Introduction
Materials and Methods
Plant Materials
Test of Antimicrobial Activity in R. coreanus Leaf Extract
Test of Antimicrobial Activity in the Antimicrobial Paper
Development of Antimicrobial Paper with Ag Nanoparticle Synthesized using R. coreanus Leaf Extracts
UV-Visible Spectrophotometry, TEM, and SEM/EDS Analyses
LC-MS/MS Analysis
Statistical analysis
Results
Preliminary Test of Antimicrobial Activity of R. coreanus Leaf Extract Against Putrefactive Pathogens
Development of Antimicrobial Paper with Ag Nanoparticles using R. coreanus Leaf Extract (R-AgNPs)
Test of Antimicrobial Activity in Antimicrobial Paper
Analysis of Components in R. coreanus Leaf Extract
Discussion
Test of Antimicrobial Activity and Synthesis of R-AgNPs
The Effect of Antimicrobial Paper Against Main Putrefactive Pathogens of Strawberry Fruit
Compounds of Antimicrobial Activity Including Antimicrobial Paper
Conclusions
Introduction
Components with biological activity in medicinal crops include secondary metabolites such as alkaloids, terpenes, and other compounds (Hyun et al., 2007). These components are superior not only in pharmacological action but also in antioxidant and antimicrobial activity. Medicinal crops contain the various ingredients according to crop type and the part of the plant, and can be used in various functional materials to delay cell senescence or suppress putrefactive bacteria (Oh et al., 2010).
Recently, studies have been conducted on the prevention of food acidification by selecting natural materials with excellent antimicrobial effectiveness (Lee and Shin, 1991; Park, 2000; Song et al., 2000; Lee et al., 2003), such as the development of food preservatives using medicinal crop extracts like Rubus coreanus (Lee et al., 2002), the control of Botrytis cinerea, one of the main putrefactive pathogens in strawberry (Kim et al., 2009; Ugolini et al., 2014), and the development of disinfectant using garlic (Paik et al., 1998).
Biologically active substances in R. coreanus are present in the leaves and stem as well as in the fruit (Lee et al., 2020). The main components are sanguiin H-4, sanguiin H-5, sanguiin H-6, gallic acid, ellagic acid types of tannins, kaempferol, and quercetin (Lee and Lee, 1995; Yang et al., 2007; Kim et al., 2012).
Phenolic and flavonoid compounds mainly in the fruit, leaves, and stems of R. coreanus have been reported to demolish cells due to decreased active oxygen and then to control oxidation of a high molecular substance; they also were reported to have antifungal and antiviral activity (Cushnie and Lamb, 2005; Kim et al., 2008). Particularly, Yang et al. (2012) reported that among phenolic compounds, kaempferol and quercetin had excellent antimicrobial and antioxidant effects.
Various methods to solve the problem of putrefactive pathogens and decomposition of postharvest strawberry have been used (Park et al., 2012): postharvest cold treatment, controlled atmospheres with CO2 treatment (Wszelaki and Mitcham, 2003; Eum et al., 2014; Kim et al., 2016), ultraviolet light treatment (Nigro et al., 2000), natural active products such as essential oils (Hernández et al., 2006; Oliveira et al., 2019), biological control with microorganisms (Wszelaki and Mitcham, 2003), and chemical control with compounds to control ethylene (Lattanzio et al., 1996; Blacharski et al., 2001; Guillén et al., 2007). Packing treatment technologies have also been used, such as MA packing (Kim et al., 1993), the use of corrosiveness films (Shin and Song, 2011), the use of film from natural materials (Chung and Cho, 2003), and application of micro-perforation films with optimum gas penetrability (Lee et al., 2011). However, there methods may adversely affect color, flavor, or texture, and the lack of packaging equipment and materials excluding to be a few commercialization (Sallato et al., 2007). Recently, the use of natural materials as a preserving agent is gaining interest because traditional packing material is made of synthetic resins that are difficult to disassemble (Hong et al., 2009).
Silver ions are known for their antimicrobial effectiveness against a wide range of microorganisms (Raji et al., 2012). Silver nanoparticles (AgNPs) are of interest because of their unique properties that make them suitable for antimicrobial applications, including dental material, as well as for composite fibers, sunscreen lotions, and bone implants (Iravani et al., 2014; Quintero-Quiroz et al., 2019).
Several physical and chemical methods have been used for synthesizing and stabilizing AgNPs. Generally, the synthesis of the AgNPs has been performed using chemical agents including metals, semiconductor, ceramics, metal oxides, etc. (Dhand et al., 2015). However, using chemical agents for nanoparticle synthesis causes environmental problems due to toxic materials with potential hazards such as environmental toxicity, cytotoxicity, and carcinogenicity (Ai et al., 2011). In recent years, natural agents such as plants extracts have emerged as an attractive alternative to chemical agents (Shah et al., 2015).
Therefore, this study was conducted to maintain the quality and extend the shelf life of strawberry fruit by controlling putrefactive pathogens with the antimicrobial paper developed from Ag nanoparticles and R. coreanus extracts.
Materials and Methods
Plant Materials
R. coreanus plants were cultivated for 7 years at the Gyeongsang National University farm in Korea, and samples (leaves) were harvested in May 2018. Samples were dried under shade after washing and ground into a fine powder using a mill. Leaf powder was extracted by reflux extraction for 3 h using a Soxhlet extractor (EAM9203-06, Mtops, Yangju, Korea) with 70% MeOH, equivalent to 10 times the dry weight. The extracts were then filtered by filter paper (Advantec No. 2, Toko Rohhi Kaisha, LTD., Tokyo, Japan) and concentrated using a rotary evaporator (R-520, Ilsin, Daejeon, Korea) under vacuum. The obtained extracts were evaporated under vacuum and lyophilized for 48 h by a lyophilizer (FD8508, Ilshin Biobase, Dongducheon, Korea). All freeze-dried extract powders were diluted 100-fold with HPLC-grade water. The samples were then filtered using a 0.22-µm syringe filter (Sartorius stedim biotech, Goettingen, Germany) and analyzed using liquid chromatography tandem mass spectrometry (LC-MS/MS). Also, 100 times dilution of R. coreanus extract was used in the development of the antimicrobial paper.
Test of Antimicrobial Activity in R. coreanus Leaf Extract
Botrytis cinerea (KACC 40573), Alternaria tenuissima (KACC 40968), and Rhizopus stolonifer (KACC 45322) were obtained from Rural Development Agriculture in Korea, and the inoculum suspension was prepared with spores obtained from 15-day-old cultures on potato dextrose agar medium (PDA), adjusted to 106 spores/mL using a hemocytometer.
The leaf extract powders were dissolved to final concentrations of 100 times dilution of extract for antimicrobial activity, which was performed by disc diffusion (10-mm diameter, Toyo Roshi Kaisha, LTD., Tokyo, Japan) using 50 µL of suspension containing 106 spore/mL of fungi, spread evenly on the surface of the nutrient agar plates. B. cinerea was incubated for 3-5 days at 25°C, A. tenuissima for 5 days at 25°C, and R. stolonifer for 3 days at 25°C to investigate clear inhibition zones around discs, which indicated the presence of antimicrobial activity. For optimal fidelity of results, each test was performed in triplicate.
Test of Antimicrobial Activity in the Antimicrobial Paper
An antimicrobial activity test was conducted by the disc diffusion method using self-produced antimicrobial paper (with R-AgNPs) instead of disc paper (10-mm diameter, Toyo Roshi Kaisha, LTD., Tokyo, Japan). General paper (Advantec No. 2, Toko Rohhi Kaisha, LTD., Tokyo, Japan) was used as a control (Table 3). The antibacterial activity was measured in daily intervals by artificially inoculating strawberry fruits with putrefactive pathogens after putting the antimicrobial paper at the bottom of an airtight container for reducing environmental pollutants (Fig. 5). Strawberry fruits were immersed with 106 spore/mL of mixed three putrefactive pathogens and then placed at the bottom of an airtight container after drying the moisture from the fruit surfaces. Two fruits were arranged in one airtight container and left at room temperature (25°C). Tests of the antibacterial activity in airtight containers were performed in five replications with two fruits per replication.
For testing the antibacterial activity in actual packing boxes, 14 fruits were arranged in two layers in one box and left at room temperature (Fig. 6). The changes in fruit weight and hardness were measured at intervals throughout the day. Fruit weight was measured by dividing the total fruit number from total fruit weight excluding the weight of packing boxes after measuring the total weight including packing boxes using an electronic balance (ARG2202, Ohaus Corporation, Parsippany, USA). Fruit hardness was measured using a fruit hardness tester (Diameter 5 mm, 083014 KM Type, Fujiwara Factory, Osaka, Japan) in three replications with three fruit per replication in different packing boxes apart from measuring fruit weight.
Development of Antimicrobial Paper with Ag Nanoparticle Synthesized using R. coreanus Leaf Extracts
Ag nanoparticle synthesis with R. coreanus extracts (R-AgNPs) was conducted by modifying the method of Kyung and Ko (2014). Aliquots (38 mL) of appropriately 100× diluted extracts, 12 mL of 10 mM or 30 mM silver nitrate, and 2 mL of 1.0 M NaOH were mixed, and distilled water was added for a final volume of 400 mL. Mixture solution and paper porridge (wood pulp) were mixed in a 10:1 ratio (400 mL:40 g). The mixture was ground and left for 5 min to react. For equal distribution of particles and synthesis with extract and silver nitrate in paper porridge, ultrasonic waves were treated with an ultrasonic bath (JAC-3010 (40 kHz, 200 W), KODO Technical Research Co., Hwaseong, Korea) at 54°C for 2 h. Then, the mixture was placed in a paper manufacturing frame, evenly spread, and dried at 60°C in a dryer.
UV-Visible Spectrophotometry, TEM, and SEM/EDS Analyses
UV-visible spectrophotometry (OPTIZEN 2120UV, Mecasys, Daejeon, Korea) was used to measure the extract (final volume 400 mL) used in the development of the antimicrobial paper at a range of 300 to -700 nm to confirm synthesis of Ag nanoparticles (AgNPs) with R. coreanus leaf extract (R-AgNPs). TEM (Tecnal 12, FEI Inc., Brno, Czech Republic) analysis for shape and size of synthesized R-AgNPs was performed by putting a drop of synthesized R-AgNPs solution in the upper corner of a copper grid and observing it when dry. SEM (emission scanning electron microscope II; MIRA3 LM, TESCAN Inc., Brno, Czech Republic) analysis for content and distribution patterns of R-AgNPs within the antimicrobial paper was conducted by cutting the antimicrobial paper into 1 × 1-cm size slices and observing the side and surface of the antimicrobial paper after Au coating. EDS (energy dispersive spectroscopy; JED-230, JEOL, Tokyo, Japan) was used to analyze the elements by K and L X-rays.
LC-MS/MS Analysis
The main components of the antimicrobial paper (10 g) produced by synthesizing Ag nanoparticles and R. coreanus extract (R-AgNPs) were extracted by reflux extraction for 3 h with 70% MeOH, and the extracts were analyzed by LC-MS/MS, which was performed by coupling a HPLC system (Agilent 1100, Agilent Technologies, CA, USA) to a Qtrap mass spectrometer (Qtrap, AB Sciex CO, CA, USA) equipped with an electrospray ionization (ESI) source for analyzing the five ingredients (gallic acid, ellagic acid, kaempferol, quercetin, and quercetin-3-O-glucoside) in R. coreanus leaves. The YMC-Pack Pro C18 RS Column (150 × 2.0 mm × I.D., 5 µm YMC Korea Co., Ltd., Seongnam, Korea) was used. The mobile phase to analyze the ellagic acid, kaempferol, quercetin, and quercetin-3-O-glucoside consisted of acetonitrile (solvent A; Daejung Chemicals and metals Co., Ltd., Siheung, Korea):H2O (solvent B; 0.1% formic acid) with a gradient elution of 0.0-7.0 min (90% B), 7.0-8.0 min (70% B), 8.0-9.0 min (50% B), 9.0-10.0 min (20% B), 10.0-11.0 min (0% B), 11.0-12.0 min (30% B), 12.0-13.0 min (50% B), 13.0-14.0 min (70% B), and 14.0-45.0 min (100% B). The flow rate was 0.2 mL·min-1 with an injection volume of 10 µL and column temperature maintained at 40°C. The mobile phase to analyze the gallic acid consisted of acetonitrile (solvent A):H2O (solvent B; 0.5% formic acid) with a gradient elution of 0.0-5.0 min (50% B), and 5.0-8.0 min (40% B), 8.0-30.0 (100% B). The flow rate was 0.15 mL·min-1 with an injection volume of 10 µL and column temperature maintained at 40°C. The mobile phase to analyze the gallic acid consisted of acetonitrile (solvent A):H2O (solvent B; 0.5% formic acid) with a gradient elution of 0.0-5.0 min (50% B), 5.0-8.0 min (40% B), and 8.0-30.0 min (100% B). The flow rate was 0.15 mL·min-1 with an injection volume 10 µL and column temperature maintained at 30°C.
The mass spectrometer was operated under positive ion and selected ion monitoring (SIM) modes. ESI was conducted using a spray voltage of 4.5 kV. The capillary voltage and the tube lens offset were fixed at −40 and −130 V, respectively. The heated capillary temperature was fixed at 350°C. Nitrogen used as the sheath and the auxiliary gas was set to 35 and 5 arbitrary units, respectively. Each ingredient was analyzed by multiple reaction monitoring (MRM) using a triple quadrupole mass spectrometer.
The mass spectra of gallic acid, ellagic acid, kaempferol, quercetin, and quercetin-3-O-glucoside were isolated at m/z 171.1, 303.0, 287.0, 303.1, and 465.0, respectively (Table 1). The standard compounds were ellagic acid hydrate (Alfa Aesar, Ward Hill, MA), gallic acid (Sigma Aldrich, St. Louis, MO, USA), kaempferol (Sigma Aldrich, St. Louis, MO, USA), quercetin (Sigma Aldrich, St. Louis, MO, USA), and quercetin-3-O-glucoside (Sigma Aldrich, St. Louis, MO, USA). The solutions for standard curve calculation were prepared from 10, 20, and 30 ppm for gallic acid and 0.1, 1, and 10 ppm for the other ingredients.
Table 1.
Antimicrobial activities in single and mixture treatments of medicinal crop extracts against Botrytis cinerea, Rhizopus stolonifer, and Alternaria tenuissima in vitro
| Treatmentz | Inhibition zone (mm)x | |||
| A. tenuissima | R. stolonifer | B. cinerea | ||
| Cont. | (Hexaconazole 2%) | 9.0 ± 1.0y | 9.1 ± 0.1 | 6.8 ± 0.4 |
| Single | Rubus coreanus | 8.7 ± 0.6 | 8.3 ± 1.1 | 6.7 ± 0.6 |
| Mixture | Japanese torreya + Rubus coreanus | 8.2 ± 0.6 | 8.7 ± 0.6 | 6.7 ± 0.3 |
| Japanese torreya + Moringa + Rubus coreanus | 8.2 ± 0.8 | 8.5 ± 0.5 | 6.5 ± 0.5 | |
Statistical analysis
The data were analyzed using one-way analysis of variance (ANOVA) using the SPSS program (SPSS version 21, SPSS Inc., Chicago, IL, USA). Statistical assessments of the differences between mean values were performed using Duncan’s multiple range test (DMRT) at p < 0.05.
Results
Preliminary Test of Antimicrobial Activity of R. coreanus Leaf Extract Against Putrefactive Pathogens
In our previous study, of the extracts of 33 medicinal crops, only R. coreanus had antimicrobial activity against all 3 of the major putrefactive pathogens (B. cinerea, A. tenuissima, and R. stolonifer) that cause strawberry fruit rot after harvesting. Single and mixture treatments were conducted to compare with extracts that had the antimicrobial activity against more than one putrefactive pathogen to increase the antimicrobial effect of R. coreanus extract (Table 1). Extracts of Torreya nucifera and Moringa oleifera had antimicrobial activity against one (B. cinerea) and two (B. cinerea and R. stolonifera) putrefactive pathogens, respectively, although these extracts were lower in antimicrobial activity than the control treatment with chemical pesticide. The results showed that the two-mixture treatment of R. coreanus and T. nucifera extracts and the three-mixture treatment of R. coreanus, T. nucifera,and M. oleifera extracts had to some degree antimicrobial activity against all three putrefactive pathogens, although these mixture treatments were lower than the control treatment (hexaconazol 2%). The antimicrobial activity between the single treatment of R. coreanus extract and the two- or three-mixture treatments including R. coreanus extract was not significant. Therefore, the single treatment of R. coreanus extract was finally selected for the development of antimicrobial paper with Ag nanoparticles.
Development of Antimicrobial Paper with Ag Nanoparticles using R. coreanus Leaf Extract (R-AgNPs)
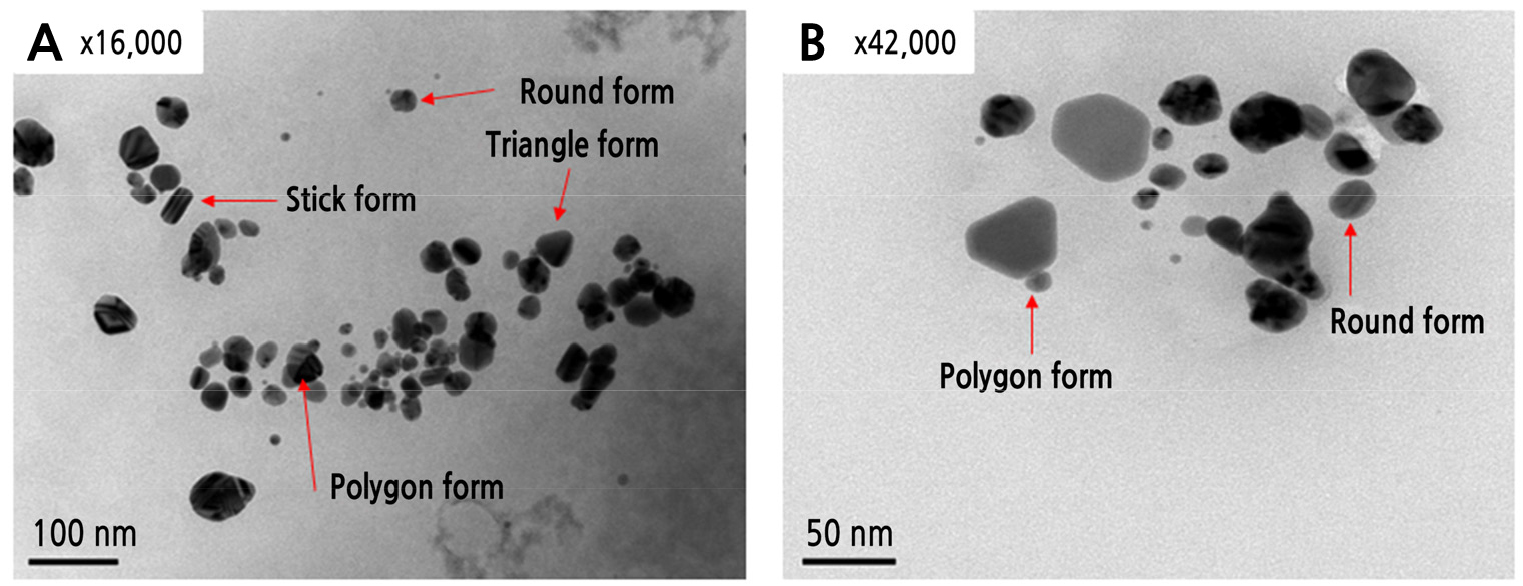
R. coreanus extract was used to synthesize the Ag nanoparticles to increase antimicrobial activity. In the case of synthesis with R. coreanus extract and Ag nanoparticles (R-AgNPs), R-AgNPs were formed, and this was confirmed by UV-visible spectrophotometry analysis through the color change of the solution. The results confirmed the synthesis of AgNPs, the R.coreanus extract did not seen peak in the wavelength of 400-500 nm. However all of the synthetic extracts (R-AgNPs) by silver nitrate concentration with R. coreanus extract (500 dilution) was observed dependently concentration with the largest peak at 400 nm, the known absorption wavelength of silver (Fig. 1A). All of the synthetic extract (R-AgNPs) by R. coreanus extract concentration with silver (0.30 mM) was observed dependently concentration with the largest peak at 400 nm, and with a change in color. This confirms that R. coreanus extract combined with the Ag nanoparticles. TEM analysis was conducted to check that the R. coreanus extract and Ag nanoparticles were properly synthesized. R. coreanus extract was combined with round nanosilver particles 100 nm or smaller, along with some triangular, polygon, and stick forms (Fig. 2).
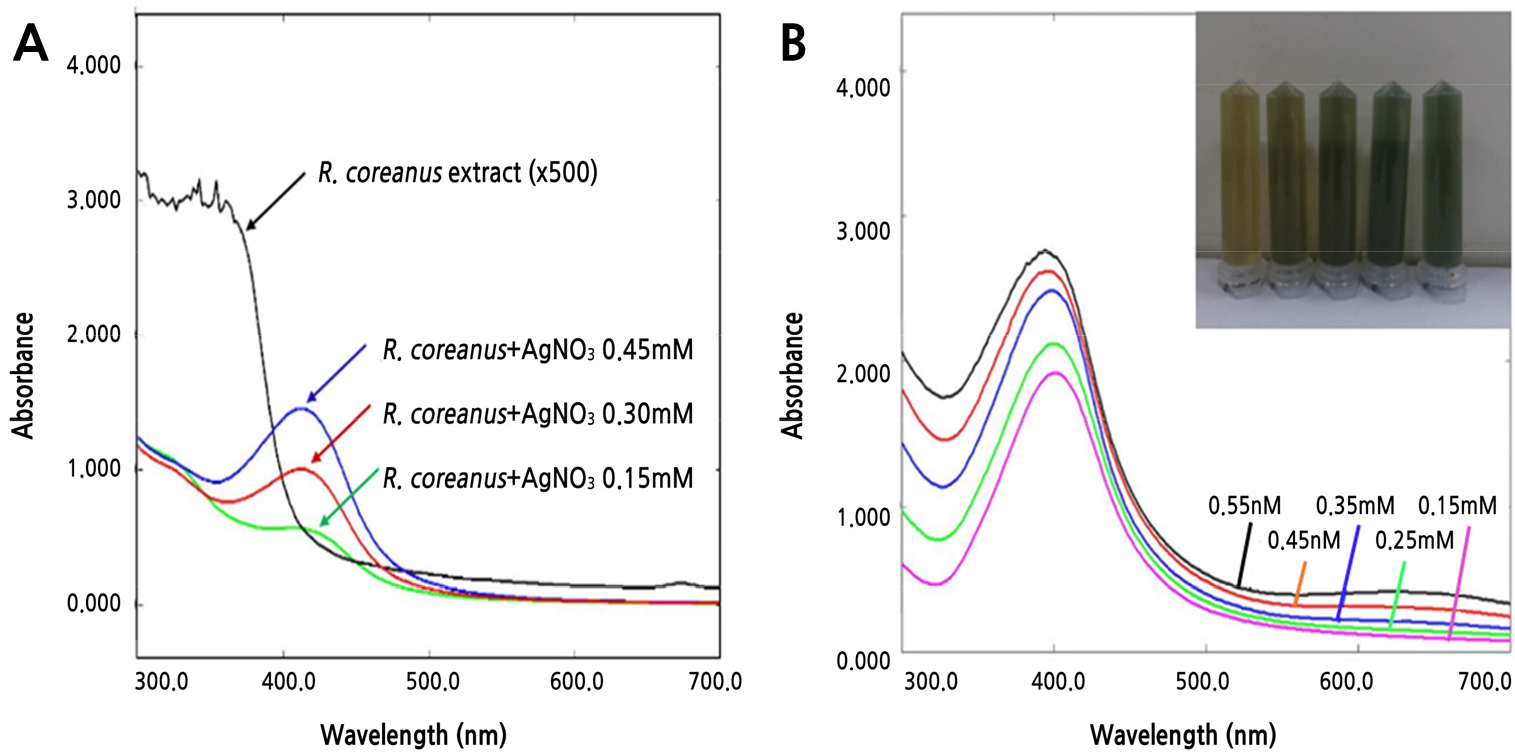
Fig. 1.
Change in absorbance by silver concentration (A) and R. coreanus extract concentration (B) in Ag nanoparticle synthesis. A indicates change of absorbance by silver concentration (0.15-0.45 mM) with 500 dilution of R. coreanus extract. B indicates change of absorbance and color by concentration of R. coreanus extract with silver concentration (0.30 mM).
Based on this result, the antimicrobial paper was developed with R. coreanus extract and Ag nanoparticles to be used as the inner paper in the packaging. Antimicrobial paper made from the mixture of R. coreanus extract and Ag nanoparticles based on the manufacturing process for general paper (210 × 297 × 0.01 mm) had a dark yellow-brown color.
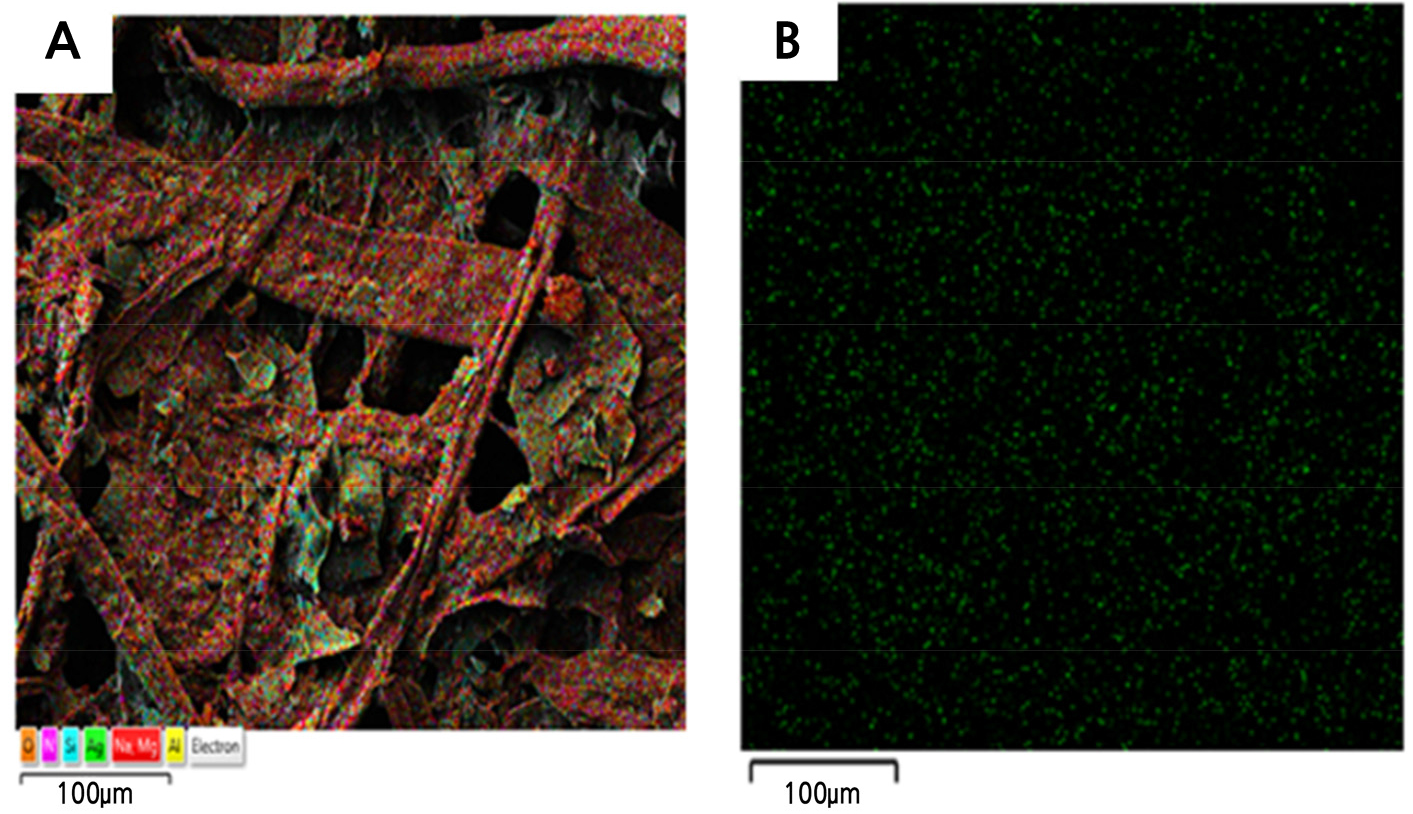
Ag content in the developed antimicrobial paper was investigated with SEM. Ag nanoparticles were not observed in general paper but were observed at the nanometer level in antimicrobial paper with a content of 5.2-7.9% in total element components per unit area (cm2) (Fig. 3 and Table 2).
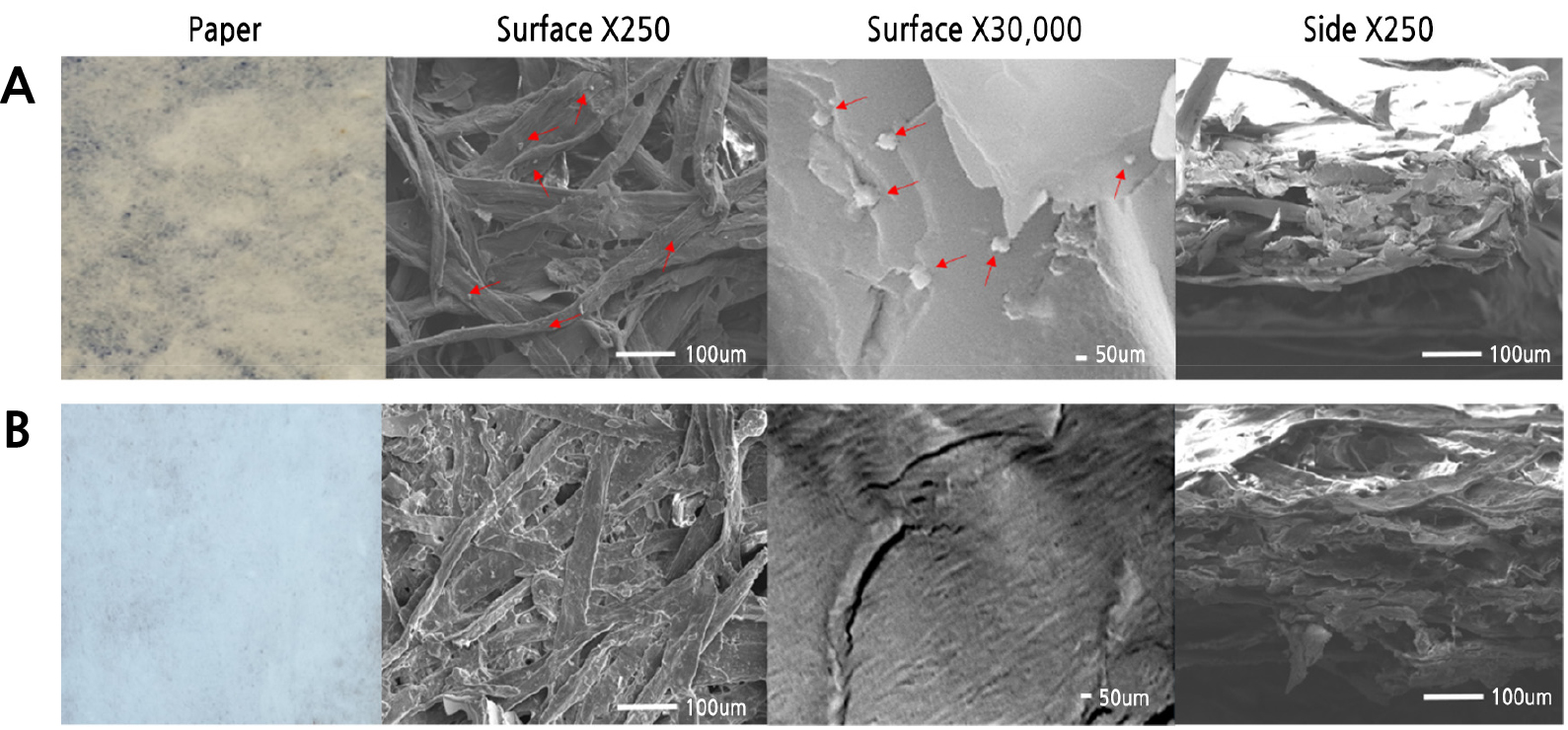
Fig. 3.
Scanning electron microscope (SEM) images of silver nanoparticles prepared with R. coreanus extract. (A) Antimicrobial paper (R. coreanus 100× + 30 mM AgNO3), (B) Control (non-treatment paper). Arrows indicate Ag nanoparticles with R. coreanus extract. Surface and side show images at 250 (surface and side X 250) and 30,000 (surface X 30,000) magnification.
Table 2.
Element contents in silver nanoparticle paper prepared with R. coreanus extract with and without ultrasonication (40 kHz) treatment
| Element | Ultrasonication | No ultrasonication | Non-treatment paper | ||||
| Surface | Side | Surface | Side | Surface | |||
| Wt %z | |||||||
| N | 4.1 f y | 3.4 de | 3.8 e | 4.19 d | 5.6 d | ||
| O | 52.7 a | 48.2 a | 54.7 a | 54.4 a | 63.6 a | ||
| Na | 1.1 g | 1.7 f | 1.1 f | 2.0 f | 3.5 e | ||
| Mg | 10.9 c | 11.0 c | 11.3 c | 12.1 c | 11.8 c | ||
| Al | 3.4 e | 3.9 d | 3.4 e | 2.9 e | 1.7 f | ||
| Si | 20.0 b | 27.7 b | 20.5 b | 20.3 b | 13.8 b | ||
| Ag | 7.9 d | 3.2 e | 5.2 d | 1.1 g | 0.0 g | ||
In this study, EDS analysis for identifying equal distribution through the antimicrobial paper found that Ag particles were distributed equally (Fig. 4). The content of Ag in antimicrobial paper with ultrasonic waves treatment was 3.2% and 7.9% of the surface and side per unit area among total element components, respectively, while the content of Ag in antimicrobial paper with no ultrasonic bath treatment was 1.1% and 5.2% of the surface and side, respectively. Ag was not detected in control paper without added Ag (Table 2).
Test of Antimicrobial Activity in Antimicrobial Paper
Compared to control paper, the antimicrobial paper had activity against all three putrefactive pathogens that cause strawberry fruit rot after harvesting. In particular, the antimicrobial paper had excellent effects against B. cinerea and A. tenuissima. On the other hand, R. stolonifer showed inhibition until 1 day (24 h) after inoculation, but then showed signs of covering the antimicrobial paper and proliferated quickly at 1.5 (36 h) days after inoculation (Table 3). Inoculation of putrefactive pathogens in strawberry fruits showed that R. stolonifer and B. cinerea started to cause disease symptoms on the fruit surface after 2 days with control paper and after 3 or 4 days with antimicrobial paper of 10 mM AgNO3 and 30 mM AgNO3, respectively. The effect of antimicrobial activity increased with increasing concentrations of Ag (Fig. 5). Also, antimicrobial paper minimally changed fruit weight and hardness compared to control paper (Fig. 6). As a result, the antimicrobial paper had the effect of distribution extension of about 1-2 days.
Table 3.
Effects of putrefactive pathogen control in antimicrobial paper synthesized by R. coreanus extract and silver nanoparticles
|
Putrefactive pathogen | Inhibition zone (mmx) | ||||||||
| A. tenuissima | R. stolonifer | B. cinerea | |||||||
| Days after treatment | 2 days | 4 days | 6 days | 1 day | 1.5 days | 1 day | 2 days | ||
|
Control (non-treatment paper) | 13.0 ay | 7.3 b | 0.0 b | 6.0 b | 0.0 a | 4.3 b | 0.0 b | ||
| Antimicrobial paper | 13.7 a | 9.3 a | 3.7 a | 10.7 a | 0.0 a | 7.3 a | 2.3 a | ||
| Inhibition zone in antimicrobial paperz | 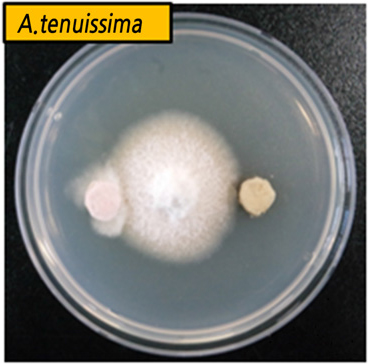 |  |  | ||||||
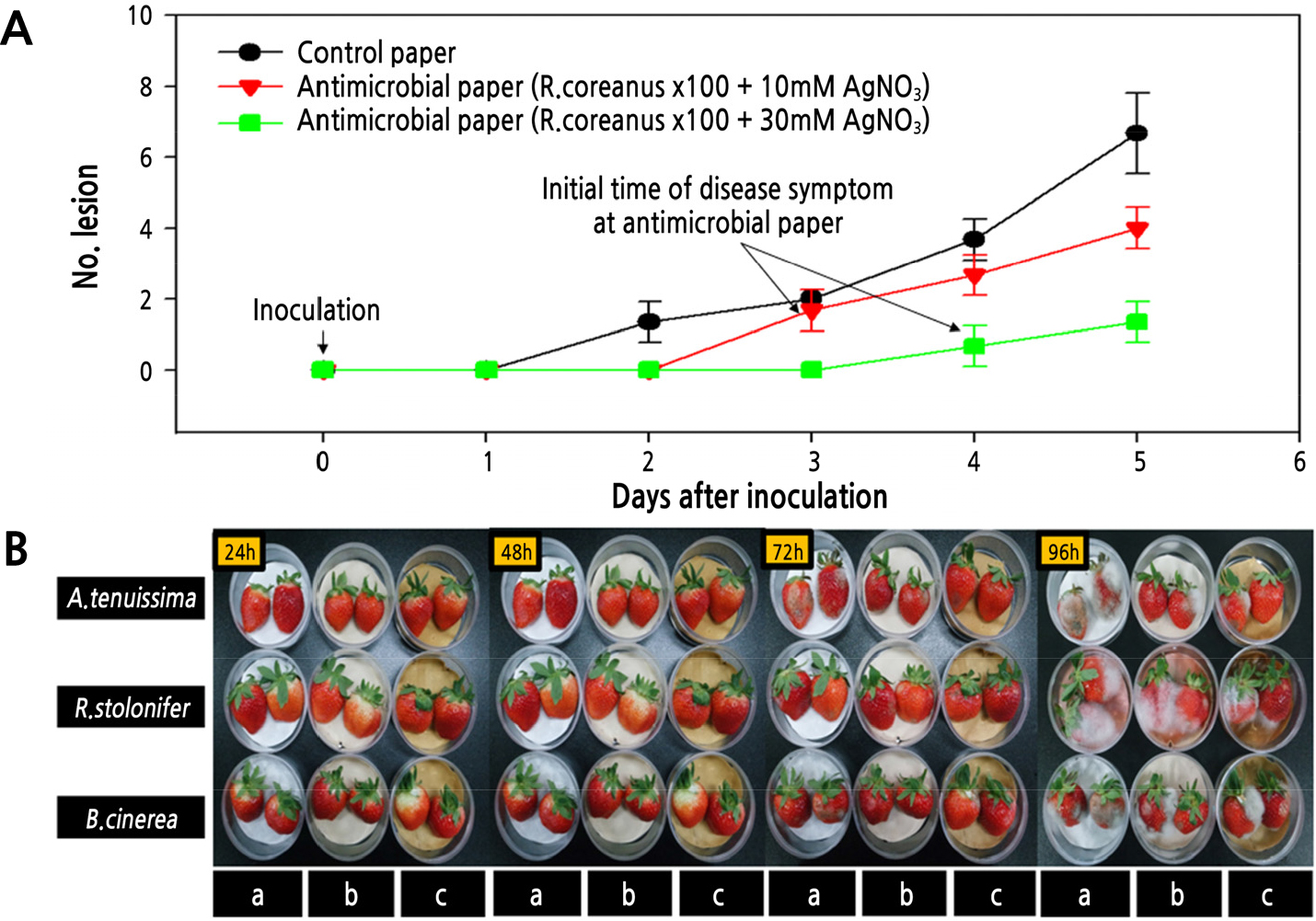
Fig. 5.
Control degree of putrefactive pathogens on strawberry fruits stored at 25℃ for 5 days after inoculating the three putrefactive pathogens with concentrations of AgNO3 in manufacturing antimicrobial paper. (A) The number of lesion over time after the initial occurrence of disease symptoms. (B) Disease symptoms after inoculation of A. tenuissima (top), R. stolonifer (middle), and B. cinerea (bottom) in a horizontal line, and control paper (a), antimicrobial paper (R. coreanus ×100 + 10 mM AgNO3 (b), and antimicrobial paper (R. coreanus ×100 + 30 mM AgNO3) (c) in vertical lines.
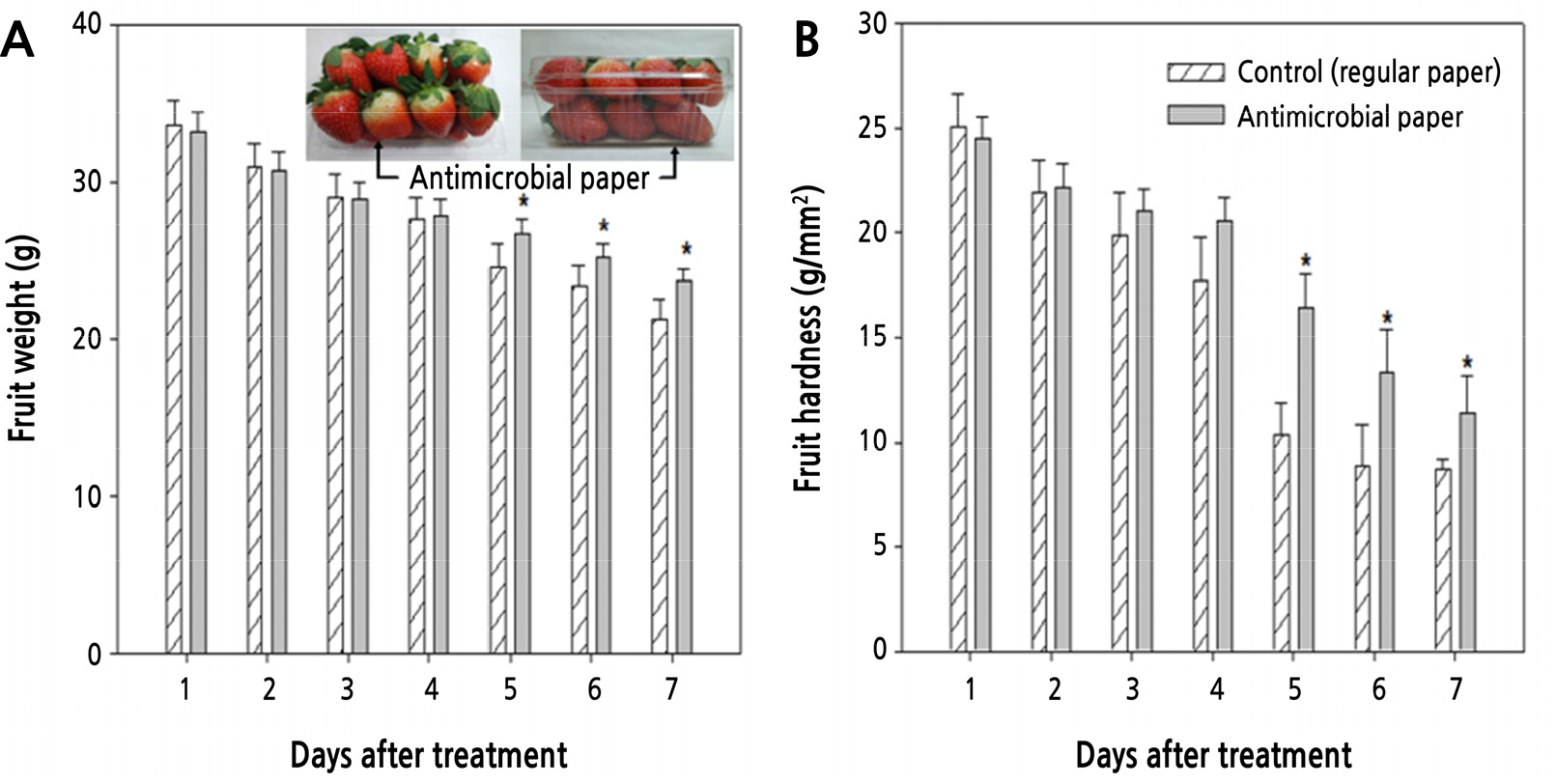
Fig. 6.
Changes in fruit weight (A) and hardness (B) in strawberry fruits during 7 days with antimicrobial paper treatment (R. coreanus ×100 + 30 mM AgNO3). Values are represented as means ± SE. * Denotes significant differences at p < 0.05. Strawberry image indicates the experiment in actual packaging boxes.
Analysis of Components in R. coreanus Leaf Extract
R. coreanus leaves were analyzed for the major ingredients of ellagic acid, gallic acid, kaempferol, quercetin, and quercetin-3-O-glucoside through MS full scanning mode with a scan range of 100-1,500 (m/z) based on a previous study (Li et al., 2016). Gallic acid produced a protonated ion [M+H]+ at m/z 171.1 and fragment ions at m/z 126.9, 125.0, 153.0, 109.0, and 80.9. The corresponding m/z ratios of the remaining compounds were as follows: ellagic acid, m/z 303.2 → 275.0, 264.9, 256.9, 229.1, and 200.8; kaempferol, m/z 287.0 → 153.0, 165.0, 137.0, and 69.1; quercetin, m/z 303.1 → 229.2, 164.9, 153.1, and 69.1; and Q-3-O, m/z 465.3 → 303.2 (Table 4). Antimicrobial paper was analyzed for the content of these components by LC-MS/MS through the reflux extraction method with 70% MeOH. The results showed that antimicrobial paper contained 1.37 mg/100g of these main components, and a range of 0.03-0.70 mg by each component in the case of antimicrobial papers produced with R. coreanus leaf extract (Table 5).
Table 4.
Compounds detected in Rubus coreanus by liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis in positive ion mode
| Compound |
Formula (molecular weight) |
Retention time (min) |
[M+H]+ (m/z) |
MS/MS Fragment ion (m/z) |
Reference fragment ions |
| Gallic acid |
C7H6O5 (170.1) | 6.72 | 171.14 | 126.90, 125.00, 153.00, 109.00, 80.90 | 127.0(Zhao et al., 2007) |
| Ellagic acid |
C14H6O5 (302.2) | 14.10 | 303.04 | 275.00, 264.90, 256.90, 229.10, 200.80 | 257.0, 275.0, 303.0 (Abu-Reidah et al., 2015) |
| Kaempferol |
C13H10O6 (286.2) | 14.39 | 287.03 | 153.00, 165.00, 137.00, 69.10 | 153.0, 121.0, 165.0(Lin et al., 2008) |
| Quercetin |
C15H10O7 (302.2) | 14.03 | 303.05 | 229.20, 164.90, 153.10, 69.10 | 153.0, 229.0, 137.0(Lin et al., 2008) |
| Quercetin-3-O-glucoside |
C20H20O12 (464.3) | 14.30 | 465.04 | 303.20 | 303.0(Jang et al., 2018) |
Table 5.
Component contents in antimicrobial paper prepared by R. coreanus extract and silver nanoparticles
| Treatment | Ellagic acid | Gallic acid | Kaempferol | Quercetin | Quercetin-3-O-glucoside | Total |
| mg/100g | ||||||
| R. coreanus extract | 52.90 az | 11.70 a | 1.52 a | 7.28 a | 24.68 a | 98.10 a |
| Antimicrobial paper | 0.39 b | 0.70 b | 0.03 b | 0.05 b | 0.20 b | 1.37 b |
Discussion
Test of Antimicrobial Activity and Synthesis of R-AgNPs
Several crops and their by-products were tested for antimicrobial activity to control the three major putrefactive pathogens (B. cinerea, A. tenuissima, and R. stolonifer) that cause strawberry fruit rot after harvesting. Of these crops, only R. coreanus leaf extract was selected for the development of antimicrobial paper. Ahn et al. (2000) reported that although Aralia cordata, Salvia miltiorrhiza, Rheum palmatum, Aloe spp, and Scutellaria baicalensis had no antimicrobial activity in a single treatment, a mixture treatment had strong antimicrobial activity due to synergistic effects. However, in this study, antimicrobial activity between the single treatment of R. coreanus extract and the mixture treatment (two or three including R. coreanus) was not significant. Xu et al. (2018) indicated that different proportions of a combination presented different antimicrobial effects. Man et al. (2018) also reported that synergy between different antibacterial compounds can be determined as multiple concentrations of two different compounds. This study aimed to extend the expiration date using antimicrobial paper for strawberry fruit. However, research on the optimum proportions of the mixture treatment require further study.
Silver nanoparticles offer significant improvements in antimicrobial activity through specific effects such as adsorption at bacterial surfaces (Ouay and Stellacci, 2015). The antimicrobial activity increased with increasing concentrations of AgNO3 in the antimicrobial paper. The maximum concentration of AgNO3 was set to 30 mM for producing antimicrobial activity; higher concentrations affected the color of the antimicrobial paper and potentially could harm the environment, including soil (Ortiz et al., 2015). R. coreanus leaf extract was most effective in 10× solution among 10×, 100×, and 1,000× solutions. Yet, there were problems with too many materials for the 10× solution and insufficient antimicrobial effects with the 1,000× solution. The maximum concentration of R. coreanus leaves extract was set to 100× dilution for producing antimicrobial paper. In a previous study, the result of testing each antimicrobial effect in AgNO3 (30 mM) and R. coreanus leaf extract (100× tdilution solution) had the significantly effect on three major putrefactive pathogens (Nam, 2019). We confirmed that the mixed treatments were more effective than single treatments. Therefore, this study was aimed to produce antimicrobial paper with a synergy effect by mixing R. coreanus extract and Ag nanoparticles. The synthesis of R. coreanus leaf extract with Ag nanoparticles (R-AgNPs) was confirmed by UV-visible spectrophotometry, TEM, and SEM analyses. Son et al. (2011) reported that Ag nanoparticles show a peak around 400 nm, which shifts to higher wavelengths as the solution concentration increases. The synthesis with R. coreanus extract and Ag nanoparticles (R-AgNPs) was confirmed to be observed dependent of concentration with the largest peak at 400 nm. Therefore, the change in wavelength of Ag nanoparticles in response to the concentration of R. coreanus extract means that R. coreanus extract combined with the Ag nanoparticles. In the TEM analysis, R. coreanus extract was combined with round nanosilver particles, along with some triangular, polygon, and stick forms (Fig. 2). This result corresponds with reports from Kyung and Ko (2014), which demonstrated that the green-synthesis solution by mixture of chitosan and silver nitrate had round nanosilver particles. In addition, EDS analysis for identifying equal distribution in antimicrobial paper found that Ag nanoparticles were distributed equally by ultrasonication treatment for 2 hours (Fig. 4). Lu and Chou (2008) reported that ultrasonication treatment was beneficial to obtaining high conversions to narrow size distributions of silver colloids from this synthesis process.
The Effect of Antimicrobial Paper Against Main Putrefactive Pathogens of Strawberry Fruit
Antimicrobial paper was tested by putting it in the bottom of the actual packaging box after artificially inoculating strawberry fruits with three putrefactive pathogens. The antimicrobial paper controlled the fruit quality 2 days longer than the control paper. The antimicrobial paper had excellent effects against B. cinerea and A. tenuissima. On the other hand, R. stolonifer showed lower inhibition than B. cinerea and A. tenuissima. It seems that each putrefactive pathogen had different growth characteristics. Odeniyi et al. (2009) reported that R. stolonifer is a zygomycete and has a filamentous growth habit with stolons and sporangiospores, while B. cinerea and A. tenuissima are ascomycetes and have sclerotium, hypha, and conidia. This filamentous growth habit of R. stolonifer causes faster growth than other putrefactive pathogens (Simmons, 2007; Williamson et al., 2007). A. tenuissima did not proliferate in the control paper, generally. A. tenuissima is slower in growth than other pathogens because it forms a hypha at 5-7 days after inoculation, and its growth is more robust with longer periods of light exposure (Rotem, 1994; Simmons, 2007). In this study, it seems that A. tenuissima proliferation was controlled by growth in the dark to match the transport process of strawberry fruits.
However, inoculation of putrefactive pathogens in strawberry fruits showed that R. stolonifer and B. cineraea started to cause disease symptoms on the fruit surface after 2 days with control papers and after 3-4 days with antimicrobial papers. Therefore, the antimicrobial paper had the effect of distribution extension of about 2 days (Fig. 5). Also, antimicrobial paper showed a smaller change in fruit weight and hardness than control paper (Fig. 6). Therefore, antimicrobial paper gave a 2-day extension in maintaining fruit quality and storage period by inhibition of putrefactive pathogens.
Sallato et al. (2007) reported that material processing such as boscalid could extend putrefactive inhibition until 5-15 days, but direct treatments such as these on fruits could have side effects on fruit color, fragrance, and texture (Holcroft and Kader, 1999; Pelayo et al., 2003).
Generally, putrefactive pathogens cause a loss in fruits weight in strawberry of 15-44.3% during distribution by B. cinerea, 65.7% by R. stolonifer, and 44.1% by Alternaria sp., and in the case of fruit hardness, cause a loss of 2.4%, 1.1%, and 3.3%, respectively (Legard and Chandler, 2000; Legard et al., 2000; Embaby et al., 2016). Therefore, this study identified that antimicrobial paper can inhibit putrefactive pathogens by minimizing changes to fruit weight and fruit hardness during shelf life because it was added as buffer paper in the box and the fruit was not treated directly.
Compounds of Antimicrobial Activity Including Antimicrobial Paper
Components with antimicrobial activity in R. coreanus leaf extract were analyzed, such as ellagic acid, gallic acid, kaempferol, quercetin, and quercetin-3-O-glucoside. Shen et al. (2014) reported that ellagic acid from blueberry extract was the most effective in inhibiting the growth of L. monocytogenes and S. enteritidis. Also, gallic acid-AgNPs and gallic acid-quercetin-Ag-SeNPs at appropriate concentration inhibited the growth of B. subtilis, C. albicans, E. coli,and S. aureus (Mittal et al., 2014; Li et al., 2015). In the case of kaempferol, Ilk et al. (2017) reported that the synthesized kaempferol-chitosan nanoparticles exhibited an immediate inhibition zone in C. violaceum. Akroum et al. (2009) reported that quercetin-3-O-glucoside among the components of Mantha lonifolia extract most effectively inhibited growth of B. cereus, B. subtilis, E. coli, P. aerugionosa, and S. aureus. Considering the above reports, it can be assumed that the main compounds in R-AgNPs have antimicrobial activity against the putrefactive pathogens.
Conclusions
Antimicrobial paper was developed using Ag nanoparticles synthesized with R. coreanus extract. The developed antimicrobial paper showed antimicrobial effects against all three putrefactive pathogens (B. cinerea, A. tenuissima,and R. stolonifer) causing strawberry fruit rot after harvesting. Strawberry fruit during distribution were confirmed to have a 2-day extension in fruit quality maintenance and storage period with antimicrobial paper laid in the box.