Introduction
Materials and Methods
Information from on-farm trials
Collection of Data for Environmental Factors, Soil Traits, Growth and Yield
Collection of Data for Chlorophyll Fluorescence Characteristics
Results
Discussion
Conclusions
Introduction
The current state of agriculture in Korea uses advanced technologies (Information Technology; IT, Information Communication Technology; ICT, Internet of Things; IoT, etc.) for environmental control for autonomous greenhouses, improving production efficiency, enhancing crop quality, and saving labor to replace the scarce labor force caused by aging and the decline of the rural population (Yoon et al., 2020). We promote research that concerns the government by carrying out annual agricultural and livestock ICT convergence modelling projects to strengthen the production of the agri-food industry through the convergence of agricultural and advanced technology. The resulting data allow producers to increase the quantity and quality of agricultural products by using smart farming approaches for precise environmental control, thus improving production. Smart farming involves informed control of shading, ventilation, irrigation, temperature, and when to ship. Notably, it has the advantage of improving the productivity and quality of agricultural products with less labor, energy, and nutrients compared with other systems, by creating an optimal growth environment based on data such as crop growth, growth environment, and agricultural work logs (Jang, 2017).
An innovative data-based farm aims to enable people who do not know about agriculture to use agricultural data to farm. Various objects in the agricultural production site are woven into a network to collect and analyze information from each object. Agricultural knowledge, which is an experience that existed only in farmers’ heads in the past, is converted into data so that the machine can use and systemize this information (Roh, 2016). Through the collected data, research is being conducted to improve the production volume by precise environmental management through big-data utilization and the improvement of some environmental facilities. However, in the domestic agricultural environment, compared to advanced countries, the cultivation area per farmhouse and the scale of horticultural facilities are small, and the proportion of small farm households is high; therefore, it is necessary to develop and distribute a smart farm model suitable for the domestic agricultural environment (Lee et al., 2019a).
Various fruits and vegetables such as cucumbers, zucchini, tomatoes, and peppers require long cultivation periods and are suitable for forcing culture, in which flowering is artificially induced by environmental conditions. Cucumbers are produced year-round, and many growers are cultivating them using insulated or heated facilities and four cultivation types, forcing culture, semi-forcing culture, early maturation culture, and retarding culture). The primary cultivation type is forcing culture, in which the planting time is the middle of October and the harvest occurs in the middle of the next July. Cucumber cultivation management varies depending on crop type, temperature, lighting quality, and management methods (Lee, 2012; Ashraf et al., 2020; Cui et al., 2020; Kowalczyk et al., 2020; Schlering et al., 2020; Wang et al., 2020). For example, early-morning heating is applied to raise temperatures (Kwon et al., 2004), and studies are being conducted on the effects of light conditions and inhibition of ventilation using shading and fog during high temperatures (Ashraf et al., 2020; Kim et al., 2020; Kowalczyk et al., 2020; Park et al., 2020). Fertigation has the advantage of being able to provide adequate water and nutrients for crop growth in a timely manner, which improves the utilization of water, improves fertilizer utilization efficiency and improves the quantity and quantity of crops by supplying appropriate fertilizers (Miller et al., 1976; Cook and Sanders, 1991). However, the yield of crops varies significantly depending on soil fertility conditions, and most of them are empirically studied. Predicting the growing pattern according to the type of facility, cultivation environment, variety, and cultivation management method will provide vital information for growers. Cucumber greenhouses equipped with cultivation support facilities allow farmers to produce a high-value crop and are mainly used in Chungnam (36°48'N 127°6'E, elevation 85 m), Gyeongbuk (36°24'N 128°9'E, elevation 59 m), and Gyeonggi (36°59'N 127°6'E, elevation 18 m) provinces of South Korea. Although cucumber is a fruit vegetable that likes relatively cool temperatures, it is highly sensitive to temperature and humidity changes. Therefore, it is particularly important that farmers manage the environmental conditions, as physiological disorders and pest infections are frequent in this crop, although this is highly dependent on the facility environment. The type of facility, growing region, time, variety, and farming techniques greatly influence the overall growth and production of cucumbers.
Therefore, this study was conducted to select leading farmers among the facility cucumber cultivation farms and collect environmental and growth information over the entire growth cycle to derive optimal environmental factors that affect growth, thereby contributing to improving productivity and reducing production costs. This was implemented to use the technique as primary data for scientific analysis, verification, modelling, and spreading to the field.
Materials and Methods
Information from on-farm trials
This experiment was conducted on three farms located in Sabeol-myeon (36°28'N 128°13'E), Ian-myeon (36°33'N 128°09'E), and Hamchang-eup (36°34'N 128°12'E) in Sangju, Gyeongsangbuk-do South Korea, the main production areas for the cultivation of cucumbers (Cucumis sativus L.). The cultivation status is shown in supplementary data (Suppl. Table 1). The grafted cucumber seedlings were purchased from a commercial nursery (Happy Spark Plug Seedling Co. Ltd., Gunwi). The method of planting seedlings was to put green mulching vinyl film, about 1.8 m in width on top of the soil, and plant the seedlings in two rows at 40-cm intervals. The cucumber variety used was a gynoecious type, meaning that the main branch fruiting habit and the first fruit set occurred after eight nodes of development of cucumber plants. Water and fertilizer were supplied by drip irrigation installed in two rows above the furrows. Each farm used slightly different fertigation methods based on standard recommended fertigation (2,000 kg/10a for composts, 9.2 kg/10a for N, 10.3 kg/10a for P2O5, 8.1 kg/10a K2O, and 200 kg/10a for CaCO3). Additional fertilizer supply in the early stages of growth applied 2 to 3 kg of nitrogen and potassium per 10a at seven- to ten-day intervals. The number of trials was increased by observing the vitality of plants. In addition, practical cultivation methods usually followed the cucumber growing manual published by the RDA of Korea (Lee et al., 2019b).
Collection of Data for Environmental Factors, Soil Traits, Growth and Yield
To measure the environment inside the facility, solar radiation (SQ-100X, Apogee Instrument Inc., USA) and temperature/humidity (ATMOS 14, Meter Environment, USA) sensors were installed at the tip of the cucumber plant, at the height of 2 m. Geothermal (6470- 6, Watchdog, USA) sensors were also buried, and data were collected at 1-h intervals. The collected data were presented as cumulative solar radiation, average atmospheric temperature, weekly average relative humidity, and average ground temperature. Soil samples were collected and analyzed in 3 locations at each point from 5th October 2018 to 31st June 2019. pH and electrical conductivity (EC) were measured in a ratio of 1:5 soil and distilled water, and effective phosphoric acid was diluted five times with distilled water after adding 20 mL of single leachate to 5 g of a soil sample by Lancaster method, shaking for 10 minutes, filtering, and colorimetric quantification at 720 nm using a spectrometer. For the base analysis of substituted cations (K, Ca, Mg, and Ca), 25 mL of single leachate was added to 5 g of dry soil, shaken for 30 minutes, filtered, and measured by inductively coupled plasma analysis (GBC, Intergra XL, Australia). Plant length and a number of nodes were measured from the surface to the point of growth, and the number of nodes greater than 2 cm was measured as the adequate number of nodes. The stem diameter was measured at the 10th node below the growth point. The length and width of leaves were also measured for leaves attached to the 10th node below the growth point, and the number of leaves was examined by counting the fully developed leaves of not less than 3 cm. The measurement period was from 3 weeks to the end of the harvest after planting, and ten plants from each farm were examined at intervals of 1 week. The harvested fruits were measured at intervals of 3 days. The price of cucumbers was marked as an average value from the past five years (November 2014 to October 2019) at Garak Market operated by the Seoul Agro-Fisheries & Food Corporation (https://www.garak.co.kr/price) Cucumbers are auctioned off with 50 pieces in one box, and quality grades are divided into four categories: premium, good, fair and poor (Suppl. Fig. 1).
Collection of Data for Chlorophyll Fluorescence Characteristics
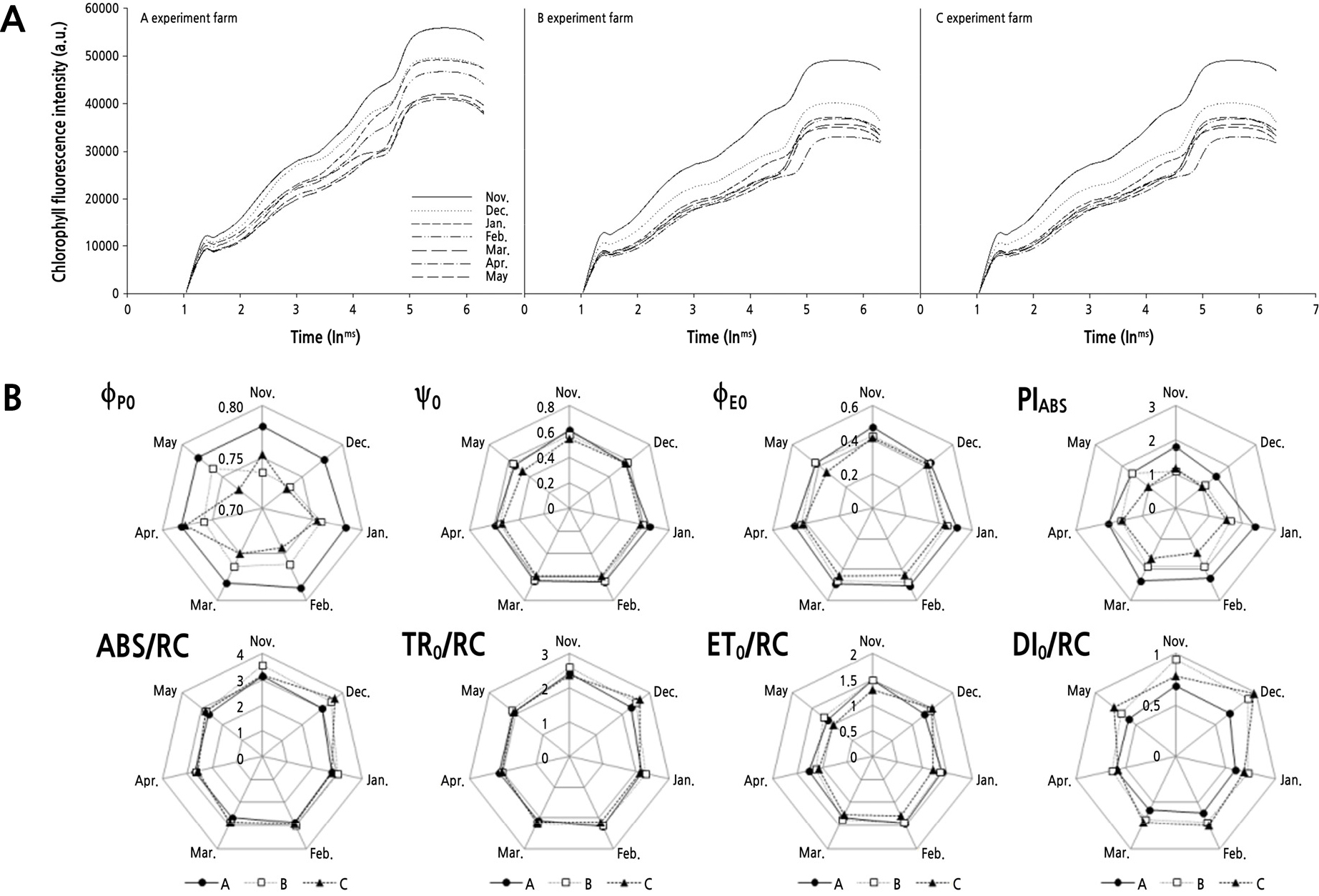
Chlorophyll fluorescence analysis (Origin Jump Intermediate Peak, OJIP) was performed using a portable chlorophyll fluorescence meter (FP-100, Photon System Instruments, Czech Republic). Fully developed leaves, the 10th below the growth point, were selected for the measurements. The leaves were dark-adapted for 15 min before starting the measures using leaf clips provided by the manufacturer (Thwe, 2014). Measurements were made at the same time each month until May 2019. Chlorophyll fluorescence parameters (ΦP0, Ψ0, ΦE0, PIABS, ABS/RC, TR0/RC, ET0/RC, DI0/RC) were calculated and presented using JIP analysis results (Strasser and Govindjee, 1992; Strasser, 2000) as shown in supplementary data (Suppl. Table 2). The OJIP curve represents the continuous reduction of the electron receptor pool in photosystem II (Govindjee, 1995). It can be derived by illuminating the dark-adapted leaf, divided into three stages (Strasser and Govindjee, 1992). In the IP section connecting I (60 ms) and P (300 ms), it means the accumulation of electron transfer activity from the reaction center of photosystem II to the plastoquinone pool (PQ pool), that is, QA-QB2- (Stirbet et al., 1998)
Results
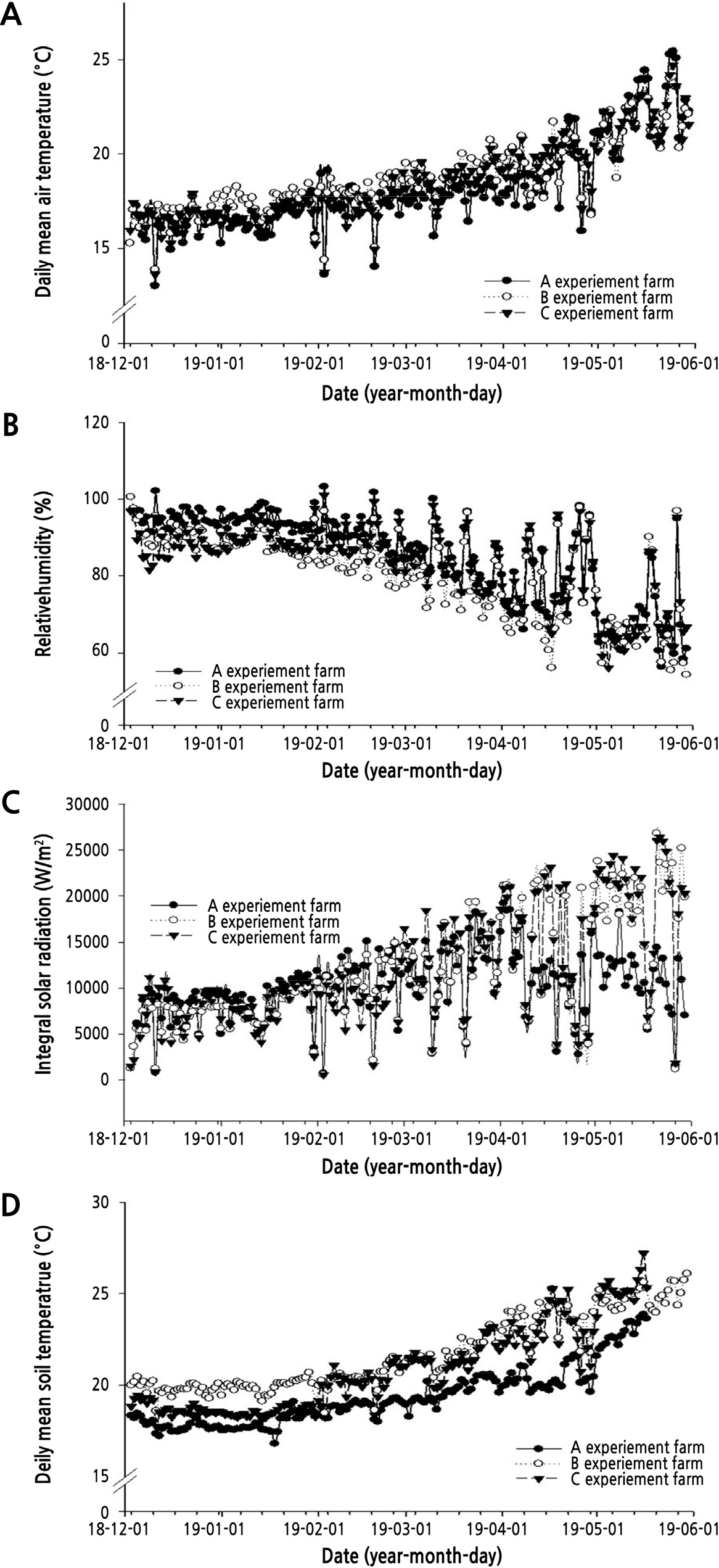
The daily cumulative solar radiation, average temperature, weekly average humidity, and average soil temperature were observed for six months (December 3, 2018 to May 30, 2019) at three farm sites (Fig. 1). The cumulative insolation from 1 month after cucumber planting to when cultivation was terminated was the highest at farm A; there were similar trends at farms B and C. When looking at the distribution of cumulative insolation by season at farm A, the winter season (December-February) was 10 to 11% higher than other points. The spring season (March to May) was 25 to 29% less. The grower at farm A shaded the light during the day (12 to 16 o'clock) using a thermal screen (90%) to block the excessive amount of solar radiation coming in on a clear day in spring. The average temperature inside the facility was maintained around 15 to 19°C in winter and 17 to 23°C in spring. Humidity suitable for cucumbers is relatively high compared to other fruits and vegetables, about 70 to 80% during the day and 90% at night. In winter, the average daily humidity was slightly higher in A and B, 80 to 90% compared to C, but relatively high humidity was maintained.
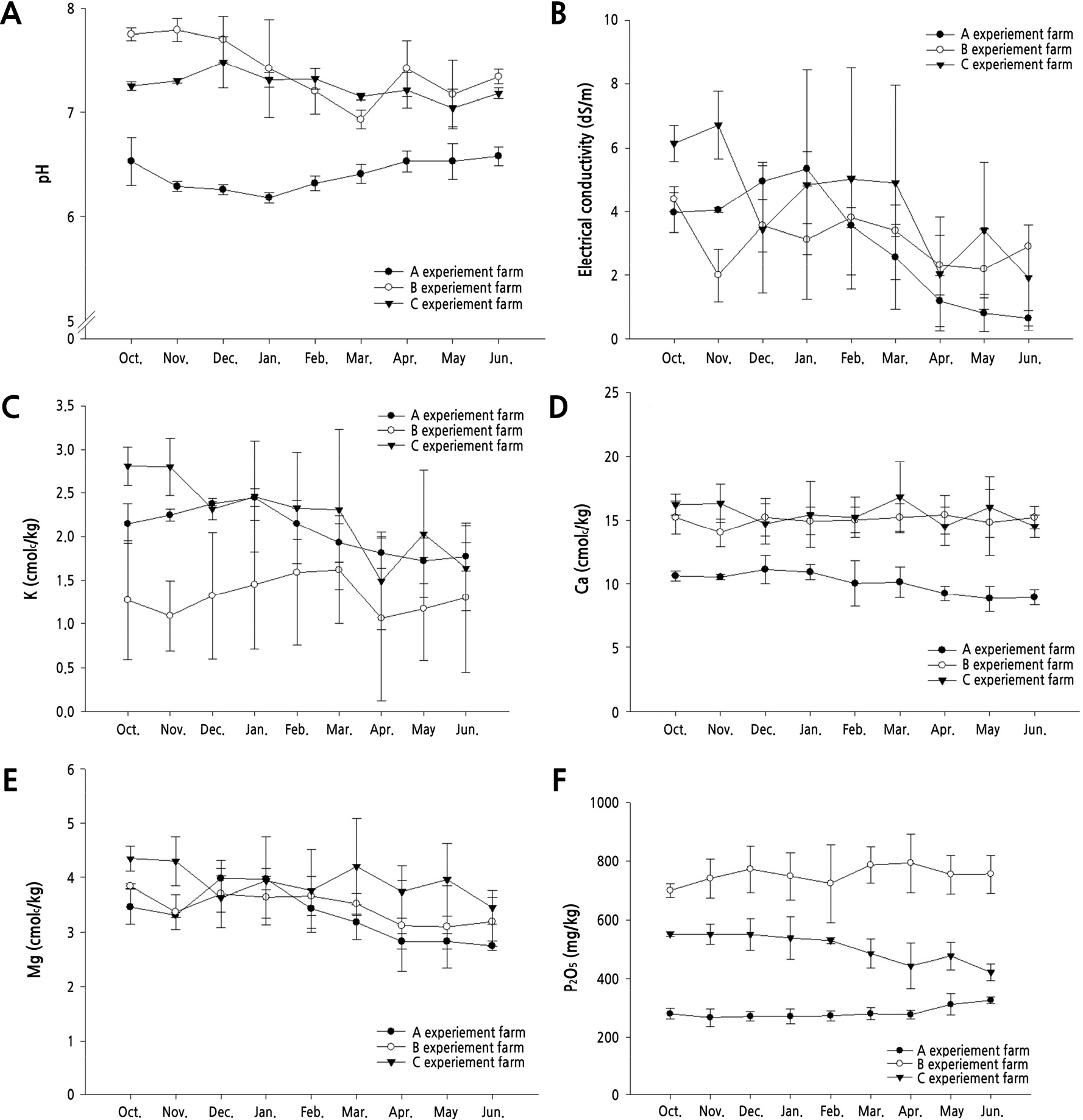
Changes in soil physicochemical properties in the three farm sites during the growth period are presented in Fig. 2. The soil pH of farm A was between 6.2 and 6.5, and farm C was between 7.0 and 7.4, both of which remained essentially unchanged over the entire growth period. However, there was a significant deviation in the pH value from 6.9 to 7.8 at farm B. Electrical conductivity in farms A and B tended to decrease toward the end of growth, and the deviation between zones within each point was relatively higher than in farm B. The available phosphoric acid content differed between the points, but there was no significant change during the entire growth period. Potassium, an exchangeable cation, tended to decrease gradually in the farms A and C in the later stages of growth, but the deviation was severe even within each measurement point. The organic matter and available phosphoric acid content differed between the farm sites, but there was no significant change during the entire growth period within the sites.
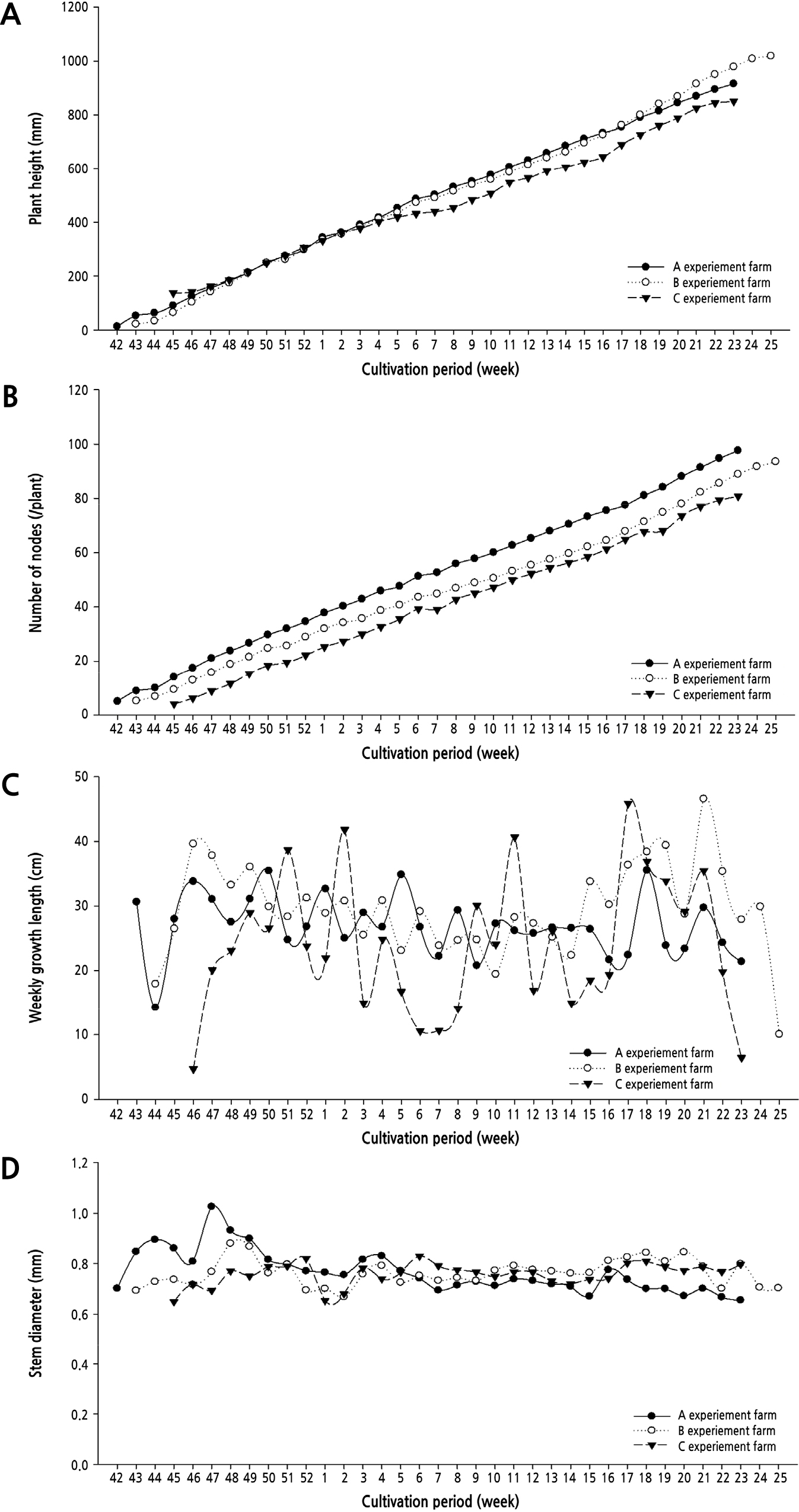
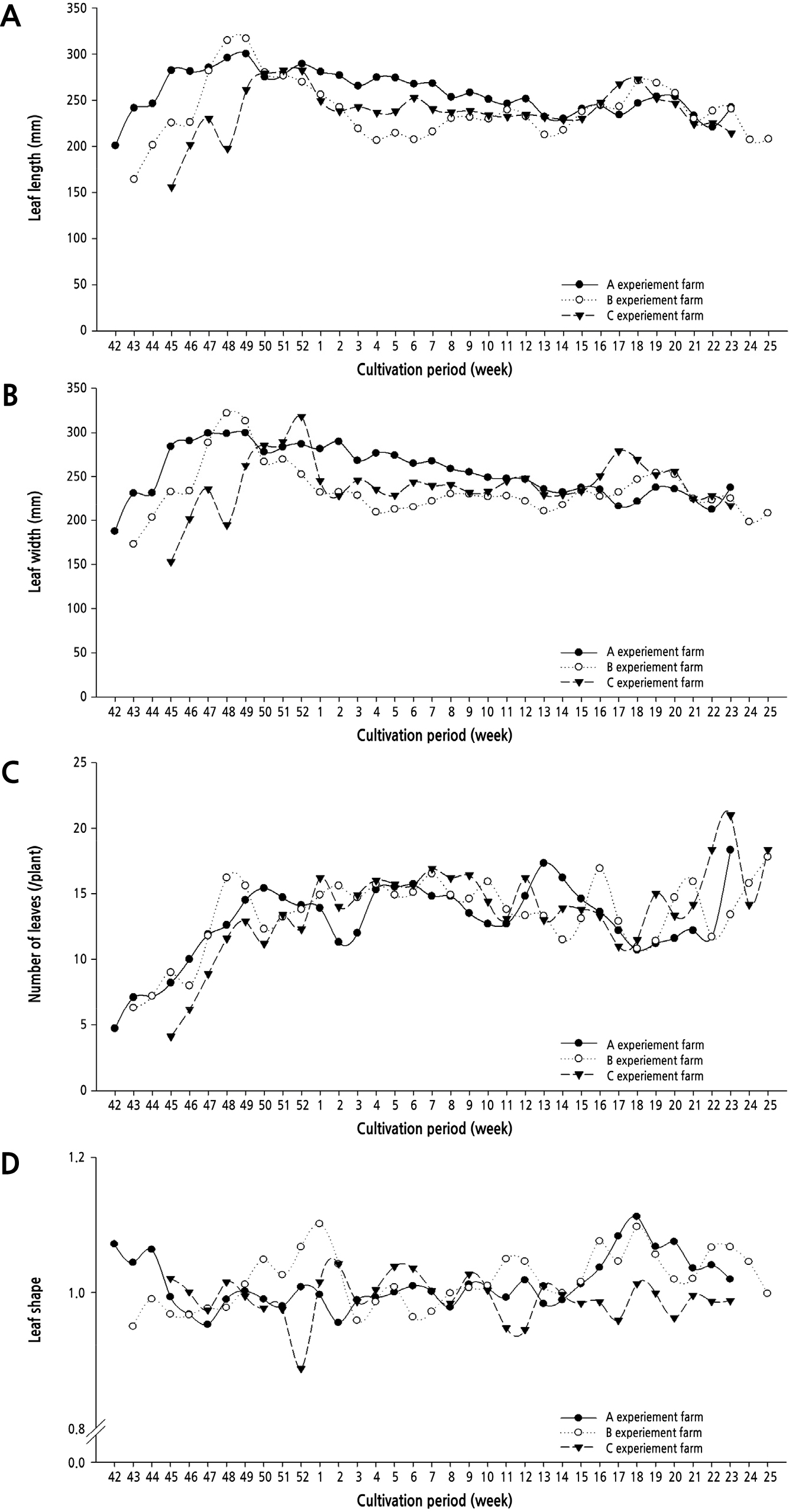
Growth characteristics of the cucumber at three farms sites from 3rd week after planting are presented in Fig. 3. The highest and lowest height of the plant was found at farm B (10,200 mm) and C (8,510 mm), respectively. The number of nodes was the highest at farm A (97.6/plant) and the lowest at C (80.8/plant). The stem diameter under the 10th maturation leaf was around 0.65–0.85 mm, and it tended to become thinner toward the later stage of cultivation in all three farm sites. The growth characteristics of the leaves were around 250–300 mm in length and width in the early stages of harvest, but the size of the leaves tended to decrease to 200–300 mm in the late stages of the crop (Fig. 4). The number of leaves remained between 12 and 15 throughout the entire growth period.
Correlation analysis between the productivity and the growth parameters (plant height, leaf length and width, number of leaves) and environmental factors (daily mean air and soil temperature, relative humidity, and integral solar radiation) was conducted for the possible association among the analyzed parameters (Table 1). The results showed a non-significant correlation between the number of leaves and the productivity of cucumbers, and a significant positive correlation (p < 0.001) was observed with plant height. In contrast, productivity negatively correlated with leaf length and leaf width. The correlations with the environmental factors and productivity were positive and high, showing the highest correlation between soil temperature and productivity among the tested factors. The higher soil temperature promoted productivity. Therefore, it is expected that production can be increased by manipulating the optimal environmental conditions (soil temperature, air temperature, integral solar radiation, relative humidity) that have the most significant influence on the productivity of cucumbers. In the results of the on-farm trial test, the practical cultivation method of a high-productivity farm was applied as a standard manual for the environmental management of other farms and will help enhance the production of cucumbers.
Table 1.
Correlation analysis of productivity (kg/plant) with growth and environmental factors in cucumber experiment farm sties
| Variable | Plant height | Leaf length | Leaf width |
Number of leaves |
Daily mean air temp. |
Daily mean soil temp. |
Relative humidity |
Integral solar radiation |
| Productivity | 0.63***z | –0.36*** | –0.42*** | 0.17NS | 0.46*** | 0.49*** | –0.45*** | 0.42*** |
As a result of monthly analysis of the change in chlorophyll fluorescence response during the growth period before cultivation at the three farm sites (Fig. 5), the value of the maximum fluorescence at farm A was higher than at other farms, and tended to decrease toward the later stage of growth. It is thought that the photosynthetic activity decreased due to aging of the plant. In particular, during the winter season (November to February), plants at farm A maintained a high peak fluorescence intensity compared to other time points. The values for plants at farm B continued to decrease sharply from December, while farm C declined sharply after January.
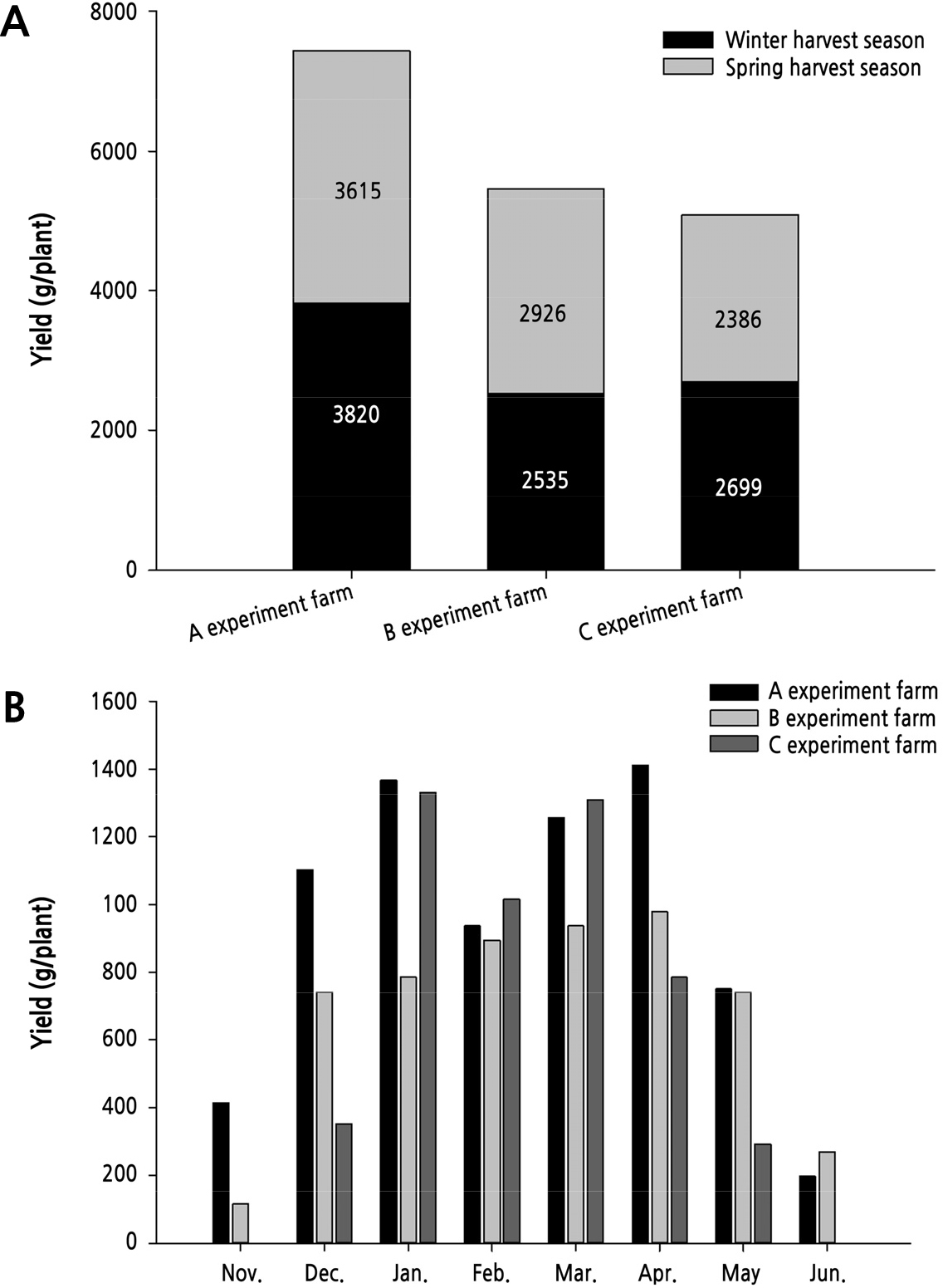
The total yield was highest at farm A (7,435 g per plant), followed by farm B (5,460 g) and farm C (5,090 g). Comparing winter harvests, farm A harvested 42 to 51% more than other farm sites, thus significantly affecting overall income (Fig. 6a). When comparing the monthly harvest, farm B showed a tendency to increase the yield after February, and the total yield was higher than at farm C, but when checking the monthly auction price, the total income was thought to be the lowest (Fig. 6b).
This is the result of a survey of the auction price of cucumbers averaged over the five years from November 2014 to October 2019 in the Garak Market (https://www.garak.co.kr/price). Based on 50 cucumbers per box, the price difference of premium and poor products by grade varied by 2 to 2.5 times. Forcing-grown cucumbers have the highest price in January, and prices begin to fall from mid-February, and prices from April to June are the lowest. The price of cucumbers is marked on average for five years from November 2014 to October 2019 at Garak Market operated by the Seoul Agro-Fisheries & Food Corporation (Suppl. Fig. 1). Cucumbers are auctioned off in boxes of 50 pieces, and quality grades are divided into four categories: premium, good, fair, and poor. The price of cucumbers was the lowest between April and July and October to November, when semi-contact cultivated cucumbers were shipped, and the highest in winter and around August, from December to March of the following year.
Discussion
The growth of crops is greatly affected by temperature and humidity. Temperature also has a significant impact on photosynthesis and ultimately influences the products. Moreover, the optimal growth temperature varies depending on the type of crop and the growth stage. So, reasonable temperature control models are required for a growth facility to be established. Cucumber has specific requirements for environmental conditions, as can be seen from the conditions where this crop originated, where it grows during the rainy season. It is highly sensitive to dry environmental conditions, which significantly inhibit the growth of leaves and stems, and the expansion of fruits (Lee et al., 2019a). In winter, the top and side windows of growth facilities rarely open to prevent the entry of cold air from outside, so it is thought that humidity can be maintained around 80 to 90% during the week. However, in spring, the humidity tended to drop due to frequently opening of the top and side windows to lower the internal temperature. Cucumbers should be kept at 15°C or higher temperatures, because they are relatively sensitive to soil temperatures and their growth stops if they fall below 12°C (Lee at al., 2019b). When soil temperature exceeds 25°C, stems and leaves grow rapidly, accelerating aging and shortening the cultivation period. After May, when there was a lot of solar radiation, soil temperature often exceeded 25°C. However, shading treatment at farm A allowed it to maintain soil temperatures of around 20 to 23°C. In the spring, the farm A site was shaded from April and the internal temperature was about 2°C lower than that of the other farm sites, but there was no difference in temperature even after the shade was applied after May. In plastic house cultivation, various types of cultivation are carried out, ranging from the winterization through the warming facility or the warming facility using double and triple plastic tunnels and insulation curtains in the rain covered state, along with only polyethene plastic covered (Lee et al., 2012).
Temperature management after planting is essential for rapid growth and improved relative growth rate. At the farm site, the night temperature was set at around 12°C due to concerns over increased heating costs in winter. The daily average temperature was between 15 and 18°C, which was lower than the optimum temperature for growth. The soil temperature was relatively low at 17 to 20°C in the season when solar radiation was insufficient, but it was kept high at 23 to 25°C in the period of high solar radiation. Therefore, management efforts should be made to reduce the soil temperature during the summer. Currently, the types of plastic houses used by growers are dual and triple plastic tunnels for keeping the plants warm, and the optical transmission and period of use of film also vary (Lee et al., 2012). Structural improvements are needed to maintain a stable environment inside the facility for smart farming. Increasing the transmittance to one layer is important rather than using multiple film layers for cucumber cultivation facilities with high optical requirements. In spring, farm A used a shading treatment on clear days after April, and the internal temperature was about 2°C lower than other points, but after May, there was no difference in temperature even after shading was applied. However, it is thought that the effects of soil temperature and plant physiological reaction may have affected stable productivity even during high temperature periods. In other studies (Woo et al., 2014; Kim et al., 2020), temporary shading (90%) reduced light stress, effectively inhibiting photorespiration, and improved productivity by reducing physiological stress.
The primary cultivation type represented forcing culture (at planting time was the middle of October and until harvest in the middle of the next July), for promoting harvest timing. After planting, nutrient management should be continuously conducted, while looking at the vitality of the plant. The acidity of the soil is an important factor that affects the solubility of nutrients, absorption, the activity of soil microorganisms, etc. Cucumbers are normally grown at a low acidity (pH 6.0–6.5) (Lee et al., 2019b). The soil pH at farm A with the highest productivity was maintained at 6.1–6.7 with no significant change. Potassium, calcium, and magnesium decreased slightly after implantation, but remained well for the entire growth period without significant fluctuations. Growth often affects cucumbers because of their high growth rate and sensitivity to unfavorable environments due to their shallow root systems. Cucumbers continue to grow well if the balance between vegetative growth and reproductive growth is even, but stable management becomes difficult if it is tilted to either side. The cause of uneven growth is thought to be affected by fruit management, the occurrence of diseases and pests, and environmental control. It has been confirmed that the pattern of nutrient absorption changed significantly five to six weeks before the transplant and tends to differ at different stages of growth before and after bearing fruit (Kim, 2014). Cucumbers are classified as non-nutritive, but are often lacking in nitrogen, potassium and magnesium (Kim, 2014). It is known that the photosynthetic ability of leaves is the highest between 20 and 30 days after full development, and the photosynthetic capacity of leaves rapidly declines after 45 days. Therefore, at three farm sites, many leaves were not secured due to difficulties such as surveying the tunnel type facility, manned work during soil cultivation, and the occurrence of diseases and pests in the old leaves.
When plants are affected by light, temperature, moisture, or chemical stress, the pattern of the OJIP curve changes (Lu and Zhang, 1999; Mathuret al., 2011; Oh and Koh, 2013; Oh et al., 2014). The previous research revealed that chlorophyll fluorescence parameters enable evaluate for plant healthy levels under environmental stress (Liu et al., 2019; Choi and Jeong, 2020; Shin et al., 2020; Sousaraei et al., 2021). Table 2 shows our measurements for the main chlorophyll fluorescence parameters. ΦP0, Ψ0, and ΦE0, which refer to the energy transfer ratio and fluorescence yield of photosystem II at each stage of the photochemical reaction. These parameters tended to be higher at farm A than at other farm sites. Among them, the ΦP0 variable, representing the maximum quantum yield in the initial photochemical reaction, remained at a stable value of 0.8 for the entire period of growth without significant change. PIABS, which represents the vitality of plants, also tended to be high at farm A, and there was a difference of 1.2–1.5 times or more compared to other points from November to March. All points showed high levels during January-March and decreased after that. PIABS refers to energy conservation efficiency in reducing electron carriers using absorbed light energy (Holland et al., 2014), and there are three significant steps to control photosynthetic activity in the reaction center of photosystem II. In other words, the total density of the active reaction center, the rate at which the photochemical process captures the energy absorbed by the reaction center, and electron transfer in the electron transport process after QA reduction are all reflected. Therefore, PIABS is used as a good indicator for evaluating stress and monitoring photosynthetic ability (van Heerden et al., 2007; Lee et al., 2014), and PIABS is known to decrease with stress (Wang et al., 2012). ABS/RC, TR0/RC, and ET0/RC, which represent the change in energy flow per reaction center, all tended to decrease toward the later stages of growth. There was no significant difference between the points, but for DI0/RC, which is the non-photochemical energy loss discarded as heat, farm A did not show a significant decrease, but farms B and C showed varying monthly reductions. These results indirectly show that the inactive center of reaction increases when subjected to stressful situations due to plant cultivation or the environment, and most of the captured energy is not sent to electron transfer. Finally, this is thought to be related to the number of products. Forcing cultivation of cucumbers is used mainly for the production of cucumbers during the winter, to increase income from December to March of the following year (Lee et al., 2012).
Conclusions
Data on growth, physiological responses, yield, and cultivation environments were accurately collected in cucumbers' main production area by each trial’s farms. In farm A, where early morning heating was performed at a relatively high temperature (20°C target) for one to two hours before sunrise in the low temperature (winter season), the chlorophyll fluorescence remained relatively high, so it is thought that the exposure to stressful conditions was limited. During the high temperature period, which is the late harvest period, a non-woven screen was used to block excessive insolation from 12 to 16 o'clock to suppress the temperature rise, thereby also reducing exposure to stressful conditions and increasing productivity. Although there were some differences in the accuracy of the information for each cucumber experiment farm, it is thought that the basic information necessary for evaluating the growth and yield of cucumbers in general farms is accurate. To apply agricultural data to improve agricultural resilience for each country, a plan was suggested to organize, process, and utilize actual data from each farm. Future cucumber production will require standard data (growth, environment, etc.) to introduce an automated and viable production system. Smart farming thus requires a convergence of information and communication technologies. By using big data and internet of things technology, a company can monitor the growth and environmental information of crops in real time with sensors. This helps growers establish an optimal growth management system to pursue digitalization and intelligent knowledge of the entire process from production system to distribution and consumption. In order to implement precision farming within smart farming, useful control information is required, and control information will vary greatly depending on facility type, region, timing, variety, farming techniques, etc. However, various cultivation strategies can be used in smart farming by establishing an optimal growth environment management model that maximizes the productivity improvement of facility-grown cucumbers.
Supplementary Material
Supplementary materials are available at Horticultural Science and Technology website (https://www.hst-j.org).
- HORT_20220021_Table_1s.pdf
Cucumber’s cultivation information of on-farm trial sites
- HORT_20220021_Table_2s.pdf
Description of chlorophyll fluorescence parameters used in this study
- HORT_20220021_Figure_1s.pdf
Changes in the average price of cucumbers in the ‘Garak’ market over the five-year period form November 2014 to 2019.