Introduction
Materials and Methods
Experimental materials
Experimental analysis methods
Results and Discussion
Introduction
Hydroponic cultivation (apart from pure hydroponics systems) primarily utilizes solid substrates, such as coir, rock wool, and perlite. In the early 2000s, rock wool and perlite were the most commonly used substrates. However, after 2005, the use of perlite decreased, whereas cultivation areas using rock wool increased significantly (RDA 2021). The inorganic substrate rock wool has several advantages, such as excellent porosity and water retention, which facilitate uniform nutrient and moisture management, thereby enhancing cultivation stability (Bussell and McKenna 2004; Lee et al. 2017). However, owing to its low biodegradability, the disposal of rock wool after use poses significant challenges (Raviv et al. 2002; Kim and Jeong 2003; Park et al. 2003; Allaire et al. 2005; Gilewska 2006; Acuna et al. 2013; Jaroszuk-Sierocińska et al. 2014). Coir substrates possess stable physicochemical properties due to their high water retention capacity and slightly acidic pH (Rincón et al. 2005; Hongpakdee and Ruamrungsri 2015; Lim et al. 2020a). Additionally, given that the use of rock wool has decreased significantly owing to the aforementioned disposal issues, the use of coir substrates has surged (RDA 2013; MAFRA 2018). As of 2019, the total area cultivated with coir substrates has increased to 1,586 hectares across various crop types, accounting for 35% of the overall hydroponic cultivation area (Choi et al. 2019; Lim et al. 2019; Lim et al. 2020b). With regard to rock wool, the International Agency for Research on Cancer (IARC) announced no evidence of carcinogenicity (IARC 2002). However, it is still classified as waste, making recycling impossible (Fig. 1). Although coir substrates and plant residues after cultivation are considered recyclable biomass, the lack of proper recycling systems is an issue. The increasing scale of smart-farm cultivation has led to a substantial increase in the volume and diversity of by-products. Environmental pollution caused by these by-products has reached a critical point, demanding immediate and comprehensive management solutions (Obi et al. 2016; Arora et al. 2023). Moreover, during the pre-soaking process of coir substrates, a dark reddish leachate is generated, and misunderstandings regarding this leachate can cause complaints and conflicts among residents. Therefore, an accurate analysis of its characteristics is necessary. The rapid expansion of smart farms has also exacerbated the problem of by-product disposal. Inadequate regulations and limited disposal options have left farmers to grapple with these by-products independently, often leading to improper disposal practices that contribute to soil contamination, water pollution, and biodiversity loss. For these reasons, immediate and comprehensive management is required. For sustainable agriculture, it is essential to establish a system for processing various by-products that can be reused as resources. To recycle agricultural by-products, it is crucial to determine the direction of recycling by assessing their potential hazards and analyzing their components.
This study analyzed the heavy metals, pesticide residues, and inorganic components in the rock wool and coir substrates used in paprika greenhouses to evaluate their recycling potential. Additionally, heavy metals, the chloride ion concentration, and salinity in the leachate generated during the pre-soaking process of the coir substrates were analyzed.
Materials and Methods
Experimental materials
To analyze heavy metals, pesticide residues, and inorganic components in rock wool and coir substrates before and after use, rock wool samples were collected from three paprika greenhouses located in Gangwon-do, Jeollabuk-do, and Gyeonggi-do, Korea. Coir substrate samples were collected from seven paprika greenhouses located in Jeollanam-do, Jeollabuk-do, Gyeongsangnam-do, Gangwon-do, and Jeju Island, Korea (Figs. 2 and 3).
Leachate samples were collected from paprika cultivation greenhouses to analyze and measure the heavy metal levels, chloride ion concentrations, and salinity of the leachate samples generated during the pre-soaking process of the corresponding coir substrates.
Experimental analysis methods
Analysis of heavy metals in substrates and leachates
To analyze heavy metals in the substrate before and after use, five rock wool substrate samples were collected both before and after use from a greenhouse in Inje-gun, Gangwon-do. The five samples collected before use were combined and analyzed as a single set, and the five samples collected after use were similarly combined and analyzed as a separate set. For the coir substrates, similar to the analysis of rock wool substrates, the five samples collected before use and the five samples collected after use were each combined and analyzed separately, with one analysis conducted for each set. The leachate samples generated during the pre-soaking of the coir substrates were collected from five sections at the Mokpo National University greenhouse. These five samples were combined into one set and analyzed once, as described above. The heavy metal contents in the rock wool and coir substrates were analyzed according to the soil contamination test standard ‘ES 07400.2c’ Metals—Inductively Coupled Plasma-Atomic Emission Spectroscopy, as prescribed by the Soil Environment Conservation Act announced by the Ministry of the Environment (ME 2017). The samples were prepared according to the test specifications and were analyzed for cadmium (Cd), chromium (Cr), copper (Cu), mercury (Hg), nickel (Ni), lead (Pb), and zinc (Zn) using an inductively coupled plasma optical emission spectrometer (ICP-OES, SpectroGreen, Spectro, Germany) at the Mokpo National University Plasma Spectroscopy Analytical Center.
To analyze the chromium detected in the rock wool substrate samples separately as Cr3+ and Cr6+, the chromium species were initially separated using ion chromatography (IC) at a flow rate of 2.0 mL/min with 40 mM NH4NO3 (pH 7.0) as an eluent. The separated species were then quantified using the ICP-OES device (SpectroGreen, Spectro, Germany). The heavy metal contents detected in the rock wool and coir substrates before and after use were compared with the soil contamination concern standard for region 1 (paddy fields, dry fields, orchards, pastures, and mineral spring sites), as specified in the enforcement rule of the Soil Environment Conservation Act announced by the Ministry of the Environment.
The heavy metal content in the leachate generated during the pre-soaking process of the coir substrates was analyzed according to the water pollution process test standard ‘ES 0400.3c’ Metals—Inductively Coupled Plasma-Atomic Emission Spectroscopy, as specified by the Water Environment Conservation Act (ME 2023) announced by the Ministry of the Environment (ME 2023). The prepared samples were analyzed according to the test standards for Cd, Cr, Cu, Hg, Pb, and Zn using an ICP-OES device (5800 ICP-OES, Agilent, USA). The heavy metal content in the leachate from the coir substrate was compared with the clean area standards specified in the discharge limits for water pollutants under the enforcement rule of the Water Environment Conservation Act (ME 2023).
Analysis of pesticide residues in substrates
To analyze the residual pesticides, five used rock wool substrate samples were collected from a greenhouse in Gimje-si, Jeollabuk-do. As above, these were combined and analyzed once. Similarly, five used coir substrate samples were collected from a paprika greenhouse in Namwon-si, Jeollabuk-do, combined, and analyzed once.
The pesticide residues in the rock wool and coir substrates were analyzed according to the multi-residue analysis method specified by the Ministry of Food and Drug Safety (MFDS 2022). The samples were prepared according to the test standards and were analyzed for 320 types of pesticide residues using GC-MS/MS (7890–7000C, Agilent, USA) and LC-MS/MS (API 4000, ABSCIEX, USA). The pesticide residues detected in the rock wool and coir substrate samples were compared with the pesticide residue limits set by the Ministry of Food and Drug Safety (MFDS 2022).
Analysis of inorganic components in substrates and leachates
For the analysis of the inorganic components of the used substrates, five used rock wool substrate samples were collected from greenhouses in Gimje-si, Jeollabuk-do, and Hwaseong-si, Gyeonggi-do. These samples were combined and analyzed in duplicate to calculate the average values. For the used coir substrates, five samples were collected from each of seven paprika greenhouses located in Gangjin-gun, Jeollanam-do; Namwon-si, Jeollabuk-do; Jinju-si and Haman-gun, Gyeongsangnam-do; and Cheorwon-gun, Gangwon-do, and Jeju-do. These samples were combined and analyzed once per greenhouse. The leachate samples generated during the pre-soaking of the coir substrate samples were collected from five sections at the Mokpo National University greenhouse, combined, and analyzed once.
To analyze the inorganic components in the rock wool and coir substrates, 3.0 g of each substrate sample was acid-digested in a mixture of 30.0 mL nitric acid and 2.0 mL hydrogen peroxide at 180°C for two hours. After digestion, the solution was filtered through a quantitative type of filter paper, diluted, and analyzed for nitrogen (N), boron (B), calcium (Ca), potassium (K), magnesium (Mg), manganese (Mn), sodium (Na), phosphorus (P), and silicon (Si) using ICP-OES.
To measure the chloride ion concentration in the coir substrate leachate, samples were prepared according to the water pollution process test standard ES 04356.1b under the Water Environment Conservation Act, as announced by the Ministry of the Environment (ME 2023). The samples were filtered through a 0.2-µm membrane filter (PVDF 0.2 µm, Waters Co., USA) to remove solid particles, after which the ion content was analyzed using an ion chromatograph (Dionex Integrion HPIC, Thermo Fisher Scientific, USA).
The salinity of the coir substrate leachate was analyzed according to the water pollution process test standard ‘ES 04310.1d’ under the Water Environment Conservation Act, as announced by the Ministry of the Environment (ME 2023). Samples prepared according to the test standards were used to rinse the EC meter cell (CM-25R, TOA-DKK, Japan) two to three times. The electrical conductivity was then measured repeatedly at a maintained temperature of 25 ± 0.5°C, and the values were recorded and converted to salinity based on the electrical conductivity.
Results and Discussion
Analysis of heavy metals in substrates and leachates
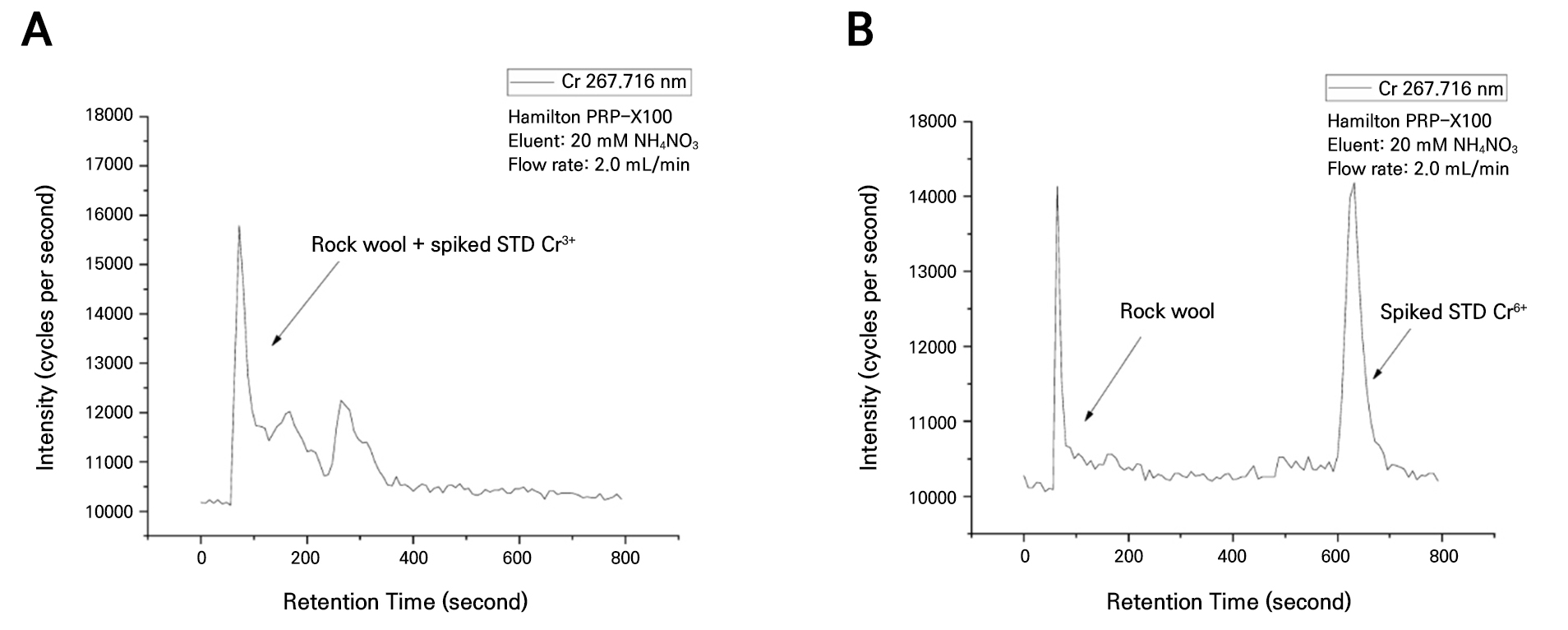
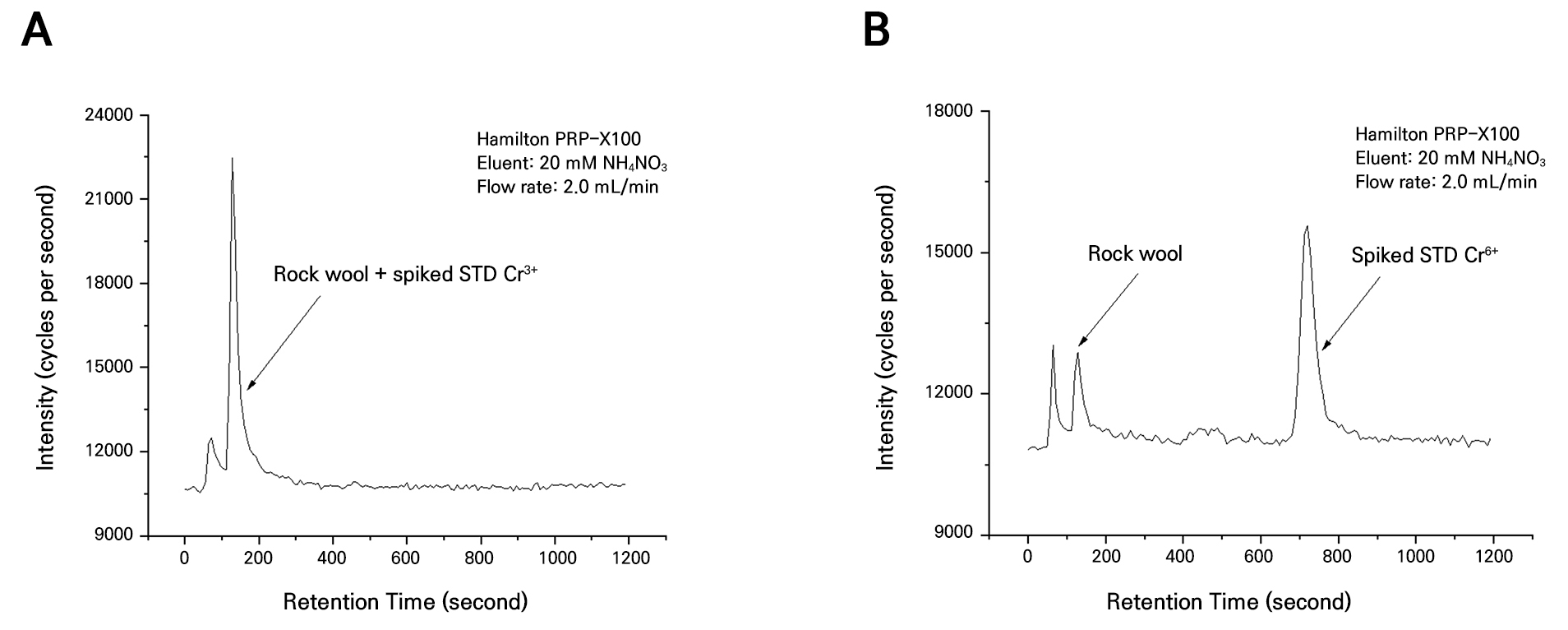
The analysis of the heavy metal content in the rock wool substrates before use showed that Cd was not detected, whereas Cu, Ni, Pb, Hg, and Zn were found to be present at concentrations of 102.0, 73.1, 51.7, 73.1, and 79.2 mg·kg-1, respectively (Table 1). The concentrations of Cd, Cu, Ni, Pb, and Zn were below the threshold levels for region 1 as specified by the soil contamination concern standard in the enforcement rule of the Soil Environment Conservation Act in South Korea. However, mercury and chromium exceeded the limits, with concentrations of 69.1 mg·kg-1 and 264 mg·kg-1, respectively.
The analysis of the heavy metals in the rock wool substrate samples indicated the absence of both Cd and Hg. While Cu, Ni, Pb, and Zn were observed at concentrations of 146.0, 31.7, 43.9, and 178.0 mg·kg-1, respectively, all were below the threshold levels for region 1 as specified by the soil contamination concern standard (Table 1). However, Cr was detected at 1,015 mg·kg-1, greater by 746 mg·kg-1 compared to the unused rock wool substrate. The heavy metals detected before use – that were not present after use – were likely washed out by the nutrient solution during cultivation. In contrast, the increased concentrations of Cr, Cu, and Zn in the used substrates compared with the unused substrates were likely due to the accumulation of these elements from the supplied nutrient solution or water during the cultivation process. In particular, Cr, which exhibited the highest concentration in the rock wool substrate, can be separated into trivalent (Cr3+) and hexavalent (Cr6+) species. Hexavalent chromium, which is produced by industrial processes, is highly irritating and corrosive and reportedly has severe effects on the skin, liver, kidneys, and respiratory system of humans. Due to these properties, Cr6+ is classified as toxic or carcinogenic. In contrast, Cr3+, which mainly originates from natural sources, has a relatively low impact on human health and the environment (Mathur et al. 1977; Suzuki et al. 1984; Kim et al. 2004). After extracting the total chromium levels from the rock wool samples before and after use, an analysis was conducted with both the rock wool extract solution and the Cr6+ standard solution, which showed a peak for Cr6+ (Figs. 4B and 5B). However, when the rock wool extract solution was analyzed with the Cr3+ standard solution, a peak was observed only for Cr3+ (Figs. 4A and 5A). These results indicate that Cr6+ is not present in the rock wool. The absence of hexavalent chromium and the presence of only trivalent chromium in the rock wool substrate indicate that the substrate used is not harmful to humans and does not contribute to environmental pollution. This suggests that the rock wool substrate, currently classified as waste and not recyclable, could potentially be recycled. Grodan, a leading rock wool substrate manufacturer in Denmark, provides a “recycling solution” service to over 62% of its customers to promote the recycling of rock wool. This initiative has enabled the annual recycling of thousands of tons of rock wool annually, achieving a 45% recycling rate (Grodan 2022). Based on these examples of international success, it is necessary to establish a domestic rock wool recycling system to convert waste rock wool into a valuable resource. The analysis of heavy metals in the coir substrates before and after use revealed that the levels of Cd, Cu, Hg, Pb, Cr, Zn, and Ni were all below the threshold levels for region 1, as specified by the soil contamination concern standard (Table 1). Currently, greenhouses are using used coir substrates as soil conditioners in fields or paddies without analyzing harmful factors such as heavy metal or residual pesticide contents (Rhie et al. 2017). However, recycling used coir substrates without analyzing these harmful factors is considered an illegal act. This situation arises because no official report has documented the results of analyses of harmful factors in used coir substrates, resulting in recyclable resources being treated as waste. The results of this study provide support for officially recognizing used coir substrates, previously recycled illegally by greenhouses, as reusable resources. Therefore, used coir substrates could serve as a valuable source of materials for recycling as a fertilizer or soil conditioner for use in crop cultivation.
Table 1.
Heavy metal contents in rock wool and coir substrates before and after use
| Growing substrate | Cultivation | Heavy metals (mg/kg) | ||||||
| Cd | Cr | Cu | Hg | Ni | Pb | Zn | ||
| Rock wool | Before | N.D.z | 269 | 102.0 | 73.10 | 73.1 | 51.7 | 79.2 |
| After | N.D. | 1,015 | 146.0 | N.D. | 31.7 | 43.9 | 178 | |
| Coir | Before | 0.02 | 1.58 | 3.59 | N.D. | 0.66 | 0.50 | 9.78 |
| After | 0.13 | 1.17 | 66.56 | N.D. | 0.54 | 0.19 | 123.37 | |
| Soil contamination concern criterion of region 1y | 4.00 | 5.00 | 150 | 4 | 100 | 200 | 300 | |
Analysis of pesticide residues in substrate samples
The analysis of pesticide residues in the rock wool and coir substrates revealed that dinotefuran was present in the rock wool substrates (Table 2). Three insecticides (chlorantraniliprole, chlorfenapyr, dinotefuran) and three fungicides (boscalid, procymidone, fluxapyroxad) were detected as well. Importantly, the levels of all detected pesticide residues in both the rock wool and coir media were below the maximum residue limits (MRLs) specified by the Ministry of Food and Drug Safety, with chlorantraniliprole at 1.0 mg·kg-1, chlorfenapyr at 0.7 mg·kg-1, dinotefuran at 2.0 mg·kg-1, boscalid at 3.0 mg·kg-1, procymidone at 5.0 mg·kg-1, and fluxapyroxad at 1.0 mg·kg-1. Maintaining pesticide residues below these threshold levels is crucial for ensuring crop safety and protecting consumer health (FAO 2011). Additionally, because coir substrates are made from natural organic materials, they are readily utilizable as soil fertilizers and conditioners with minimal processing, provided that the pesticide levels remain below regulatory limits.
Table 2.
Residual pesticide levels in rock wool and coir substrates after use
| Growing substrate | Classification | Pesticide | mg/kg |
| Rock wool | Insecticide | Dinotefuran | 0.036 |
| Avermectin B1 and 318 other components | N.D.z | ||
| Coir | Insecticide | Chlorantraniliprole | 0.01 |
| Chlorfenapyr | 0.056 | ||
| Dinotefuran | 0.113 | ||
| Fungicide | Boscalid | 0.282 | |
| Procymidone | 0.030 | ||
| Fluxapyroxad | 0.098 | ||
| Avermectin B1 and 313 other components | N.D. | ||
Analysis of inorganic components in substrates and leachates
The analysis of the inorganic components in the used rock wool substrates showed that they contained N at 17.8 g·L-1, B at 0.06 g·L-1, Ca at 46.4 g·L-1, K at 5.24 g·L-1, Mg at 2.51 g·L-1, Mn at 3.20 g·L-1, Na at 6.74 g·L-1, P at 18.72 g·L-1, and Si at 154.0 g·L-1 (Table 3). Rock wool is a produced by melting basalt and dolomite at temperatures above 1400°C, primarily containing silicon dioxide (SiO2) and calcium oxide (CaO) (Yörükoğlu et al. 2020; Yap et al. 2021; Hossein et al. 2023). Therefore, in this study, the pre-treatment process conducted before analyzing the inorganic components of the rock wool substrate likely resulted in higher measurements of Si and Ca compared to those for the coir substrates. Although Si is not an essential element for plant growth, it plays a crucial role in increasing resistance to pathogens, fungi, and pests and in strengthening physical structures to prevent lodging (Epstein 1994; Datnoff et al., 1997). Silicon has been reported to enhance resistance to environmental sources of stress, such as salinity, and to improve photosynthetic efficiency (Al-aghabary et al. 2004; Lee et al. 2010). Silicon is the only element known to not cause toxicity in soil, even at high concentrations (Ma and Takahashi 2002). Calcium, the second most abundant element in rock wool substrates, is an essential macronutrient for plants. Calcium is primarily involved in the formation of the middle lamella during cell plate formation and cell division and is particularly associated with the physical protection of cell membranes, enhancing disease resistance (Xi et al. 2012). In addition, calcium acts as an initial signal transducer under cold and frost stress, triggering responses from various plant hormones (White and Broadley 2003; Xi et al. 2012). Therefore, the presence of various inorganic components, particularly high levels of Si and Ca, in the rock wool substrates suggests that they can be effectively recycled into various forms of fertilizers and soil conditioners, including silicate-based fertilizers.
Table 3.
Inorganic components in rock wool (n=2) and coir substrates (n=7) after use
| Growing substrate | N | B | Ca | K | Mg | Mn | Na | P | Si |
| g/L | |||||||||
| Rock wool | 17.80 ± 2.4z | 0.06 ± 0.004 | 46.40 ± 15.17 | 5.24 ± 1.27 | 2.51 ± 1.17 | 3.20 ± 1.51 | 6.74 ± 0.84 | 18.72 ± 3.42 | 154 ± 28.51 |
| Coir | 10.72 ± 2.34 | 0.067 ± 0.02 | 5.42 ± 1.66 | 5.86 ± 3.0 | 1.62 ± 0.52 | 0.19 ± 0.16 | 0.55 ± 0.31 | 4.74 ± 2.9 | 0.0009 ± 0.0004 |
Table 4.
Heavy metals, chloride ion concentration, salinity, and pH of the coir substrate leachate before use
| Leachate | Cd | Cr | Cu | Hg | Pb | Zn | Cl- | salinity |
| mg/L | % | |||||||
| coir | N.D.z | N.D. | 0.043 | N.D. | N.D. | 0.156 | 1,849.1 | 0.44 |
Nitrogen, the most abundant element in coir substrates, is the most important macronutrient, playing a critical role in plant growth through the synthesis of proteins. Therefore, it is the most commonly used fertilizer in crop cultivation (Taiz and Zeiger 2009). In addition to protein synthesis, nitrogen is utilized in the synthesis of nucleic acids, chlorophyll, and plant growth regulators (Below 2002). When applied to soil, organic fertilizers can rapidly mineralize organic nitrogen, thereby promoting early plant growth (Gale et al. 2006). Consequently, coir substrates rich in various inorganic nutrients and with high nitrogen levels can be easily recycled as soil conditioners, fertilizers, or artificial soil, making them valuable resources for various agricultural applications.
The chloride ion concentration in the leachate generated during the soaking process of the coir substrates before use was measured and found to be 1,849 mg·L-1 (Table 4). This value is below the proposed discharge limit of 3,000 mg·L-1 for chloride ions suggested by the Ministry of the Environment, indicating a low likelihood of causing water pollution (ME 2021). Although an official discharge limit for chloride ions has not yet been established by the Ministry of the Environment, the measured concentration is considered within a range that ensures environmental safety. The measured salinity of the leachate generated during the soaking of the coir substrates before use was 0.44%. High salinity can inhibit microbial activity, and the sudden influx of salts during wastewater treatment has been reported to have more detrimental effects than a gradual influx (Kincannon and Gaudy 1966, 1968; Burnett 1974; Woolard and Irvine 1995; Muyzer 1999; Abou-Elela et al. 2010). Wastewater with salinity below 3% can be effectively purified using traditional physiological methods, whereas wastewater with higher salinity levels is more difficult to purify (Woolard and Irvine 1995; Muyzer 1999). Given that the salinity of the coir substrate leachate in this case was 0.44%, it was considered very low and unlikely to cause problems during wastewater treatment or to exacerbate environmental pollution.
In addition, the recycling of growing media plays a vital role in sustainable agricultural practices. The increasing scale of smart-farm cultivation necessitates the establishment of systems for cultivation and the management of by-products. Therefore, based on the findings of this study, it is essential to reassess rock wool, which is currently classified as waste, and develop a system that can repurpose it as a valuable resource. This potential for recycling could help reduce environmental pollution through resource circulation. The findings of this study suggest that rock wool, previously classified as non-recyclable waste, could potentially be repurposed as a soil conditioner or fertilizer.