Introduction
Materials and Methods
Plant materials
Cold hardiness determination of bud tissues
Cold hardiness determination of cane tissues
Results
Cold hardiness of dormant bud tissues
Cold hardiness of cane tissues
Discussion
Conclusion
Introduction
Low temperatures during overwintering are essential for temperate fruit crops to fulfill chilling requirements and acquire cold hardiness (Luedeling et al., 2011; Salama et al., 2021). Due to global warming, unseasonable warm spells during overwintering are becoming increasingly common (Pagter and Arora, 2013), causing reduced winter chilling, insufficient acclimation, and premature deacclimation in temperate fruit crops (Arora and Rowland, 2011; Salama et al., 2021). In particular, insufficiently acclimated or prematurely deacclimated temperate fruit crops can be damaged when exposed to subsequent temperature drops after warm spells (Arora and Rowland, 2011; Londo and Kovaleski, 2019; Vyse et al., 2019). To cope with this problem, temperate fruit crops need to be cultivated in colder regions. However, low mid-winter temperatures in the colder regions can hinder the survival and productivity of temperate fruit crops, including grape (Vitis spp.), and therefore limit their geographical distribution (Vyse et al., 2019; Rahemi et al., 2022).
Grape is an increasingly popular temperate, sub-temperate, and sub-tropical fruit crop, with cultivation areas expanding into cold climate regions (North et al., 2021; Rahemi et al., 2022). However, cold hardiness levels of grapevines greatly vary depending on the species, cultivar, and even tissue (Fennell, 2004; Londo and Kovaleski, 2017; Horiuchi et al., 2021). Generally, V. vinifera cultivars are preferred for commercial cultivation due to desirable fruit quality traits. However, these cultivars are more cold-sensitive than other Vitis interspecific hybrid cultivars or wild species, which have genetic backgrounds including V. labrusca, V. riparia, and V. amurensis (Grant and Dami, 2015; Londo and Kovaleski, 2017, 2019; Svyantek et al., 2020; Zhao et al., 2020). Cold hardiness levels in a range of grapevine germplasm resources have also been reported to be associated with osmoregulants, such as soluble sugars and proline, and water contents, which greatly affect the stability of the cell membrane (Ershadi et al., 2016; Karimi, 2020). Accordingly, mid-winter cold hardiness levels of grape cultivars must be evaluated in detail to predict their likelihood of winter survival and productivity in specific cold regions and to establish cultural and breeding strategies that will help overcome freezing stress (Londo and Kovaleski, 2017; Schrader et al., 2019; Jun et al., 2021; North et al., 2021).
When plants are exposed to freezing temperatures, ice forms in extracellular spaces, decreasing the water potential and then causing dehydration of any unfrozen protoplasts (Pearce, 2001; Wisniewski et al., 2014). As the temperature continues to decrease, the formation of intracellular ice crystals causes mechanical damage to the cellular membranes (Pearce, 2001; Arora, 2018). Cell dehydration and membrane disintegration inflict freezing injuries on plant cells (Yamazaki et al., 2009; Arora, 2018). Thus, cold hardiness is often assessed by directly or indirectly analyzing the physiological changes of plant cells or tissues in response to freezing temperatures. During ice formation, abrupt increases in tissue temperatures, called exotherms, arise due to the latent heat release of fusion (Quamme et al., 1972; Burke et al., 1976). Cold hardiness can be evaluated based on the appearance of exotherms during a thermal analysis (Mills et al., 2006; Kaya and Köse, 2017; Londo and Kovaleski, 2017; Yu and Lee, 2020). In particular, low-temperature exotherms (LTEs) caused by intracellular ice formation, which is lethal to the cells, serve as a measure of cold hardiness in dormant buds, which utilize a process of deep supercooling to survive freezing temperatures (Burke et al., 1976; Kaya and Köse, 2017; Londo and Kovaleski, 2017; Svyantek et al., 2020). In practice, differential thermal analysis (DTA) has commonly been used to measure cold hardiness of grapevine buds (Andrews et al., 1984; Mills et al., 2006; Londo and Kovaleski, 2017; Kasuga et al., 2020). Given that cellular leakage is also a symptom of membrane damage caused by freezing stress (Lindén et al., 2000; Arora, 2018), the amount of cellular leakage provides an estimate of the freezing injury (Yu et al., 2017; Yu and Lee, 2020). Indeed, electrolyte leakage (EL) has been the most frequently measured parameter for evaluating plant tissue injuries, in part because EL can be determined continuously and rapidly with an electrical conductivity (EC) probe, requires small sample amounts, and provides quantitative data (Lindén et al., 2000; Lee et al., 2012; Lee et al., 2013; Yu et al., 2017).
In this study, mid-winter cold hardiness levels in the bud and cane tissues of 24 grape cultivars were evaluated by means of LTE and EL analysis methods, respectively, to categorize these cultivars into groups according to their relative hardiness levels. This information can be a useful tool in cold injury mitigation cultural practices. A grape breeder may find this information useful for pedigree consideration if developing cold-tolerant cultivars.
Materials and Methods
Plant materials
Twenty-four five- to seven-year-old grape cultivars grown in an experimental vineyard of the National Institute of Horticultural and Herbal Science in Wanju (35°50ʹN, 127°01ʹE; altitude of approximately 30 m), Korea were used in this study. The 24 grape cultivars, originating from Japan (8), Korea (7), the USA (6), Brazil (1), France (1), and Romania (1), included 19 table grapes and five wine grapes (Table 1). Nine table grapes, ‘Autumn black’, ‘Beni Balad’, ‘Emerald Seedless’, ‘Greaca’, ‘Hongju Seedless’, ‘IFG6’, ‘Ruby Okuyama’, ‘Ruby Seedless’, and ‘Stella’, and one wine grape, ‘Cabernet Sauvignon’, were V. vinifera cultivars, while others were Vitis interspecific hybrid cultivars with different parentage ratios of V. vinifera (Table 1). The own-rooted grapevines were planted at a spacing of 1.8 × 3.0 m, trained to a bilateral cordon, and pruned to two buds per spur. The grapevine trunk heights ranged from 1.3 to 1.4 m and the diameters at 30 cm above the ground were 3.5 to 5 cm. The grapevines were grown under a rain-sheltered system (3.0 × 2.7 m, height × width) with polyethylene film. Each grapevine was irrigated weekly with 60–80 L of water from March to November. Other cultural practices, in this case fertilization, thinning, and pest management, were conducted according to the recommended procedures of the National Institute of Horticultural and Herbal Science. Approximately 50-cm cane segments 0.8–1.2 cm in diameter were collected from the third to eighth nodes of three or four grapevines from each cultivar in mid-January. Minimum, average, and maximum air temperatures in this area were –4.6, 0.1, and 5°C in January of 2022. The collected samples were immediately packed in plastic bags to prevent dehydration and were stored at 4°C until use to analyze the cold hardiness of the bud and cane tissues.
Table 1.
Grape cultivars used in this study
Cold hardiness determination of bud tissues
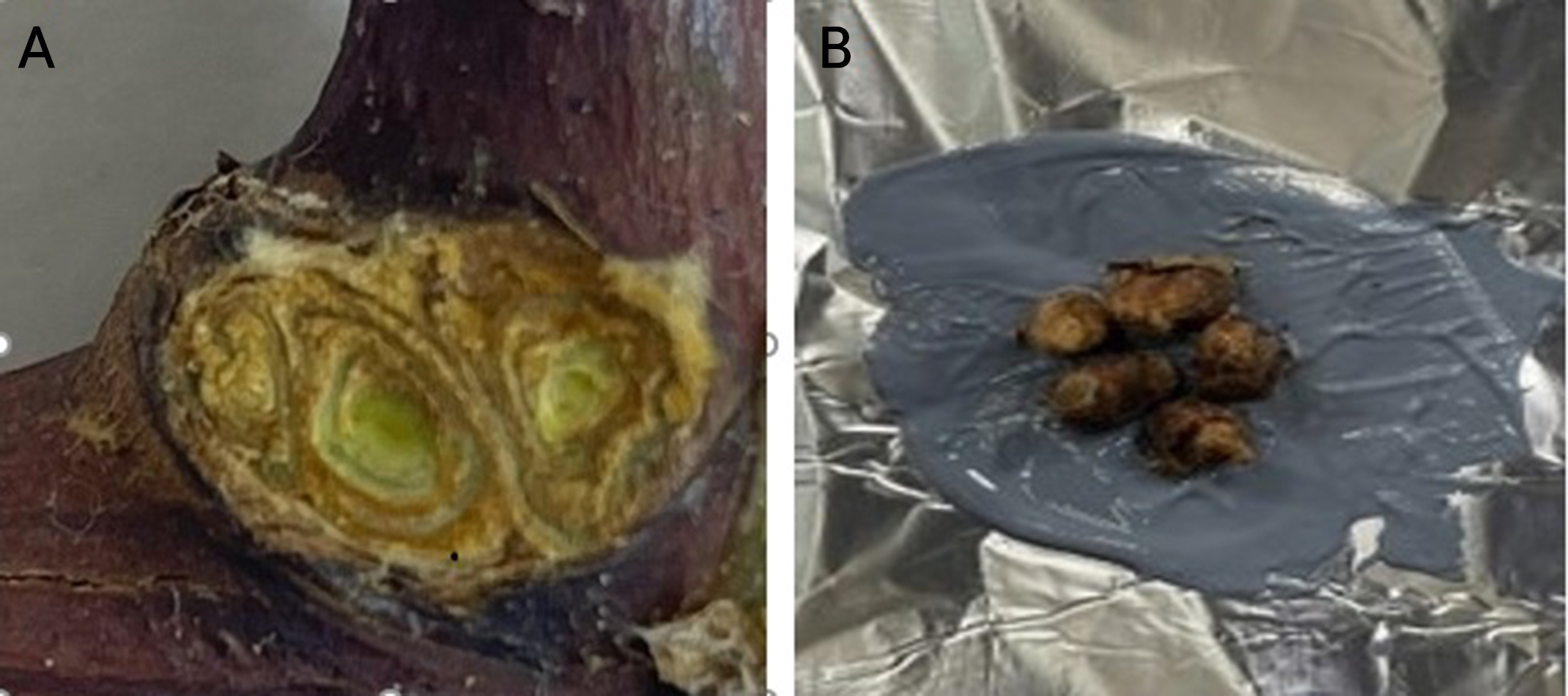
The cold hardiness of the bud tissues was determined via an LTE analysis during the DTA, as described by Jun et al. (2021) with slight modifications. Here, 25 to 30 buds of each cultivar were used to determine the average LTEs. Dormant grapevine buds for the DTA were excised at the basal site of each compound bud from the sampled cane segments (Fig. 1A). The excised buds were immediately stuck to a thin layer of heat sink silicone compound on aluminum foil (Fig. 1B) to prevent the tissue from drying and to ensure high thermal conductivity between the bud tissue and a copper-constantan thermocouple attached to the outer surface of the aluminum foil. Temperature differentials between completely dried reference and fresh sample buds were analyzed during cooling to ‒35°C at a rate of ‒5°C/h in a programmable bath circulator (RW-2040G, Jeio Tech, Seoul, Korea) equipped with a temperature controller. Temperatures during the DTA process were recorded every 10 s using a data logger (CR-1000M, Campbell Scientific, Inc., Logan, UT, USA). The initial base point of each LTE peak was recorded as the LTE temperature of each bud.
Cold hardiness determination of cane tissues
The cold hardiness of the cane tissues was determined by means of an EL analysis following the method of Jun et al. (2021) with slight modifications. Internodes of the cane segments were cut into 8 cm pieces and randomly divided into five groups. Three out of the five groups were cooled at a rate of ‒2°C/h in the programmable bath circulator (RW-2040G, Jeio Tech) until they reached each of three target temperatures ranging from ‒5 to ‒35°C, after which they maintained at each target temperature for 2 h and then thawed at 0°C. Temperatures during freezing and thawing were recorded every minute using a data logger (CR-1000M, Campbell Scientific, Inc.) with copper-constantan thermocouples. The other two groups were separately incubated in a refrigerator at 4°C and in a freezer at ‒80°C, respectively, for the non-injured and completely injured controls. Following the freezing and thawing treatment, the cane internodes were cut into 0.5 cm pieces, and five pieces of each sample were shaken in a 50-mL tube containing 10 mL of distilled water at 125 rpm on an orbital shaker (Supertech Orbital Shaker, SeouLin Bioscience, Seoul, Korea) at room temperature for 24 h. The initial EC of each aliquot was then measured using an EC meter (Orion Star A215, Thermo Fisher Scientific, Waltham, MA, USA). After autoclaving the samples at 120°C for 30 min, the total EC was measured.
Injury percentages were calculated as % EL = (initial EC/total EC) × 100 and were adjusted with percent injury data at 4 and –80°C to represent 0 and 100%, respectively, using an equation reported by Yu et al. (2017) (Suppl. Table S1). Using the percent-adjusted injury data, a quantitative estimate of cold hardiness, i.e., the median lethal temperature (LT50), was calculated using the Gompertz function, an asymmetric sigmoid curve appropriate for use in the data fitting of plant responses to temperature stress (Lim et al., 1998) (Suppl. Fig. S1). The percent-adjusted injury data of each cultivar were resampled 30 times as described by Arora et al. (2004) to obtain efficient LT50 estimates without repeating the entire experiment.
Results
Cold hardiness of dormant bud tissues
Most of the excised dormant buds tested in this study did not produce high-temperature exotherms (HTEs), instead producing one or two LTEs during the DTA. The LTE values of the 24 cultivars ranged from ‒13.6 to ‒19.9°C on average.
Based on the difference between the lowest and highest averages of the LTEs, the 24 cultivars were classified into the following five groups at intervals of 1.5°C: very cold-sensitive, cold-sensitive, moderately cold-hardy, cold-hardy, and very cold-hardy (Table 2). Generally, V. vinifera cultivars produced higher LTEs than Vitis interspecific hybrid cultivars. V. vinifera ‘Autumn Black’, ‘Hongju Seedless’, and ‘Cabernet Sauvignon’ were evaluated as very cold-sensitive. Their LTE values exceeded ‒14.5°C. Five V. vinifera, ‘Stella’, ‘Beni Balad’, ‘Ruby Seedless’, ‘Ruby Okuyama’, and ‘Emerald Seedless’, and three interspecific hybrid cultivars, ‘Shooting Star’, ‘Shine Muscat’, and ‘Violet King’, were determined to be cold-sensitive. Notably, the cold-sensitive interspecific hybrid cultivars had a high parentage ratio of V. vinifera (Table 1). The interspecific hybrid ‘Ageude’, V. vinifera ‘IFG6’, and the unknown ‘Shouheikou’ were evaluated as moderately cold-hardy. The unknown ‘Shouheikou’ is believed to be from a chance seedling of European red grape (Table 1). Six interspecific hybrid cultivars, ‘Beta’, ‘Campbell Early’, ‘Gaeryangmeoru’, ‘Bailey Alicante A’, ‘Shigyoku’, and ‘Tano Red’, were cold-hardy. These include a wide range of Vitis species, including wild types apart from ‘Shigyoku’, which is a mutant of V. labruscana ‘Takasumi’ (Table 1). ‘Cheongsoo’, ‘Muscat Bailey A’, and ‘Shiny Star’, which are interspecific hybrid cultivars mostly from a cross between V. vinifera and V. labruscana, were very cold-hardy, with LTE values below ‒19.6°C (Table 2). However, the evaluation of V. vinifera ‘Greaca’ showed it to be very cold-hardy as the interspecific hybrid cultivars.
Table 2.
Mid-winter cold hardiness in the bud tissues of 24 grape cultivars estimated by a low-temperature exotherm (LTE) analysis
| Cultivar | LTE (°C) | Cold hardiness level |
| Autumn Black | ‒13.6 ± 2.3z |
Very cold-sensitive (LTE > ‒15.0°C) |
| Hongju Seedless | ‒14.4 ± 2.7 | |
| Cabernet Sauvignon | ‒14.5 ± 0.5 | |
| Stella | ‒15.4 ± 2.3 |
Cold-sensitive (‒16.5°C < LTE < ‒15.0°C) |
| Shooting Star | ‒15.4 ± 2.3 | |
| Beni Balad | ‒15.9 ± 2.2 | |
| Ruby Seedless | ‒16.0 ± 2.9 | |
| Ruby Okuyama | ‒16.0 ± 3.4 | |
| Emerald Seedless | ‒16.3 ± 2.5 | |
| Shine Muscat | ‒16.3 ± 3.2 | |
| Violet King | ‒16.4 ± 2.6 | |
| Ageude | ‒17.1 ± 3.3 |
Moderately cold-hardy (‒18.0°C < LTE < ‒16.5°C) |
| IFG6 | ‒17.1 ± 4.2 | |
| Shouheikou | ‒17.2 ± 3.1 | |
| Beta | ‒18.0 ± 3.0 |
Cold-hardy (‒19.5°C < LTE < ‒18.0°C) |
| Campbell Early | ‒18.0 ± 3.6 | |
| Gaeryangmeoru | ‒18.1 ± 3.1 | |
| Bailey Alicante A | ‒18.1 ± 3.8 | |
| Shigyoku | ‒18.4 ± 4.5 | |
| Tano Red | ‒19.1 ± 3.2 | |
| Greaca | ‒19.5 ± 3.6 |
Very cold-hardy (LTE < ‒19.5°C) |
| Shiny Star | ‒19.6 ± 3.4 | |
| Muscat Bailey A | ‒19.9 ± 3.2 | |
| Cheongsoo | ‒19.9 ± 3.9 |
Cold hardiness of cane tissues
In cane tissues, the percent-adjusted injury EL data at freezing temperatures exhibited a sigmoidal curve, fitting well to the Gompertz function (Suppl. Fig. S1), indicating that EL of the cane tissues provided a reproducible LT50. The LT50 values of the 24 cultivars ranged from ‒20.6 to ‒37.7°C.
Based on the difference between the lowest and highest averages of the LT50 values, the 24 cultivars were classified into five groups at intervals of 3.5°C (Table 3), as with the bud tissues. In the cane tissues, Vitis interspecific hybrid cultivars were cold-hardier than V.vinifera cultivars. Among the interspecific hybrid cultivars, ‘Ageude’, ‘Shine Muscat’, ‘Beta’, and ‘Campbell Early’ were evaluated as very cold-hardy, while ‘Shooting Star’ was very cold-sensitive with the V.vinifera ‘Emerald Seedless’, ‘IFG6’, and ‘Autumn Black’. The very cold-sensitive interspecific hybrid cultivars had a high parentage ratio of V. vinifera. The LT50 values of the very cold-hardy and the very cold-sensitive cultivars were below ‒35.5°C and above ‒24.0°C, respectively (Table 3). Unlike in the bud tissues, the V. vinifera ‘Cabernet Sauvignon’ was evaluated as cold-hardy, as were the interspecific hybrid cultivars of ‘Shiny Star’, ‘Bailey Alicante A’, ‘Cheongsoo’, ‘Tano Red’, and ‘Gaeryangmeoru’. The interspecific hybrid ‘Violet King’ was cold-sensitive, along with the unknown ‘Shouheikou’ and the V. vinifera ‘Ruby Seedless’, ‘Hongju Seedless’, ‘Stella’, and ‘Ruby Okuyama’, while the interspecific hybrid cultivars of ‘Shigyoku’ and ‘Muscat Bailey A’ were moderately cold-hardy, like the V. vinifera ‘Beni Balad’ and ‘Greaca’.
Table 3.
Mid-winter cold hardiness (LT50) in the cane tissues of 24 grape cultivars estimated by an electrolyte leakage analysis
| Cultivar | LT50 (°C) | Cold hardiness level |
| Emerald Seedless | ‒20.6 ± 0.6z |
Very cold-sensitive (LT50 > ‒25.0°C) |
| IFG6 | ‒21.3 ± 1.3 | |
| Autumn Black | ‒23.5 ± 0.9 | |
| Shooting Star | ‒24.0 ± 2.6 | |
| Shouheikou | ‒25.1 ± 0.7 |
Cold-sensitive (‒28.5°C < LT50 < ‒25.0°C) |
| Ruby Seedless | ‒25.5 ± 3.4 | |
| Violet King | ‒26.5 ± 0.9 | |
| Hongju Seedless | ‒26.6 ± 1.2 | |
| Stella | ‒27.5 ± 1.9 | |
| Ruby Okuyama | ‒27.7 ± 1.8 | |
| Shigyoku | ‒28.5 ± 1.3 |
Moderately cold-hardy (‒32.0°C < LT50 < ‒28.5°C) |
| Greaca | ‒29.1 ± 0.3 | |
| Beni Balad | ‒30.5 ± 1.6 | |
| Muscat Bailey A | ‒31.2 ± 1.5 | |
| Gaeryangmeoru | ‒32.2 ± 1.0 |
Cold-hardy (‒35.5°C < LT50 < ‒32.0°C) |
| Cabernet Sauvignon | ‒32.6 ± 4.6 | |
| Tano Red | ‒33.3 ± 0.3 | |
| Cheongsoo | ‒34.0 ± 2.2 | |
| Bailey Alicante A | ‒34.3 ± 1.9 | |
| Shiny Star | ‒34.6 ± 5.4 | |
| Campbell Early | ‒35.5 ± 4.1 |
Very cold-hardy (LT50 < ‒35.5°C) |
| Beta | ‒35.6 ± 1.6 | |
| Shine Muscat | ‒35.7 ± 4.1 | |
| Ageude | ‒37.7 ± 1.5 |
Discussion
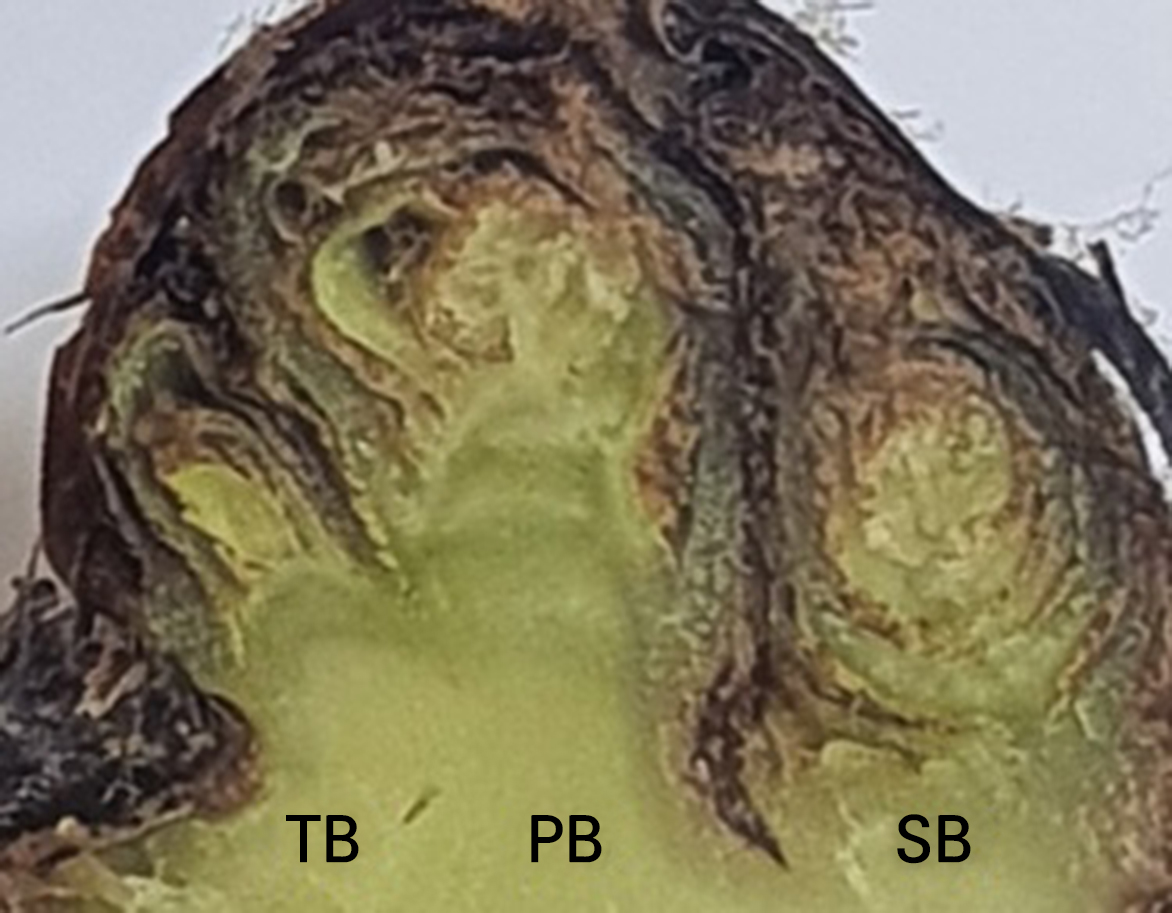
Dormant grapevine buds are composed of primary, secondary, and tertiary primordial buds (Fig. 2; Kasuga et al., 2020; Horiuchi et al., 2021). During a DTA, supercooled water in primary, secondary, and tertiary primordial buds can separately be frozen (Kasuga et al., 2020), suggesting that a dormant grapevine bud could produce up to three HTEs and LTEs, each with differing locations and magnitudes during the DTA (Andrews et al., 1984; Köse, 2006; Kaya and Köse, 2017; Kasuga et al., 2020). The primary primordial bud, which is the most developed bud, commonly produces higher levels and larger amounts of HTEs and LTEs than the secondary or tertiary primordial buds (Wolf and Cook, 1994; Fennell, 2004). As grapevine primordial bud tissues do not have large intercellular spaces for extracellular ice formation (Kasuga et al., 2020), the HTEs in dormant grapevine buds correspond to ice formation at the basal areas of the bud axis and scales, a process known as extraorgan freezing, as opposed to the extracellular freezing in primordial buds (Köse, 2006; Kasuga et al., 2020). Extraorgan ice crystals that initially form at freezing temperatures could induce partial dehydration of primordial buds and promote the supercooling capacity of dormant grapevine buds (Kasuga et al., 2020; Horiuchi et al., 2021). However, the bud tissues may not produce HTEs during a DTA if their basal areas for extraorgan freezing are removed. Because the excised dormant grapevine buds were blocked by silicone compounds to prevent the tissue from drying in this study, the primordial bud tissues of the excised buds were not dehydrated and thus their LTEs were detected at higher temperatures than those of intact buds (Andrews et al., 1984; Köse, 2006; Kasuga et al., 2020; Horiuchi et al., 2021).
The number of detectable LTEs in a dormant grapevine bud can vary depending on the bud structure and size (Andrews et al., 1984; Mills et al., 2006; Kasuga et al., 2020; Küpe and Köse, 2020). In general, DTA is not applied to fruit crops with small dormant buds, as small dormant buds are less likely to contain water to freeze nor produce LTEs (Neuner et al., 2019). Küpe and Köse (2020) reported that dormant buds with an area of less than 0.01 mm2 in ‘Karaerik’ (V. vinifera) and ‘53 Pazar 01’ (V. labrusca) grapevines did not produce LTEs. Thus, secondary and tertiary primordial buds are too small to contain enough water to form ice during a DTA when excised from cane segments. Consequently, most of the dormant grapevine buds’ LTEs as determined in this study are likely to represent the cold hardiness of primary primordial buds. The LTEs monitored during the DTA were not affected greatly by cooling rates within 10°C, while the LT50 values according to the EL analysis were relatively overestimated according to the experimental conditions such as the cooling rate or exposure time to target freezing temperatures (Yu and Lee, 2020).
Any process that damages the plant cell membrane will cause cellular leakage (Steponkus, 1984; Lindén et al., 2000; Arora, 2018). Cellular leakage is a symptom of membrane damage caused by freezing stress (Lindén et al., 2000; Arora, 2018). Such leakage can occur without a membrane rupture, but is more pronounced when the membrane ruptures and the entire cell contents are released (Yamazaki et al., 2009). Thus, recording the amount of cellular leakage provides an estimate of freezing injury in many temperate fruit crops (Lee et al., 2012; Lee et al., 2013; Moghadam et al., 2015; Yu et al., 2017). Many different types of cellular contents can be sampled to determine cellular the degree of leakage. However, EL is the most frequently measured parameter for evaluating tissue injury given that it can be determined continuously with an EC probe and does not require destructive sampling (Lindén et al., 2000; Lee et al., 2012; Lee et al., 2013; Yu and Lee, 2020). EL analyses have been used to evaluate freezing injury in grapevine canes (Jones et al., 1999), red raspberry canes (Lindén et al., 2000), blueberry shoots (Lee et al., 2012; Lee et al., 2013), and peach trunk bark and wood tissues (Yu et al., 2017). However, an EL analysis of buds is difficult due to the small amounts of sample materials. Although EL is not a direct measurement of cellular injury caused by freezing, the values from an EL analysis have correlated well with cold hardiness in many temperate fruit crops (Lindén et al., 2000; Lee et al., 2013; Yu et al., 2017; Jun et al., 2021).
Cold hardiness of grapevines generally depends on their genetic backgrounds. V. vinifera cultivars grown in a cool climate can often undergo freezing damage during overwintering (Grant and Dami, 2015; Schrader et al., 2019). Generally, cold-hardy cultivars of V. riparia or V. labrusca parentage can survive the low temperatures of a harsh winter, while those with a high parentage ratio of V. vinifera are vulnerable to such temperatures (Bourne et al., 1991; Fennell, 2004; North et al., 2021). Notably, V. riparia, a wild species that originates from North America, is one of the most cold-hardy species and has frequently been used in programs for breeding new cold-hardy hybrid cultivars (Bourne et al., 1991; Fennell, 2004; Londo and Kovaleski, 2019). In this study, however, the bud tissue of V. vinifera ‘Greaca’ was rated as very cold-hardy (Table 2). This suggests that the genetic background is not the only factor determining cold hardiness (North et al., 2021; Rahemi et al., 2022). The ranking based on mid-winter cold hardiness may differ depending on the cultivation time or site, as cold hardiness is not static but varies based on environmental conditions and cultural practices (Fennell, 2004; North et al., 2021; Rahemi et al., 2022). Ambient temperatures, crop load, training and pruning system affecting light penetration within the canopy, and fertilization and irrigation practices may influence physiological conditions, which can greatly affect cold hardiness levels of bud and cane tissues. In particular, physiological indices such as soluble sugars, proline, and water contents have been reported to be highly consistent with cold hardiness levels in a range of grapevine germplasm resources (Ershadi et al., 2016; Karimi, 2020). Therefore, identifying the genetic potential of different cultivars, which is influenced by environmental and cultural factors, for maximal cold hardiness is necessary for the successful management of vineyards and the development of new cultivars with stronger cold hardiness. In any case, moderately cold-hardy V. vinifera cultivars such as ‘Greaca’ could be useful as parental materials for breeding cold-hardy intraspecific hybrid cultivars with desirable fruit quality levels. Similarly to the case of V. labrusca, V. flexuosa and V. lincecumii, which are wild species native to Korea (Moon et al., 2017) and the USA (Lisek, 2012), respectively, are likely to affect the cold hardiness of interspecific hybrid cultivars (Tables 2 and 3). V. flexuosa was reported to be more tolerant to both biotic and abiotic types of stress than most cultivars (Moon et al., 2017).
The cold hardiness levels of the 24 grape cultivars were differently ranked depending on the tissue type, for example ‘Cabernet Sauvignon’, ‘Greaca’, and ‘IFG6’ (Tables 2 and 3). Unlike in the findings of Wang et al. (2022), in this study, the cane tissues of ‘Cabernet Sauvignon’ were evaluated as cold-hardy, as were some interspecific hybrid cultivars, including ‘Bailey Alicante A’ (Table 3). The mechanisms responsible for cold hardiness can often differ within the same plant over relatively short distances (Jones et al., 1999; Gusta and Wisniewski, 2013). Grapevine bud and cane tissues have different strategies by which to adapt to freezing temperatures: bud tissues avoid freezing by supercooling, while cane tissues tolerate extracellular freezing (Jones et al., 1999). These differences mean that mid-winter cold hardiness of bud and cane tissues can be ranked differently. Because LTE and EL analyses were found to be reliable for evaluating the cold hardiness of grapevine buds and canes, respectively, their combined application could be useful for assessing the tolerance of grape cultivars against freezing stress.
Conclusion
Mid-winter low temperatures greatly limit the survival and productivity of some grape cultivars, as well as their geographical distribution. In this study, mid-winter cold hardiness levels in the bud and cane tissues of 24 cultivars were compared by means of LTE and EL analyses, respectively. Based on the cold hardiness estimates on the bud and cane tissues, the 24 cultivars were classified into five groups: very cold-sensitive, cold-sensitive, moderately cold-hardy, cold-hardy, and very cold-hardy. The cold hardiness levels of the 24 cultivars greatly depended on their genetic backgrounds. Generally, Vitis interspecific hybrid cultivars such as ‘Cheongsoo’, ‘Shiny Star’, ‘Beta’, and ‘Campbell Early’ were more apt to withstand cold, i.e., were cold-hardier, than the V. vinifera cultivars ‘Autumn Black’, ‘Hongju Seedless’, and ‘Emerald Seedless’ according to tests of both tissues. Certain interspecific hybrid cultivars, which were evaluated to be relatively cold-sensitive, included a high parentage ratio of V. vinifera. In some cultivars, however, the cold hardiness levels differed by tissue type regardless of their genetic backgrounds. For example, V. vinifera ‘Greaca’ bud and cane tissues were evaluated as very cold-hardy and moderately cold-hardy, respectively. Therefore, different evaluation methods, including field assessments of tissue browning in buds and canes, are needed to predict the field hardiness levels of grape cultivars reliably. Based on the combined cold hardiness estimates of both tissues, ‘Shiny Star’ and ‘Cheongsoo’ were relatively cold-hardier than other cultivars, while ‘Autumn Black’ and ‘Hongju Seedless’ were relatively more cold-sensitive. These results will help establish cultural and breeding strategies to overcome freezing stress on grapevines.
Supplementary Material
Supplementary materials are available at Horticultural Science and Technology website (https://www.hst-j.org).
- HORT_20240057_Fig_S1.pdf
Supplementary Fig. S1. An asymmetric sigmoid curve fitted to the percent-adjusted injury data by the Gompertz function for estimating the median lethal temperature (LT50) in the canes of ‘Ageude’ grapevines.
- HORT_20240057_Table_S1.pdf
Supplementary Table S1. Sample calculation of percent-adjusted injuries at various temperatures through an electrolyte leakage (EL) analysis in the canes of ‘Ageude’ grapevines