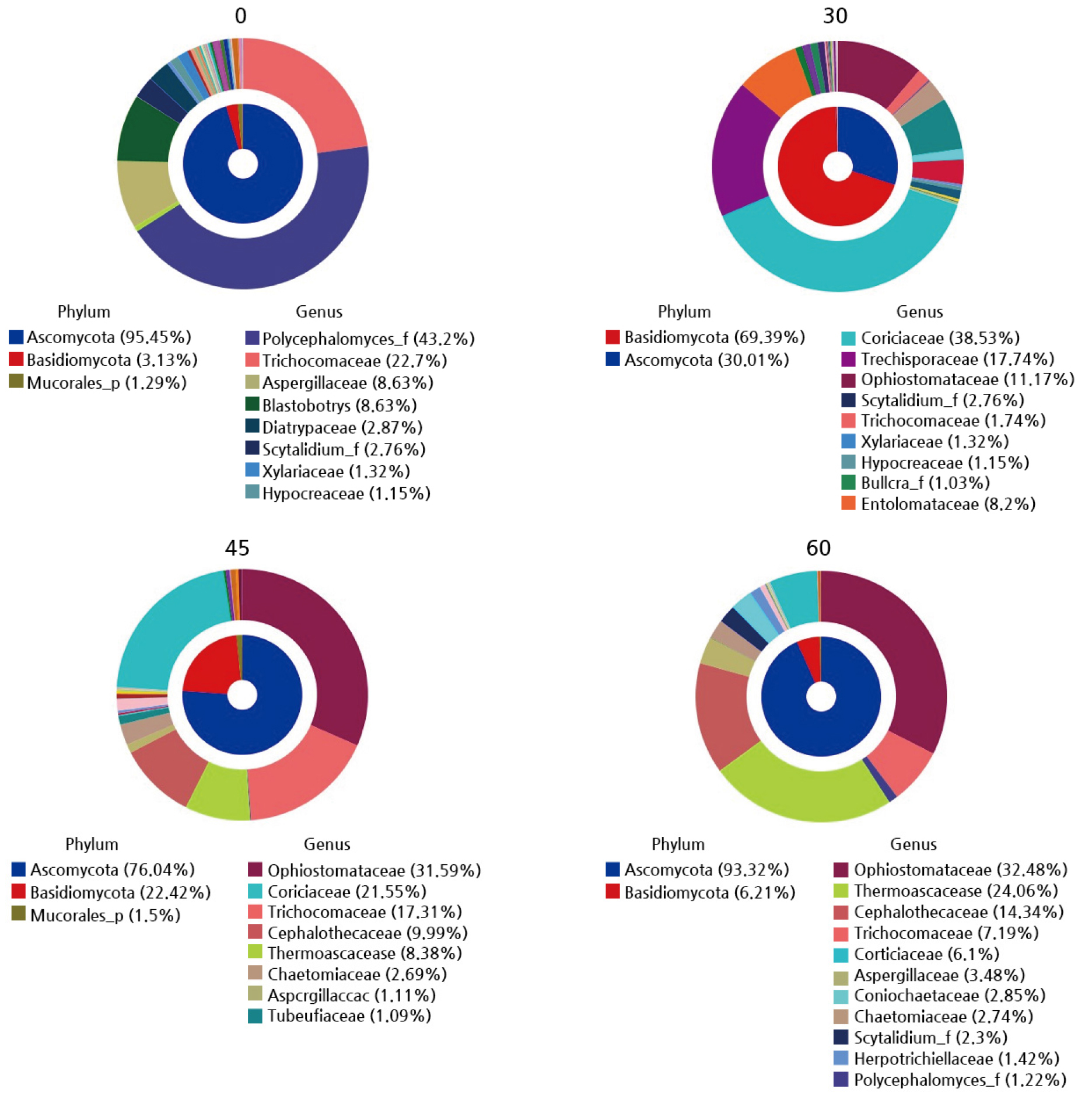
Fig. 6.
Fungal community composition in oak sawdust at 0, 30, 45, and 60 days of fermentation. An NGS analysis was carried out with a 454 GS FLX titanium system (Lee and Eom, 2016; Ahn et al., 2021). Phyla and either the families or genera of fungi with more than 1% proportional abundance in each treatment are presented (inner and outer areas are Phylum and Family/Genus, respectively). 0: non-fermentation, 30: 30 days of fermentation, 45: 45 days of fermentation, and 60: 60 days of fermentation.
Discussion
This study demonstrated that the germination viability and sprout morphology of peanut seeds were affected by the period of oak sawdust fermentation. Peanut seed grown in 60-day-fermented oak sawdust had a significantly higher seed germination rate and greater seedling growth, consequently showing the highest value of the total vigor index among all four sawdust fermentation treatments given the presence of fungal communities favorable for peanut seed germination and seedling growth. In addition, the levels of the fungal genera Ophiostomataceae, Trichocomaceae, and Thermoascaceae were increased in the sawdust fermented for 45 and 60 days (Fig. 6). This observation suggests that oak sawdust fermentation can increase and decrease populations of specific fungal genus, which can in turn be used to enhance the germination and growth of peanut sprouts. Optimized conditions of the temperature, moisture, and pH increase seed germination viability, which is important for plant biomass productivity (Pallas et al., 1977; Bochet et al., 2007; Cho and Lee, 2018). Indeed, the Ophiostomataceae are a family in fungi as pathogens of deciduous trees (Cannon and Kirk., 2007), causing changes in the physical and chemical characteristics of oak tree sawdust for seed germination (Koo et al., 2014). In a previous study, we reported that a certain fermentation period of sawdust as a plant growth medium (Ahn et al., 2021) and hypocotyl end down seed orientation (Ahn et al., 2017) enhanced the seed germination rate and seedling growth. In that earlier work (Ahn et al., 2021), we showed that bacterial communities with a high population of the Alicyclobacillus genus were established in 45- and 60-day fermentation periods. However, we did not investigate fungal community changes. In this study, the peanut seeds sown in sawdust fermented for 0, 30, 45, and 60 days showed significantly increased germination rates (3.9, 0, 30.8, and 65.4%, respectively), suggesting the 45 and 60 days of fermentation enhance seed germination. In addition, the sawdust with the 60-day-fermention period had the seedlings with the highest growth in all measurements and the most developed morphologies, while in the two treatments with the shortest fermentation periods, 0 days and 30 days, seeds almost completely failed to germinate, with only one and zero germinating seeds, respectively. It is speculated that the less fermented sawdust did not provide ideal medium conditions for the irrigation rate and texture including proper microbial communities. The seeds in the 45-day-fermented sawdust produced intermediate growth in all categories; moreover, they had relatively short lateral roots less dense with lateral hairs. Therefore, these results indicate that using oak tree sawdust fermented for 60 days can enhance the growth of lateral roots for better uptake of minerals and water from the soil (Choi and Cho, 2019). These results are not consistent with earlier work (Ahn et al., 2021) that found that a 45-day fermentation period resulted in the best sprout growth at a harvesting time of eight days. It may be that a longer sprout growth period by even two days could provide better hypocotyl and root growth in sawdust fermented for 60 days. Thus, it is recommended that if peanut sprouts with long hypocotyls and roots are desired, a ten-day growth period is recommended rather than eight days. Indeed, previous studies reported that some components of sawdust can be decomposed into xylose and glucose, which are simple compounds, harboring cellulose and lignin complex compounds (De Bont and Leijten, 1976). However, some enzymes of actinomyces could decompose recalcitrant components of sawdust during the fermentation process. Earlier work by the authors demonstrated that the bacterial community and peanut seed germination viability were increased by 45 and 60 days of oak sawdust fermentation (Ahn et al., 2021). It is speculated that a certain fungal community in sawdust could enhance peanut seed germination and seedling growth physiology (Moreno-Gavíra et al., 2020). Sawdust fermentation establishes beneficial microbial communities to produce siderophores for increased nutrient availability and IAA hormones for plant growth while also inhibiting pathogenic microbes, ultimately enhancing plant seed germination viability (Oh et al., 2003; Koo et al., 2014; Moreno-Gavíra et al., 2020; Gariglio et al., 2022; Solomons, 2022). Taken together, the current study is valuable in that it determines the conditions of the optimal oak sawdust matrix with a beneficial microbial community to cultivate peanut sprouts.


