Introduction
Materials and Methods
Experimental Materials and Culture Conditions
Induction Culture
Proliferation and Differentiation Cultures
Rooting Culture
Seedling Refining and Transplanting
Data Processing and Analysis
Results
Effects of Different Explants on Bud Induction
Effects of Different Hormone Ratios on the Induction of Explants
Effects of Different Hormone Ratios on Proliferation and Differentiation
Effects of Different Hormone Ratios on Rooting Induction
Seedling Refining and Transplanting
Discussion
Conclusion
Introduction
Since the foreign scholar Haberlandt introduced the concept of tissue culture (1902), researchers have been using tissues or individual cells for in vitro regeneration (Ikeuchi et al., 2016). One study published the composition of the Murashige and Skoog (MS) culture medium (Sahebi et al., 2016). Plant tissue culture technology has achieved significant inroads in recent years, becoming increasingly sophisticated and profoundly influencing modern agriculture, playing important roles in rapid plant propagation, variety improvements and genetic engineering breeding (Oseni et al., 2018; Pradhan et al., 2021). Tissue culturing is the process of culturing living plant organs or cells under aseptic conditions to obtain cells, tissues, or whole plants that could continue to grow (Chen, 2018). During the tissue culture process, explants, medium compositions, culture conditions, plant growth regulators, and culture methods all influence the growth of plants and the synthesis of the target products (Fan et al., 2022). Accordingly, it is necessary to study the various conditions required in plant tissue culturing to establish an optimal plant tissue culture system.
Successful cases of pineapple tissue culturing have been reported by foreign scholars (1960) (Aghion and Beauchesne, 1960). After years of research, it is well established that axillary, sucker, and crown buds can serve as explants for pineapple tissue culturing, but crown buds are usually sold together with the pineapple fruit, and they are few in number (Reinhardt et al., 2018). The basic medium was a MS medium with 30 g/L sucrose and 8 g/L agar (Murashige and Skoog, 1962). Many studies have investigated the types and contents of hormones added to the culture medium. Among these, hormones 6-benzylaminopurine (6-BA) and naphthaleneacetic acid (NAA) are mostly used in the induction, multiplication and differentiation stages, and indolebutyric acid (IBA) is mostly used in the rooting stage (Zhang et al., 2017). Moreover, a few studies have reported the use of hormones 2, 4-dichlorophenoxyacetic acid (2, 4-D), and kinetin (KT) (Zhang et al., 2017; Harahap et al., 2019). The tissue culture requirements between different species and even between different genotypes can differ, and the process and conditions must be adjusted when undertaking different explant tissue culturing (Anjanappa and Gruissem, 2021).
Pineapple (Ananas comosus L.), a perennial herb of the genus Bromeliad in the family Bromeliaceae, is a well-known tropical and subtropical fruit. Pineapples are popular, being nutrient-rich and having an attractive color, aroma, and flavor. The traditional propagation of pineapples was mainly through asexual reproduction by sucker buds. However, this method has a low reproduction coefficient, uneven plant growth in terms of height, weight, and vegetative cycle; and is prone to accumulate and spread pests, diseases, and poisons (Luan et al., 2020), which impact the production and scientific research of pineapples. Using plant tissue culture technology, we can both mass-reproduce pineapple plants rapidly and obtain non-toxic and pest-free regenerated plants while maintaining the good properties of the parents, thereby solving the problems associated with pineapple asexual propagation. Most pineapples in the market must be peeled, gouged, and soaked in saltwater. However, shredded pineapple can be cut into small chunks and eaten directly by hand. This variety is one of the best fresh pineapple varieties, as it has a large fruit type, good quality, and is relatively convenient to eat (Carlier et al., 2007).
Currently, many imperfections are discussed in reports on the tissue culturing of shredded pineapple. Most studies only document the process of proliferation and seedling strengthening, proliferation, and rooting, providing little information on the hormone concentration range and combination (Sheng, 1991; Luo and Cheng, 2002). A complete regeneration system of shredded pineapple has yet to be established. In this study, the crown and sucker buds of shredded pineapple were used as explants to explore the optimal medium for the induction, proliferation, differentiation, and rooting of regenerated plants and the optimal time for seedling refining of shredded pineapple. Importantly, we established and optimized a pineapple regeneration system to provide a reference for the propagation and cultivation of pineapple.
Materials and Methods
Experimental Materials and Culture Conditions
The experimental materials were collected from shredded pineapple grown in Xuwen County, Zhanjiang City, Guangdong Province (20°120N 110°240E) in December of 2020. In this experiment, we used the crown and sucker buds of shredded pineapple as explants. MS medium was used as the basic medium, agar 7 g/L and sucrose 30 g/L were added, and the pH of the medium was 5.8. The medium was put into a high-pressure steam sterilization pot for sterilization at 121°C for 20 minutes and used after cooling. Culturing was carried out in a controlled environment with a temperature of 24 ± 2°C, light intensity of 2000 Lux, light treatment for 16 h and dark treatment for 8 h, and the external environment was disinfected using UV lamps once a day to reduce the risk of bacterial contamination.
After airing the crown buds and sucker buds of the collected shredded pineapple for five days, all leaves and the brown roots were cut off and washed under tap water. They were disinfected with 0.1% mercuric chloride (HgCl2) for 8 min, 2% NaClO for 6 min, 0.1% HgCl2 for 5 min. After disinfecting three times, all explants were cleaned with sterile water each time. After the last cleaning, the explants were cut into small fan-shaped pieces 0.5 cm thick and inoculated on the culture medium.
Induction Culture
The crown buds and sucker buds were used as explants. After cleaning, disinfection and cutting, they were inoculated on an MS + 2.0 mg/L 6-BA + 2.5 mg/L NAA medium (He et al., 2007) to assess the effects of different explants on bud induction. Then, the sucker buds were inoculated on ten induction mediums with different contents of 6-BA and NAA (Table 1) to assess the effects of different hormone ratios on the induction of explants. Three repeats were performed for each treatment, ten bottles per replicate and five pieces of tissue per bottle, with 100 bottles in total. The induction rate was observed and counted after 30–60 days.
Proliferation and Differentiation Cultures
The induced adventitious bud tissues were inoculated on MS + 2.0 mg/L 6-BA + 2.5 mg/L NAA medium (He et al., 2007) to grow many adventitious bud tissues. The callus was cut into soybean grain size and inoculated on ten mediums with different contents of 6-BA and NAA (Table 1). The weight of the callus before inoculation and the weight after 30 days of inoculation were recorded. Three repeats were performed for each treatment, ten bottles per replicate and five pieces of tissues per bottle, with 100 bottles in total. The proliferation was counted after 30 days.
Table 1.
Formulation of Induction / Proliferation and Differentiation Medium
| Treatment | 6-BA (mg·L-1) | NAA (mg·L-1) |
| MS | 0 | 0 |
| B1N1 | 1.0 | 1.0 |
| B1N2 | 1.0 | 2.0 |
| B1N3 | 1.0 | 3.0 |
| B2N1 | 2.0 | 1.0 |
| B2N2 | 2.0 | 2.0 |
| B2N3 | 2.0 | 3.0 |
| B3N1 | 3.0 | 1.0 |
| B3N2 | 3.0 | 2.0 |
| B3N3 | 3.0 | 3.0 |
Rooting Culture
Different contents of IBA were added to the MS medium, and seven types of induction medium were, established in each case (Table 2). The robust regenerated plants with consistent growth and no roots were inoculated on the culture medium. Each treatment had three replicates, ten bottles per replicate, and five regenerated plants were used for each bottle. The rooting situation was observed and recorded after 30 days.
Table 2.
Formulation of Rooting Medium
| Treatment | MS | I0.5 | I1 | I1.5 | I2 | I2.5 | I3 |
| IBA (mg·L-1) | 0.0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
Seedling Refining and Transplanting
When all regenerated plants in the rooting medium grew to a height of 5–7 cm, the culture bottle caps were loosened and placed in a half-shaded area outdoors for refining seedlings. Seedling refining was conducted under weak light conditions for 0, 3, 7 and 10 days. Each treatment consisted of 15 regenerated plants and three biological repeats. After seedling refining, the plants were removed from the culture flask and the culture medium was cleaned. The plants were put in the shade to air excess moisture and planted in a cultivation matrix of peat soil and vermiculite at 3:1. After planting, the planting basin was placed in water to let the cultivation matrix absorb enough water and the planting basin was then taken out and placed outdoors. Daily management was carried out, and the growth rate was observed and recorded after 30 days.
Data Processing and Analysis
IBM SPSS Statistics 20 was used for data processing and analysis. A one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test was used to determine significant differences among the means (p < 0.05). The results obtained from three repeated assays were given as the mean ± standard deviation (SD).
Results
Effects of Different Explants on Bud Induction
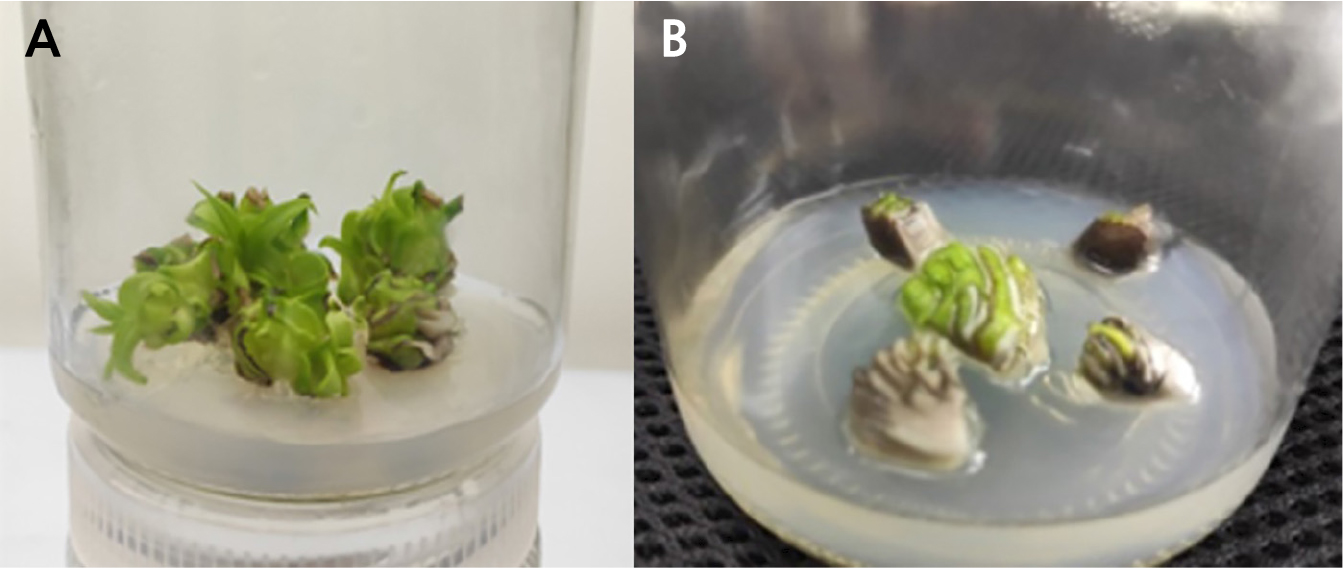
The sterilized explants were inoculated in MS + 2.0 mg/L 6-BA + 2.5 mg/L NAA medium for callus induction. The contamination rates of crown and sucker bud explants were 12% and 22%, respectively, and the sucker buds were relatively high. The crown buds began to swell and sprout at the base 15 days after inoculation, with the budding duration ranging from 15 to 25 days, with the average number of germination days equal to 20 days. The sucker buds began to swell and sprout at the base at 20 days, and lasting for a maximum of 35 days. Moreover, crown buds were the explants with the highest induction rate (78%), 29% higher than sucker buds (Table 3). As shown in Fig. 1, after 60 days of induction, the crown bud tissues exhibited significant growth, with the original leaves gradually turning green. The leaves then continued to grow, and the buds germinated (Fig. 1A). The growth of sucker bud tissue was relatively slow, with an older original leaf base and a slower rate greening, while the old leaves did not continue to grow. However, the buds germinated, and the incision became brown and expanded (Fig. 1B). Interestingly, the crown buds exhibited a better state than the sucker buds. These findings suggested that crown buds should be used as explants in the tissue culture of shredded pineapple.
Table 3.
Effects of Different Explants on Bud Induction
| Material | Contamination rate (%) | Days required for budding | Induction rate (%) |
| Crown buds | 12 b | 15–25 | 78 a |
| Sucker buds | 22 a | 20–35 | 49 b |
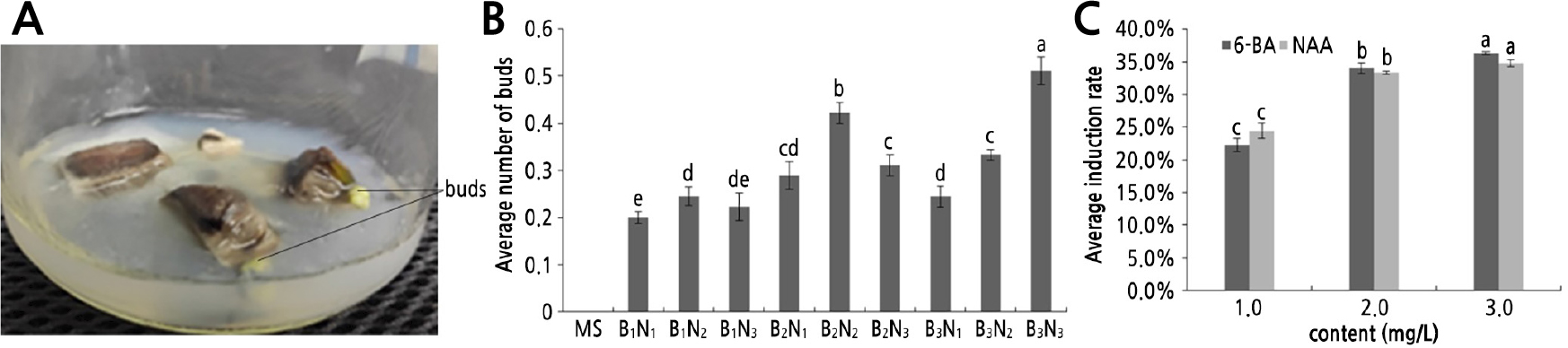
Fig. 2.
Induction state of sucker buds: (A) induction state of crown buds and sucker buds, (B) effects of different hormone ratios on pineapple induction, (C) effects of hormones on the induction rate of pineapple buds. B1N1, B1N2, B1N3, B2N1, B2N2, B2N3, B3N1, B3N2 and B3N3 represent MS+1.0 mg/L 6-BA+1.0 mg/L NAA, MS+1.0 mg/L 6-BA+2.0 mg/L NAA, MS+1.0 mg/L 6-BA+3.0 mg/L NAA, MS+2.0 mg/L 6-BA+1.0 mg/L NAA, MS+2.0 mg/L 6-BA+2.0 mg/L NAA, MS+2.0 mg/L 6-BA+3.0 mg/L NAA, MS+3.0 mg/L 6-BA+1.0 mg/L NAA, MS+3.0 mg/L 6-BA+2.0 mg/L NAA and MS+3.0 mg/L 6-BA+3.0 mg/L NAA medium, respectively. Different letters indicate significant differences (p < 0.05).
Effects of Different Hormone Ratios on the Induction of Explants
After inoculating the sucker buds of shredded pineapple on the induction mediums containing different hormone ratios for 20–30 days, the explants began to expand slowly, the incision exhibited a brown and dark green color, and the axillary buds turned green and germinated. Subsequently, light green or dark green calluses or buds could be seen (Fig. 2A). Rapid germination occurred in treatments B3N1 and B3N2 and began to expand and sprout at 20 days. The slowest germination occurred in treatment B1N1 and began to expand and sprout at 30 days (Fig. 2B and Suppl. Table 1s). Among them, the greatest percentage of induction (51.1%) occurred in treatment B3N3, which had 22 budding days. The induction rates of B1N1, B1N2, B1N3, B2N1 and B3N1 did not exceed 30%; they were 20.0%, 24.4%, 22.2%, 28.9% and 24.4%, respectively. The induction rates of B2N2, B2N3, and B3N2 were 42.2%, 31.1%, and 33.3%, respectively; none reached 50%. Moreover, the optimal medium for the induction of shredded pineapple in this experiment was MS + 3.0 mg/L 6-BA + 3.0 mg/L NAA.
When the contents of 6-BA and NAA in the medium were 1.0 mg/L, the average induction rates of the buds were 22.2% and 24.4%, respectively (Fig. 2C and Suppl. Table 2s). When the contents of 6-BA and NAA in the medium were 2.0 mg/L, the average induction rates were 34.1% and 33.3%, respectively. Peak induction was observed when the contents of 6-BA (36.3%) and NAA (34.8%) were 3.0 mg/L. Overall, the average induction rates increased with an increase in the hormone contents.
Effects of Different Hormone Ratios on Proliferation and Differentiation
Adventitious bud tissue proliferated when the proliferation coefficient exceeded 1 after 30 days of inoculation of adventitious bud tissue into the proliferation and differentiation medium with different hormone ratios (Fig. 3A and Suppl. Table 3s). The proliferation coefficients for MS and B3N1 exceeded 3.00 (3.74 and 3.10, respectively), and the differences between these two treatments were significant. The buds exhibited significant growth in the number and size and better differentiation in the MS medium (Fig. 3B-1); the tissues exhibited a large and uniform size after the B3N1 treatment (Fig. 3B-8). The proliferation coefficients of treatments B1N1, B1N3, B2N1, B2N2, B2N3, B3N2, and B3N3 ranged from 2.00 to 3.00 (2.18, 2.33, 2.13, 2.22, 2.51, 2.12, and 2.46, respectively). Among these, the tissues generally grew well with the B1N3, B2N3, and B3N3 treatment (Fig. 3B-4, B-7, B-10, and Suppl. Table 3s), with the rest exhibiting poor growth (Fig. 3B-2, B-5, B-6, B-9, and Suppl. Table 3s). The lowest proliferation coefficient was observed with the B1N2 treatment (1.98), where the tissues exhibited poor growth (Fig. 3A and 3B-3). According to the proliferation coefficient and growth, the buds exhibited significant growth, especially with the MS treatment, but this treatment was not conducive to the proliferation of adventitious bud tissues and could be selected in the seedling growth stage (Fig. 3B-1). The adventitious bud tissues exhibited better growth after the B3N1 treatment, which was more suitable for the subculture proliferation stage (Fig. 3A, 3B-8 and Suppl. Table 3s). Taken together, the above findings suggested that the most suitable proliferation medium for shredded pineapple is MS + 3.0 mg/L 6-BA + 1.0 mg/L NAA, with the most suitable seedling growth medium being MS medium.
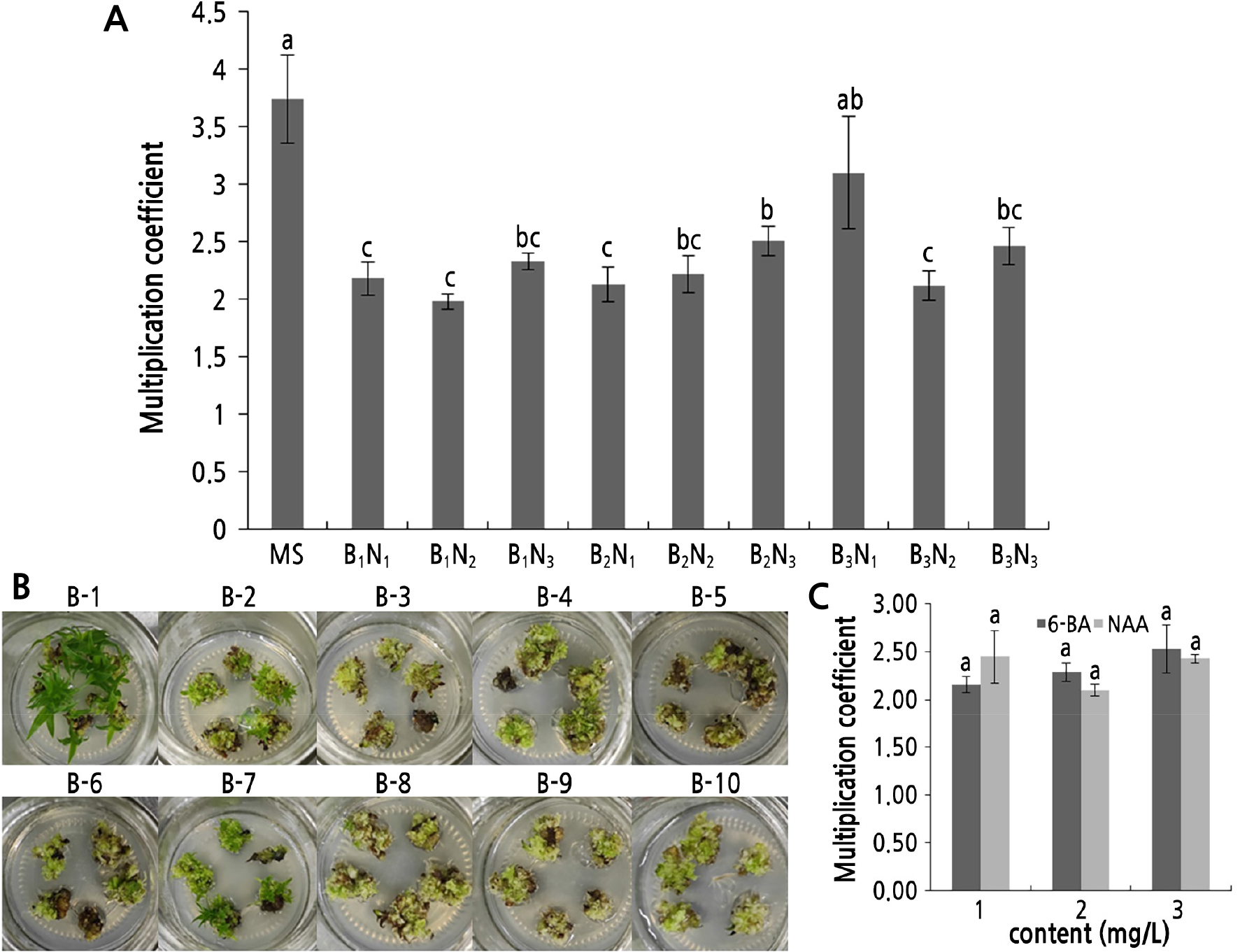
Fig. 3.
Proliferation and differentiation status: (A) effects of different hormone ratios on proliferation and differentiation after inoculation for 30 days, (B) status of proliferation and differentiation of different hormone ratios after inoculation for 30 days, (C) effects of hormones on proliferation and differentiation coefficients. B1N1, B1N2, B1N3, B2N1, B2N2, B2N3, B3N1, B3N2 and B3N3 represent MS+1.0 mg/L 6-BA+1.0 mg/L NAA, MS+1.0 mg/L 6-BA+2.0 mg/L NAA, MS+1.0 mg/L 6-BA+3.0 mg/L NAA, MS+2.0 mg/L 6-BA+1.0 mg/L NAA, MS+2.0 mg/L 6-BA+2.0 mg/L NAA, MS+2.0 mg/L 6-BA+3.0 mg/L NAA, MS+3.0 mg/L 6-BA+1.0 mg/L NAA, MS+3.0 mg/L 6-BA+2.0 mg/L NAA and MS+3.0 mg/L 6-BA+3.0 mg/L NAA medium, respectively. Figures b-1 to b-10 show the proliferation and differentiation growth statuses of MS, MS+1.0 mg/L 6-BA+1.0 mg/L NAA, MS+1.0 mg/L 6-BA+2.0 mg/L NAA, MS+1.0 mg/L 6-BA+3.0 mg/L NAA, MS+2.0 mg/L 6-BA+1.0 mg/L NAA, MS+2.0 mg/L 6-BA+2.0 mg/L NAA, MS+2.0 mg/L 6-BA+3.0 mg/L NAA, MS+3.0 mg/L 6-BA+1.0 mg/L NAA, MS+3.0 mg/L 6-BA+2.0 mg/L NAA and MS+3.0 mg/L 6-BA+3.0 mg/L NAA medium, respectively. Different letters indicate significant differences (p < 0.05).
We found that an increase in the content of hormone 6-BA in the culture medium was paralleled by a rise in the proliferation coefficient of shredded pineapple adventitious bud tissues (Fig. 3C and Suppl. Table 4s). At 6-BA concentrations of 1.0 mg/L, 2.0 mg/L and 3.0 mg/L, the proliferation coefficients were 2.16, 2.29, and 2.53, respectively, with no significant differences. However, the shredded pineapple showed a different response in NAA as the proliferation coefficient of adventitious bud tissues decreased initially and then increased. The proliferation coefficients at the NAA contents of 1.0 mg/L and 3.0 mg/L were comparable (2.45 and 2.43, respectively), while the proliferation coefficient was 2.10 at 2.0 mg/L, with no significant differences found in this case as well.
Effects of Different Hormone Ratios on Rooting Induction
The regenerated plants with a height of 3–5 cm with no roots were inoculated in mediums supplemented with different contents of IBA. Table 4 shows that the roots began to develop at 6–10 days. The rooting rates of MS, I0.5 and I3.0 were less than 100% at 93.2%, 93.2%, and 96.6%, respectively, with corresponding average numbers of roots of 4.07, 4.23, and 4.13. The roots of MS and I3.0 were thin and short, with fewer lateral roots (Fig. 4B-1 and B-7). In contrast, the roots of I0.5 were thick and long, with fewer lateral roots (Fig. 4B-2). The rooting rates of treatments I1.0, I1.5, I2.0, and I2.5 all reached 100%, and the average numbers of roots was 4.53, 5.23, 5.43, and 4.86, respectively. The roots of I1.0 were thick and long, with few lateral roots (Fig. 4B-3). The roots of I1.5 were thick and long, with extensive lateral roots (Fig. 4B-4). The roots of I2.0 were thick and long, with more extensive lateral roots and exhibiting the best root conditions (Fig. 4B-5). The roots of I2.5 were thick and long, with more lateral roots (Fig. 4B-6). From Fig. 4A, it can be seen that the average number of roots increased first and then decreased with the IBA content, and the greatest number of roots was observed when the IBA content was 2.0 mg/L. Consequently, the optimal medium for rooting induction of shredded pineapple was found to be MS + 2.0 mg/L IBA.
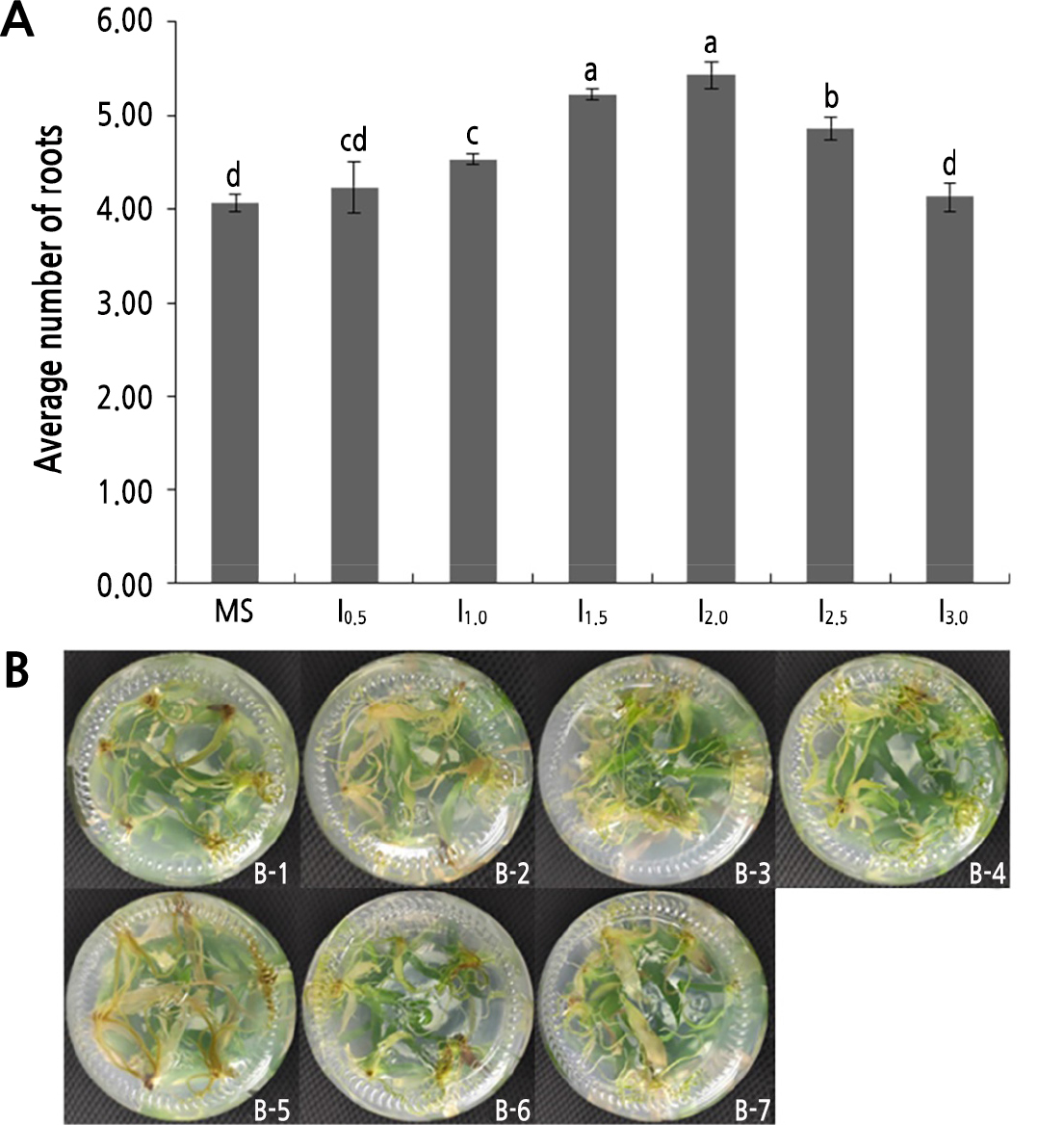
Fig. 4.
Root growth status: (A) effects of different hormone ratios on rooting after inoculation for 30 days, and (B) rooting of pineapple tissue culture seedlings after inoculation for 30 days. I0.5, I1.0, I1.5, I2.0, I2.5, and I3.0 represent MS+0.5 mg/L IBA, MS+1.0 mg/L IBA, MS+1.5 mg/L IBA, MS+2.0 mg/L IBA, MS+2.5 mg/L IBA and MS+3.0 mg/L IBA medium, respectively. Figures b-1 to b-7 show the root growth conditions of the MS, MS+0.5 mg/L IBA, MS+1.0 mg/L IBA, MS+1.5 mg/L IBA, MS+2.0 mg/L IBA, MS+2.5 mg/L IBA and MS+3.0 mg/L IBA medium, respectively. Different lowercase letters indicate significant differences (p < 0.05).
Table 4.
Effects of Different Hormone Ratios on Rooting after Inoculation for 30 Days
Seedling Refining and Transplanting
For seedling refinement and transplanting, the regenerated plants at 5–7 cm exhibiting good growth conditions were used. The regenerated plants underwent seedling refinement for 0, 3, 7, and 10 days, depending on the sample group (Fig. 5A). After seedling refinement, the plants were planted in the cultivation matrix (Fig. 5B). Table 5 showed the plants' growth conditions 30 days after planting. At baseline, the plants had a survival rate of 66.7%, the growth status of the plants was relatively worse as they, exhibited a smaller size, and the leaves were dried and yellow (Fig. 5C-0 d). Three days after seedling refinement, the plants had a survival rate of 73.3%, with poor growth status, smaller plants, dry and yellow leaves, and many plants wilted, which was not conducive to growth during the later stages (Fig. 5C-3 d). The survival rates of plants at seven and ten days were 100%. The growth phenotype was better at seven and ten days with larger plants, and the leaves were less dry and yellow. At seven days (Fig. 5C-7 d), the leaf tip was drier than at ten days (Fig. 5C-10 d), and growth during the later stages was not affected. Consequently, the selection of regenerated plants for transplantation should be conducted seven days after seedling refinement.
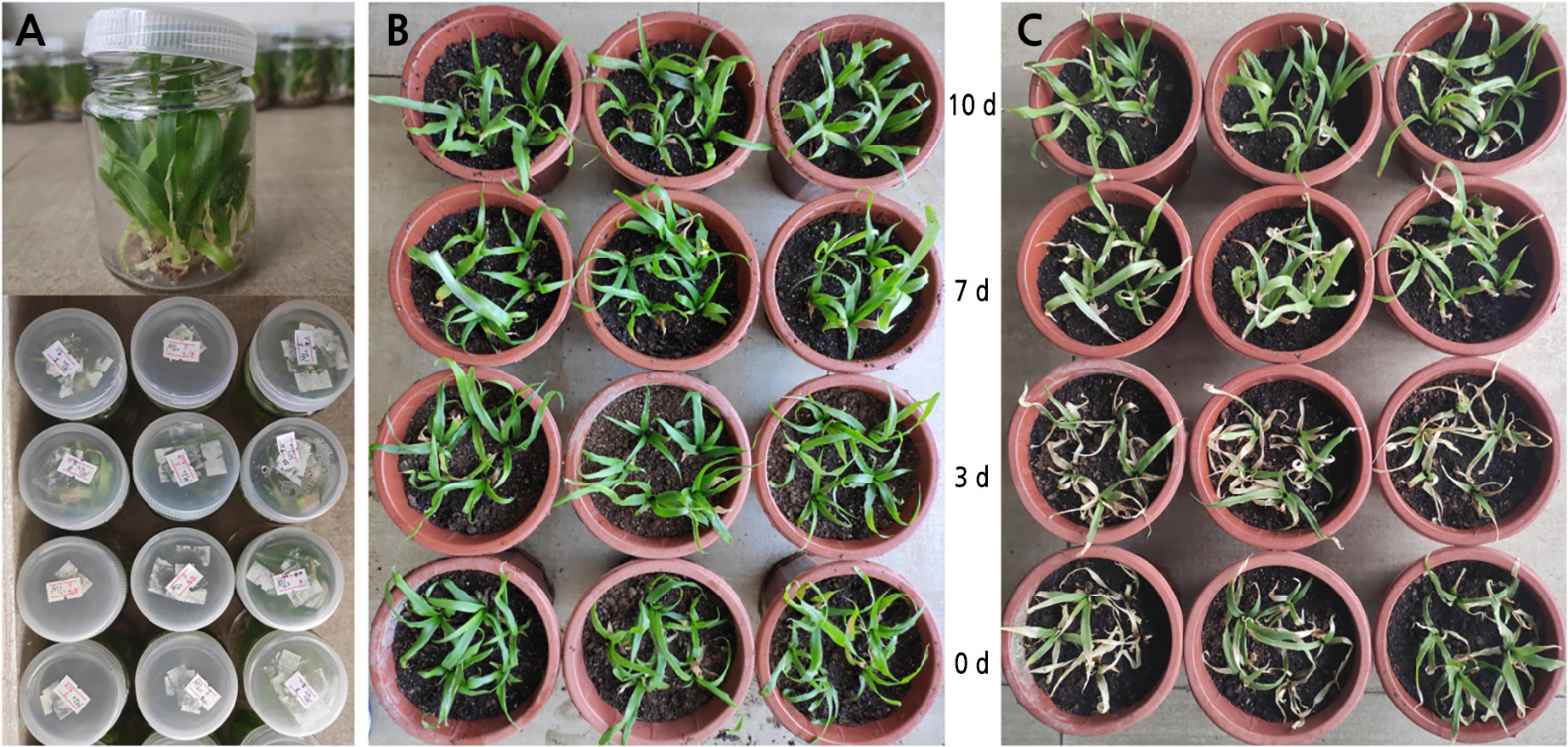
Fig. 5.
Seedling refining and transplanting: (A) loosening the cap of the culture bottle and refining the seedlings, (B) status of regenerated plants at the beginning of transplanting, and (C) Status of regenerated plants 30 days after transplanting. In figures (B) and (C), the seedlings are treated for 0 d, 3 d, 7 d and 10 d from bottom to top.
Table 5.
Survival Rates of Regenerated Plants at Different Refining Times
Discussion
During tissue culturing, any tissue or organ of the plant can be used as explants in theory, but in different plants, different varieties and different organs exhibit heterogeneous cell dedifferentiation, redifferentiation, and totipotency abilities. Accordingly, the tissue culture effect and the regeneration ability of explants are also different. The findings here suggest that selecting the correct explant is conducive to establishing a feasible regeneration system. This study found that crown buds should be selected as explants for the tissue culturing of shredded pineapple, consistent to a certain extent with a previous study (Zheng et al., 2005; Zhang, 2011). Nonetheless, inconsistencies with other studies (Huang et al., 1993) may be related to the variety, sampling time, and inoculation time. Huang et al. studied material sampling and inoculation in different seasons, finding that the growth state of buds differed, and that the pollution rate and induction rate of buds also changed (Wang et al., 2021; Lin et al., 2022). Interestingly, spring and autumn reportedly had higher survival rates and lower pollution rates (Yang et al., 2011). In this study, materials were taken and inoculated in winter, and the cold and dry conditions, with fewer pollutants attached to the leaves and leaf axils, which could reduce the pollution rate of the tissue cultures. Therefore, selecting the correct explant is crucial to establish an in-vitro culture successfully.
Plant regeneration can be conducted either by direct induction of the buds from the explants to form regenerated plants or by the induction of the callus from the explants, followed by differentiation and culturing to form regenerated plants (He et al., 2010). Consistent with that study, we found that during the induction of the shredded pineapple explants, the buds sprouted directly without any obvious callus and the original leaves of the explants gradually turned green (Fig. 2A) (Rahman et al., 2001; Zhang et al., 2010). In this experiment, the induction rate of adventitious buds increased with an increase in the concentrations of 6-BA and NAA (Fig. 2C), consistent with earlier work (Zheng et al., 2005; Zhang et al., 2010). MS supplemented with 3.0 mg/L 6-BA and 3.0 mg/L NAA was selected as the best media type for adventitious buds induction in this experiment and was associated with a high induction rate. This may have resulted because the combination of higher concentrations of 6-BA and NAA hormones was more conducive to the induction of pineapple explants. The effect of higher hormone ratios on the induction rate warrants further investigation.
Importantly, we found that the combination of 6-BA and NAA was more effective for adventitious bud proliferation and differentiation than NAA or 6-BA alone, consistent with earlier work (Huang et al., 1993; Yang et al., 2011). Auxin and cytokinin are vital to regulate plant growth and development (Nordström et al., 2004). Together they regulate various aspects of a plant’s growth and development through interaction. The combined effect of 6-BA and NAA can better promote the proliferation and differentiation of adventitious buds (Zhou et al., 2021). When adventitious buds were cultured for proliferation and differentiation on a MS medium without hormones, the largest proliferation coefficient was observed, but the tissue mass was not large; instead, several larger plants were differentiated (Fig. 3B-1). These findings suggested that the MS medium without hormone supplementation was optimal for the growth of seedlings. When adventitious buds were cultured for proliferation and differentiation on a MS medium supplemented with 3.0 mg/L 6-BA and 1.0 mg/L NAA, the second-highest proliferation coefficient was found, but the tissue mass was large and uniform (Fig. 3B-8), highlighting that it was the best proliferation medium.
In the rooting culture, the root growth of pineapple plants was less responsive to plant growth regulators and the plant formed roots when inoculated in MS without hormones. The formation of roots may be due to the endogenous hormones of regenerated plants or explants (Elmongy et al., 2018; Mostafa et al., 2020). In the present study, we found that the root growth was slow and that roots were few in number and weak without hormone supplementation (Fig. 4B-1). For root induction, different concentrations of IBA and NAA were determined. At all concentrations, IBA was more significant and effective than NAA for rooting (Priyanka, 2013). IBA is a growth regulator which is superior and effective with regard to stimulating rooting activities (Harahap et al., 2021). To speed up rooting, an appropriate amount of the IBA could be added. In this study, with an increase in IBA content, the number of pineapple roots increased initially and then decreased (Fig. 4A), likely because the concentration of IBA in the plant was too high, thus inhibiting root growth (Šípošová et al., 2019). Based on the root initiation duration, the average root number, and the root state, the optimal medium for rooting induction was MS supplemented with 2.0 mg/L IBA. At IBA contents of 1.5 mg/L and 2.0 mg/L, there were no significant differences in the average number of roots. We could therefore explore the rooting effect of the hormone content between 1.5 mg/L to 2.0 mg/L further. Yang et al. explored the effects of inorganic salts on pineapple rooting and found that supplementing with 0.1 mg/L NAA and 0.1 mg/L IBA was more suitable for rooting culture when using a 1/2 MS basic medium (Yang et al., 2011).
In the present study, during seedling refinement and transplanting, the duration of seedling refinement exerted a certain effect on the survival rates of the transplants, as the lowest overall survival rate was observed in plants that did not undergo seedling refinement (Fig. 5C-0 d). This finding may stem from the fact that the regenerated plants were in a sterile closed environment with a constant temperature and constant humidity, which suddenly turned into an open natural environment, making adaptation difficult (Yao et al., 2022). The plant survival rate at three days of seedling refinement was less than 90%, providing evidence of poor growth conditions (Fig. 5C-3 d), inconsistent with earlier findings (Luo and Cheng, 2002; Min et al., 2014). This discrepancy may be related to heterogeneity in the seedling refinement techniques and the transplanting seasons (Baskaran and Jayabalan, 2009; Yan et al., 2021). In this experiment, seedling refinement and transplanting were carried out in winter, when the winter wind was strong (in Guangdong province), and the potting soil and plant leaves easily dried out, resulting in a water shortage. Moreover, the temperature difference between day and night was considerable, making it difficult for the plants to adapt to the short refinement time. Seedling refinement of seven or ten days was associated with a plant survival rate of 100%, and these cases exhibited good growth. Our results suggest that seven days of seedling refining should be selected for transplanting to improve the efficiency of seedling refinement.
Conclusion
The present study of the influential factors of the tissue culturing of pineapple concluded that crown buds should be selected as explants and that MS+3.0 mg/L 6-BA+3.0 mg/L NAA should be selected as the induction medium. The MS medium was the most suitable for seedling culturing and the MS+3.0 mg/L 6-BA+1.0 mg/L NAA medium was the most suitable as the proliferation medium. The MS+2.0 mg/L IBA medium was the most suitable rooting culture of regenerated plants. Regenerated plants exhibited the best transplant yield at seven days of seedling refinement. This study successfully established and optimized a tissue culture and rapid propagation regeneration system for shredded pineapple and provides technical support for the rapid propagation and genetic transformation of pineapples.
Supplementary Material
Supplementary materials are available at Horticultural Science and Technology website (https://www.hst-j.org).
- HORT_20230021_Table_1s.pdf
Effect of Different Hormone Ratio on Induction of Pineapple
- HORT_20230021_Table_2s.pdf
Effect of Hormones on the Induction Rate of Pineapple Buds
- HORT_20230021_Table_3s.pdf
Effects of Different Hormone Ratios on Proliferation and Differentiation after Inoculation for 30 Days
- HORT_20230021_Table_3s.pdf
Effect of Hormones on Proliferation and Differentiation Coefficient