Introduction
Materials and Methods
Strains and Cultivating Conditions
Morphological Analysis
Physiological Analysis
Data Analysis
Results
Plant Morphology
Leaf Color
Chlorophyll and Carotenoid Content
Photosynthesis
Essential Oil Content and Yield
Correlation of Characteristics
Discussion
Introduction
Common sage (Salvia officinalis) is represented by more than 900 species which are widely distributed over the world (Mozaffarian, 1996). Sage has been characterized as one of the “sun species” of the Lamiaceae family (Castrillo et al., 2005) and an evergreen plant of the Mediterranean region. It is currently cultivated in several countries for use in medicine, food, and perfume (Santos-Gomes et al., 2002). It is an aromatic perennial subshrub native to Minor Asia and Southern Europe. Since antiquity, dried sage leaves have been used in folk medicine for the treatment of colds, bronchitis, tuberculosis, hemorrhage, and menstrual disorders (Topcu, 2006). The effectiveness of sage is mostly related to the activity of several secondary metabolites, especially essential oils and phenolic compounds, which have, among others, antioxidant (Pizzale et al., 2002; Santos-Gomes et al., 2002), hepatoprotective (Lima et al., 2005, 2007), antimicrobial, spasmolytic, and antiviral properties (Bozin et al., 2007). S. officinalis has recently been used as a popular landscape plant and an ornamental garden subshrub (Devecchi, 2006).
Many morphological and physiological processes in plants are affected by irradiance, which is one of the most important environmental factors affecting plant survival, growth, reproduction, and distribution (Keller et al., 2005; Kumar et al., 2011). However, light intensity is the most difficult environmental factor to control (Wang et al., 2007). A considerable amount of light energy is required to reduce carbon, where it is combined with CO2 to produce oxygen, carbohydrates, ATP, and NADPH. The carbohydrates are translocated from leaf cells and green stems to other parts of the plant. Carbohydrates can be converted into other compounds such as amino acids, hormones, and essential oils. These processes result in plant growth, which can be detected as an increase in dry matter (Taiz and Zeiger, 2002). Plants growing under high irradiance (1,000 or 2,000 µmol·m-2 ·s-1 PAR) absorb excessive light energy (Dole et al., 2004). As a result, inactivation of the photosynthetic apparatus or impairment of chlorophyll containing reaction centers of the chloroplasts might occur (Aro et al., 1990; Bertaminia et al., 2006). Demming-Adams and Adams (1996) reported that the number of inactive PSП reaction centers in soybean leaves increased under strong light and decreased in subsequent darkness. Therefore, photo-inhibition might reduce photosynthetic activity (Osmond, 1994). In contrast, under low irradiance conditions, insufficient ATP production leads to the reduction of carbon assimilation and plant growth (Dai et al., 2009). Kumar et al. (2013) and Corre et al. (1983) also reported that low irradiance causes a reduction in stomatal conductance and photosynthesis rate, and therefore the plant growth rate.
S. officinalis is an economically interesting plant. Together with Rosmarinus officinalis L. (rosemary), it has the highest antioxidant activity among the herbs (Santos-Gomes et al., 2002). The essential oil content of sage varies depending on its geographical habitat and environmental condition (Singh et al., 2008). Studies on the production of essential oil under different light intensity levels have shown that each species responds to shade levels differently. For example, Thymus vulgaris (thyme) (Li et al., 1996) and Matricaria chamomilla (camomile) (Saleh et al., 1973) exhibited increased essential oil content when grown under intense light (full sunlight), while Anethum graveolens (dill) (Halva et al., 1992), S. officinalis (sage) (Li et al., 1996) and Pothomorphe umbellate (pariparoba) (Mattana et al., 2010) had higher essential oil contents when cultivated under shade.
The purpose of the present study was to determine the optimum light intensity for the growth of sage by quantifying the effects of different shading treatments on plant morphology, chlorophyll and carotenoid content, photosynthetic capacity, and other characteristics in a semi-arid region of Iran.
Materials and Methods
Strains and Cultivating Conditions
S. officinalis plants were obtained from the Agriculture and Natural Resources Research Center of Isfahan, Iran. Two-month old rooted cuttings were planted in pots containing 22% sand, 37% clay, 41% silt, and 0.067% organic matter. All plants were grown under a shade cloth (about 30% of full sun light) for an acclimation period of 10 days. After that, pots were placed in mini-tunnels 1 m high, 2 m long, and 1 m wide at the agricultural department of Isfahan University of Technology. Shading structures were oriented with the longitudinal axis running north-south (51.5° 31' 55.2" N and 31.7° 43' 4.8" E).
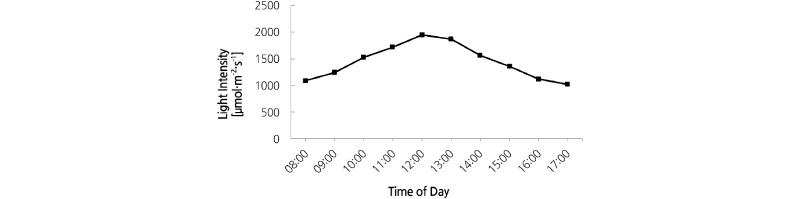
Plants were randomly divided into four groups which were subjected to four different light intensities obtained with shade cloth providing 0, 30, 50, or 70% of natural incident irradiance. Shading was accomplished by using one or two layers of commercial green polyethylene shade cloth for 5 months starting from April 1, 2013. The experimental design was completely randomized with three replicates. The mean daily variation in full sunlight from April to August in Isfahan, measured with a ST-1309 Digital Lux Meter (ST, Taiwan), is displayed in Fig. 1. Maximum monthly ambient temperatures were between 37°C and 51°C inside and outside the tunnel, respectively. Drip-irrigation was provided during the growing season as needed. The photosynthetic active radiation (PAR) incident on leaf surfaces was about 2,000 µmol·m-2·s-1 for full sunlight on a sunny day.
Morphological Analysis
Leaf area was determined by Delta-T Scan Image Analysis (Delta-T Devices, DTS-COM110, Cambridge, England). Thickness of recently expanded leaves was measured by digital coulis (Hans bear AG, Japan). The color coordinate CIE L*a*b* was scanned with a reflection spectrophotometer (Texflash, Data Color, Swiss). Healthy, completely matured young
leaves (3rd node from the top of the shoot) were selected to evaluate L (lightness), C (chroma), and h (hue angle) (Hunter, 1975; Brand, 1997) at the midpoint between the basal and distal ends of the leaf. The plant height and number of leaves per plant were recorded monthly and the mean values were reported. The total fresh weight of dry leaves and shoots was measured at harvest time by cutting the main stem at the soil line. Samples were dried to a constant weight in an oven at 85°C for 48 h.
Fresh sage leaves between the 1st and 3rd node from the top of the shoot were harvested at the end of the experiment. They were dried to constant weight in the laboratory at room temperature (25°C) for 5 d. 50 g of dry leaves from the four treatments were separately hydro distilled for 4 h with a Clevenger-type apparatus to extract essential oils. The oils were stored in a dark glass bottle at 4°C and reported on a v/w basis. The essential oil productivity (kg·ha-1) was calculated by multiplying the essential oil content by the specific gravity of oil (0.9) and the aerial biomass yield of the respective treatments.
Physiological Analysis
Photosynthetic yield (Pn) and stomatal conductance response were recorded using an LCi portable photosynthesis system with software version 1.10 (ADC, Bio Scientific Ltd., England). The parameters were recorded from the youngest fully expanded leaf (3rd node from the top of the shoot) between 09:00 and 11:00 a.m. on a clear bright day. Leaves of three plants
per treatment were measured twice within a month. The leaf sample was placed in a cuvette (27°C) and exposed to 2,000, 1,400, 1,000 and 600 µmol·m-2·s-1 PAR corresponding to full sunlight, 30%, 50%, and 70% shade, respectively, with an
ambient CO2 concentration of 350 µmol·m-2·s-1. Water use efficiency (WUE) was calculated as Pn/E (µmol CO2 /µmol H2O transpiration) (Galmes et al., 2007). Assimilation was recorded for each condition following a 10 min acclimation period.
Expanded young leaves were collected for monthly determination of chlorophyll and carotenoid content (Chl a, Chl b, Chl a +b, Chl a/ b,and Cx+c). Photosynthetic pigments were extracted from fresh leaves and absorbance (A) was measured using a scanning spectrophotometer (UV-600A, Japan). The specific absorption coefficients of Chl a, Chl b, and total carotenoids reported by Lichtenthaler (1987) were used and the mean values of the data were recorded.
Data Analysis
Analysis of variance (ANOVA) for each trait and the correlation between traits was assessed using SAS version 9.1 (SAS Ins., Cary, NC, USA). Differences between treatment means were separated by the least significance difference (LSD) at the 95% confidence level (p < 0.05).
Results
Plant Morphology
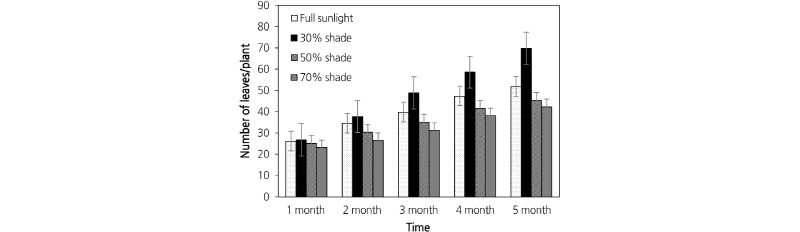
Leaf morphology was significantly affected by shade treatment. Plants grown under 50% (Fig. 2c) and 70% shade (Fig. 2d) had the largest leaves, while the leaves of plants grown under full sunlight (Fig. 2a) were the smallest. As shown in Table 1, leaf thickness decreased with increasing shade levels. Shading had a significant effect on plant height (p < 0.05) with plants grown under intense shade levels (50% and 70% shade) being taller than other treatments and those grown under full sunlight being shortest. The leaf number/plant in 30% shade was significantly greater than other treatments (p < 0.05). The mean values of leaf number/plant during the 5 month experiment are shown in Fig. 3. Leaf number increased from 70% to 30% shade, but decreased in 0% shade. The influence of irradiance on fresh biomass was evident throughout this study. The highest fresh biomass was obtained in plants grown under 30% shade, with dry biomass exhibiting a similar response (Table 1).
Table 1. Effect of shade levels on morphological and physiological characteristics of sage
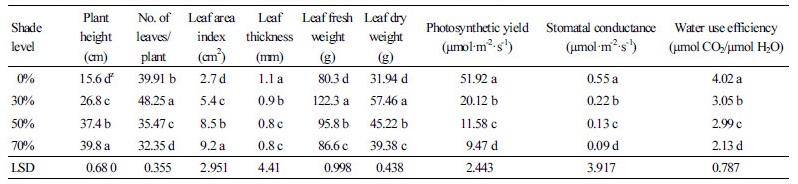 | |
zMeans with the same letters in a column are not significantly different at p < 0.05, using LSD. | |
Leaf Color
Shading significantly influenced the leaf color in all treatments (p < 0.05). The leaf color of plants grown under 30% shade (Fig. 2b) was the darkest gray- green, while those grown under 50% (Fig. 2c) and 70% shade (Fig. 2d) were lighter. The lowest L * and C * values for leaf color were observed in 30% shade with the highest values in plants grown under 50% and 70% shade
(Table 2). 30% shading resulted in a higher C value that made the foliage color more vivid (Table 2). The decrease in L and C * were consistent with a change in hue angle from gray-green to yellowish-green.
Chlorophyll and Carotenoid Content
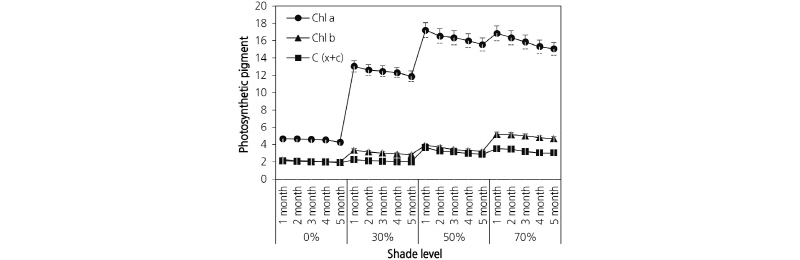
Photosynthetic pigments were measured monthly and are shown in Fig. 4. Chlorophyll and carotenoid content were significantly affected (p < 0.05) by the different light treatments (Fig. 4). Plants grown under 50% and 70% shade had the greatest Chl a, Chl b, Chl a/b, total chlorophyll, and carotenoid content. Chl a content was much higher than Chl b content across all treatments. The lowest Chl a, Chl b, total chlorophyll, and carotenoid content was observed in plants grown in full sunlight and 30% shade.
Photosynthesis
The photosynthetic rate and stomatal conductance showed a strong positive correlation with light intensity (Table 1). Plants grown under 70% shade (600 µmol·m-2·s-1) showed a substantial decrease compared with plants grown under full sun light
(2,000 µmol·m-2·s-1). WUE also decreased as the light intensity decreased (Table 1). The highest water use efficiency was observed under full sun light and the lowest under the 70% shade treatment.
Essential Oil Content and Yield
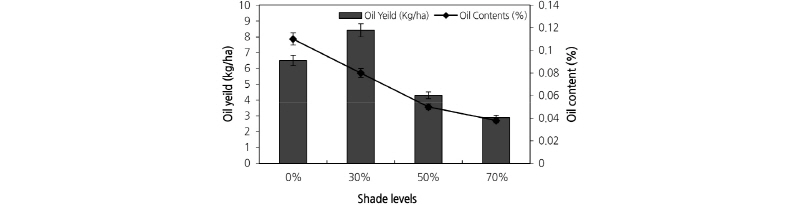
Essential oil content also decreased with increasing shade levels (Fig. 5). Sage plants grown under 30% and 50% shade levels had similar essential oil contents. The yield of essential oil was highest in plants grown under 30% shade and decreased in full sunlight and with increasing shade levels (Fig. 5).
Correlation of Characteristics
We examined correlations between different morphological and physiological characteristics of sage in different shade levels and observed a linear relationship between photosynthetic yield, stomatal conductance, and WUE with height, number of leaves, leaf area, and fresh and dry weights (Table 3).
Discussion
Changing the microclimate by subjecting S. officinalis to different shade levels resulted in observable growth response changes. Minimum and maximum leaf areas were observed in full sunlight and in 50% and 70% shade, respectively (Fig. 2). According to previous studies, shade-plants develop larger and thinner leaves to increase light harvest (Lambers et al., 1998; Taiz and Zeiger, 2002). Our results agree with the results of Kumar et al. (2013) on S. sclarea (clary sage), where leaf area increased with increasing shade levels up to 50% and declined thereafter.
The number of leaves/plant was highest with 30% shade. Leaf number then decreased with increasing shade levels up to 50% and 70% (Table 1). Zervoudakis et al. (2012) reported that the largest number of leaves per sage plant was observed at full sunlight and the number of leaves/plant then decreased as the plants were shaded up to 50% and 75% shade. This result is also supported by Mendes et al. (2001).
In the present study, plant height was taller under shade and the tallest plants were observed under the 70% shade treatment (Table 1). Plants grown under high shade levels resembled etiolated plants, which have an unusually tall appearance (Taiz and Zeiger, 2002) and display more apical dominance in order to limit low light stress (Mendes et al., 2001; Moniruzzaman et al., 2009). The taller plants observed under higher shade indicates a phototropism response to modify plant leaf distribution in order to help the plants receive enough light (Takemiya et al., 2005; Yang et al., 2007; Wang et al., 2009; Mapes et al., 2014).
Total fresh and dry biomass per plant was also significantly higher under 30% shade (Table 1) and fresh and dry biomass decreased under full sunlight and with increasing shade. This is in agreement with the results of Kumar et al. (2013) which found that both 50% and 75% shade reduced plant size and fresh weight of clary sage. Zervoudakis et al. (2012) found that dry mass was greatest under full sun light (1,400 µmol·m-2·s-1 corresponding to 30% shade treatment in this study) and decreased as the plants were shaded. These results indicate that sage grown under low-light conditions were less productive than those grown under high-light, as has been shown for several other plants (Pegoraro et al., 2010). However, it has been reported that intermediate light conditions (about 50% of full sun light) led to higher levels of biomass production in some species (De Carvalho Gonçalves et al., 2005).
**
At 30% shade, plants had dark green leaves (Fig. 2) with the lowest L and C values for leaf color (Table 2). At higher shade
* * º
levels, L and C increased and h values changed, with sage foliage color generally becoming lighter green. Leaf color can be
used to identify stress caused by adaptation to environmental changes (Brand et al., 1998).
Leaf photosynthetic pigment increased with progressive shade. Chl a, Chl b, Chl a+b, and the Chl a/b ratio increased with
decreasing light intensity from full sunlight. As shown in Fig. 5, Chl a content showed a similar behavior to total chlorophyll
content and Chl a content was much higher than Chl b. These results are similar to several studies on other plants (Czeczuga,
1987; Adamson et al., 1991; Muraoka et al., 2002; De Carvalho Gonçalves et al., 2005; Yang et al., 2007; Mielke and Schaffer, 2010). As theChl b content was remarkably lowcomparedto Chl a, the Chl a/b ratio appears tobe determined solely by theamount ofChl a insage. Beneragama and Goto (2010) also reported that higher Chl a/b values existed underlower light intensities in Euglena gracilis. The marked increase in leaf chlorophyll content in the 50% and 70% shaded conditions demonstrate the plant’s ability to maximize the light harvesting capacity under light-deficit conditions and the efficient use of light captured in photosynthesis with decreased respiration costs for maintenance (Kura-Hotta et al., 1987; Lei et al., 1996; Dai et al., 2009).
Adaptable plantsdevelop strategies to adapt to changingenvironments, including largerand thinnerleaveswith a three-fold increase in total chlorophyll content (Taiz and Zeiger, 2002). The synthesis and degradation of photosynthetic pigments are associated with acclimation to different environments. Chlorophyll is usually synthesized and photo-oxidized in the presence of light. Nevertheless, excess light can cause greater degradation and consequently decrease chlorophyll levels (De Carvalho Gonçalves et al., 2005). On the other hand, under limiting light, plants set into motion a series of compensatory mechanisms, such as a substantial increase in photosynthetic pigments. This response allows the plant to maintain a photosynthetic antennae sufficient to capture the required light energy (Czeczuga, 1987) considering that highly pigmented leaves show a higher light absorption efficiency per leaf, which may allow the plant to achieve a carbon balance under light-deficit conditions (Dai et al., 2009).
The highest carotenoid content was observed under high shade (Fig. 4). In high solar radiation environments, an increase in chlorophyll photo-oxidation depends upon the carotenoid concentration which can prevent chlorophyll photo-destruction (Czeczuga, 1987; De Carvalho Gonçalves et al., 2005). In low light environments however, carotenoids may play a more important role in light absorption and its transfer to chlorophyll (Czeczuga, 1987; Taiz and Zeiger, 2002).
The highest photosynthesis yield, stomatal conductance, and WUE were obtained in plants grown under full sunlight and linearly decreased with increasing shade levels (Table 1). Similar results have been reported by Gregoriou et al. (2007) and Hou et al. (2010) for Olea europaea L. (olive) and Glycyrrhiza uralensis (chinese liquorice), respectively. Low-light stress usually inhibits plant assimilation and production by affecting gas exchange (Wei et al., 2005; Gregoriou et al., 2007). Kumar et al. (2013) also observed a reduction of the photosynthesis rate and stomatal conductance in S. sclarea (clary sage) under strong shading (50% and 75%). Low irradiance leads to a reduction in photosynthesis and therefore growth rate (Corre et al., 1983). Ramalho et al. (2000) showed that a 15 d exposure to intense light slightly elevated the maximum photosynthesis (Amax), and more than doubled the irradiance required to reach Amax in CO2 saturated leaves of Coffea arabica L. (coffee), indicating the presence of alternative dissipation mechanisms. Zervoudakis et al. (2012) demonstrated that S. officinalis responds to high irradiance by directly restricting photosynthesis, thus altering its vegetative growth among other changes. These findings indicate that long exposure to high light intensities could damage the photosynthetic apparatus in sage.
The essential oil content was highest under full sunlight (Fig. 5). A reduction in essential oil content under low light conditions has been reported in Mentha japonica (japanese mint) by Dutta (1971), Mentha servina (pepper mint) by Ahlgrimm (1956), Mentha cordifolia (philippine mint) by Cantoria and Gacutan (1974), and Ocimum basilicum L. (basil) by Chang (2008). Mapes and Xu (2014) suggested that full solar radiation might be required for the biosynthesis of essential oil in sage. The essential oil yield increased under 30% shade and then decreased with increasing shade levels (Fig. 5). Mattana et al. (2010) reported that the highest essential oil yield in Pothomorphe umbellata L. (pariparoba) was observed with 30% shade. The highest aerial biomass yield and essential oil yield were obtained in plants grown under 30% shade (Fig. 5). Chang
Table 3. Coefficients of morphological and physiological characteristics of sage under different shade levels
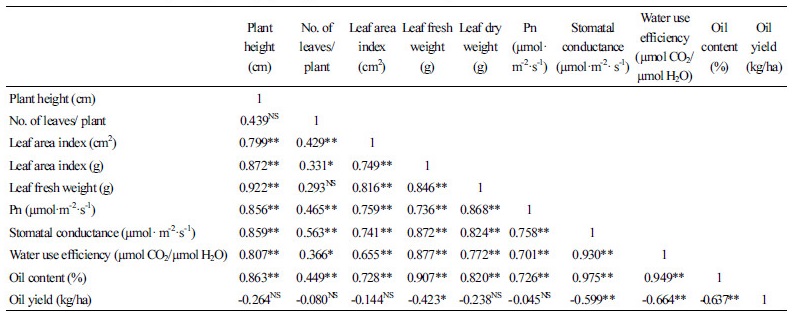 | |
NS, *, ** Non-significant or significant at p <0.05 and 0.01. | |
et al. (2008) found that essential oil synthesis, as a secondary metabolite, has a linear relationship with primary metabolism; thus, the more photosynthates produced, the more secondary metabolites accumulated.
The correlation of sage characteristics under different shade levels indicates a liner relationship between photosynthetic yield and stomatal conductance with height, number of leaves, and fresh and dry biomass weight (Table 3). Despite the necessity of light for autotrophic organisms, no plant is capable of using 100% of the maximum solar irradiation for photosynthesis. When irradiance exceeds that which can be used for photochemistry, other protective mechanisms must be used to dissipate excess excitation energy or damage will occur.
In these experiments, plants grown under 30% shade had a higher biomass than those grown under full sunlight. Maximum monthly ambient temperatures were between 37°C and 51°C inside and outside the tunnel, respectively. The shade treatment probably reduced both air and root temperature, which could have led to a lower respiration rate in the leaf and root, and low-light grown plants may have had a lower respiration rate than plants grown under full light (Sorrentino et al., 1997). A low respiration rate may consume less sucrose in leaves (source) by transferring more sucrose to the root (sink). Therefore, there must be different patterns of biomass allocation between low-light and high-light grown plants. On the other hand, the highest light intensity led to remarkable damage to chlorophyll, especially Chl a. This deterioration was restricted whenplants received a light shade (Table 1). Hence, the results suggest that sage needs a light shade (30% shade) to reach its maximum growth potential and could by successfully grown and established in low-light or shady conditions. To conclude, the results indicate that while photosynthetic capacity reached a peak under full sunlight, the highest leaf number, biomass productivity, and essential oil yield were obtained under 30% shade level. This study also revealed that sage has the ability to correctly regulate its metabolism and adapt itself to different light conditions.