Introduction
Materials and Methods
Plant Materials
Temperature Treatment
Flowering Responses and Floral Organ Counts
RNA Extraction and qRT-PCR
Statistical Analysis
Results
Flowering Responses
Floral Organ Formation
Relative Gene Expression
Discussion
Introduction
Roses are iconic cut flowers with a high economic value in the global flower market (Ha et al., 2020; Yeon and Kim, 2020a, 2020c; Ha et al., 2021). Cut roses have been developed over several centuries, resulting in various cultivars with different phenotypes, such as different floral shoot lengths, flowering types, flower sizes, petal numbers, and colors (Dubois et al., 2010; Ma et al., 2015). Cut roses are distributed to the flower market with a floral shoot length of 40–90 cm, which is a standard requirement to ensure high flower quality (Yeon and Kim, 2017). Flowers with many petals are considered to have higher market value (Gattolin et al., 2020). Dubois et al. (2010) reported that new cultivars that have been bred for double flowers—those with more than 20 petals—are preferred in the flower market. Many commercial flower crops with double petals, such as rose, chrysanthemum, marigold, lily, stock, and camellia, are popular in the ornamental horticulture industry (Ma et al., 2015; Irani and Arab, 2017; Gattolin et al., 2020).
Flowering responses such as days to flowering, the floral shoot length, the petal size and color, and the vase life are affected by abiotic conditions such as the temperature, relative humidity, and light period (Cho et al., 2017; Yeon and Kim, 2017; Im et al., 2021; Lee et al., 2021; Roh and Yoo, 2021; Shi et al., 2021). In particular, the ambient temperature is an essential factor that controls the flowering time, quality, and floral organ development (Cho et al., 2017; Yeon and Kim, 2020b). In Korea, cut roses are generally harvested 30–50 days after the emergence of floral shoots. In particular, heat stress causes early-flowering short shoots with poor flower quality outcomes, such as decolorized flowers with smaller petals and a diminished scent (Lee and Kim, 2015; Yeon and Kim, 2017; Han et al., 2018; Yeon and Kim, 2020b; Lee et al., 2021).
Unsuitable temperature conditions affect floral organ formation during development (Ma et al., 2015). Lower temperature conditions induce physiological disorders, such as blindness and bullhead in roses, thus reducing their market value (Seo and Kim, 2013). Many plants are sensitive to heat stress during the reproductive growth stage, which affects the formation and function of floral organs, including the rate of pollen germination and carpel hyperplasia (Wang et al., 2021; Desta et al., 2022). Any abnormality in floral organ formation undermines the commercial value (Yeon and Kim, 2017).
Some studies on flower development have reported that MADS-box genes control floral organ formation in many species, including Arabidopsis thaliana, Castanea mollissima, Rosa chinensis, R. damascena, and R. hybrida (Dubois et al., 2010; Ma et al., 2015; Rusanov et al., 2019; Liu et al., 2021). The expression of such genes with A- and C-functions as the interaction with B-function genes contributes to the production of sepals, petals, stamens, and pistils, such as the AGAMOUS and APETALA2 homologs RhAG and RcAP2, respectively, and changes with the temperature conditions, thereby affecting floral organ formation (Ma et al., 2015; Han et al., 2018; Rusanov et al., 2019).
To date, only a few studies have investigated floral organ formation in response to environmental factors. As double flowers are an economically valuable trait in the market, the mechanism of floral organ differentiation, including that of petals, stamens, and carpels, needs to be better understood with respect to the proper temperatures for ideal growth. In this study, we determined whether sub- and supra-optimal temperatures influence flower development and floral organ formation in the R. hybrida ‘Vital’ in a temperature range of 10–35°C, aiming to improve our understanding of the impact of temperature conditions on flower quality outcome.
Materials and Methods
Plant Materials
Cut rose (R. hybrida) cultivar ‘Vital’ plants were used owing to their thermal plasticity. The plants were propagated by cutting and planted in 2 L pots filled with a commercial soil (Baroker, Seoul Bio, Korea) and perlite mix (2:1 v/v); a controlled-release fertilizer (Osmocote, ICL Specialty Fertilizers, Netherlands) at a concentration of 9.3 g·L-1 was added on March 23, 2021. The potted plants were grown in a glasshouse at the University of Seoul with two flowering cycles and were transported to an environmentally controlled growth chamber on June 30, 2021.
Temperature Treatment
The plants were cultivated in three growth chambers (HB-301S-3, Hanbaek Scientific Co., Bucheon, Korea) with different day/night temperature treatment regimens: 18/10°C (low temperature, LT; sub-optimal temperature), 35/25°C (high-temperature, HT; supra-optimal temperature), and 25/18°C (optimal temperature, OT). For each treatment, five potted plants were used for ten flowering shoot harvests. All three treatments were performed under a 16/8 h light/dark cycle and at 50 ± 5% relative humidity and 440 ± 7 µmol·m-2·s-1 light intensity using white LED and high-pressure sodium mixed lamps. Additionally, 200 mL of a nutrient solution was supplied regularly to each pot (EC 1.2 dS·m-1, pH 5.8) (Shin et al., 2022). The nutrient solutions were composed of KNO3, Ca(NO3)2·4H2O, Mg(NO3)2·6H2O, (NH4)2PO4, (NH4)6MO7O24·4H2O, EDTA-Fe, MnSO4·5H2O, ZnSO4·7H2O, H3BO3, and CuSO4·5H2O (2,323, 1,841, 204.8, 575, 64.5, 0.88, 12.05, 8.63, 9.27, and 1.25 mg·L-1, respectively), with H2SO4 provided as needed (Shi et al., 2021).
Flowering Responses and Floral Organ Counts
Cut rose flowers were harvested at a specific flowering stage (all petals open, and visible stamens not in the senescence stage) following the methodology of Ma et al. (2015) with modifications to explore floral organ differentiation. Six to ten flowers were cut for each of the three treatments. The time to flowering (days to flowering) was determined from the bud-break of the shoots. Shoot and peduncle lengths, stem diameters, petal sizes, and fresh weights were measured when the flowering shoots were harvested (Yeon and Kim, 2020c). Floral organs (sepals, petals, stamens, carpels, or petaloid stamens) were counted from the harvested flowers. Petal/stamen chimeras were separately counted as petaloid stamens, which appear as irregular or intermediate petal/stamen shapes (Ma et al., 2015). The relative differentiation rate in floral organs was calculated according to the days to flowering and the integrated temperature as the sum of the treated temperature per hour throughout the flowering period of the shoots (Kim and Lieth, 2012).
RNA Extraction and qRT-PCR
For the quantitative real-time PCR (qRT-PCR) analysis, floral buds were sampled when they attained a diameter of 3.0 ± 0.2 mm, referred to as floral bud stage 3, in which stamen primordia emerge and develop (Ma et al., 2015). Whole buds were frozen in liquid nitrogen before storage at -80°C. Total RNA was extracted using a plant RNA extraction kit (Takara MiniBEST Plant RNA Extraction Kit, Takara, Japan). Complementary DNA (cDNA) was synthesized from 1 µL of the extracted RNA using a cDNA synthesis kit (PrimeScript 1st strand cDNA synthesis Kit, Takara, Japan). All subsequent steps were performed according to the manufacturer’s instructions. cDNA (3 µL) of each sample was mixed with a PCR premix solution (AccuPower® 2X GreenStar Master Mix, Bioneer, Korea), and amounts of 15 µL of the reaction mixtures were prepared for the qRT-PCR analysis. The qRT-PCR analysis (Exicycler™ 384 Real-Time Quantitative Thermal Block, Bioneer, Korea) was conducted with the following cycle parameters: 40 cycles at 95°C for 5 s, 58°C for 25 s, and 72°C for 30 s. Raw cycle threshold (Ct) values were calculated using the 2-ΔCt method, and RhACT1 was used as a reference gene. The relative expression levels of six genes expected to be related to floral organ formation in rose plants were determined based on OT. The six target genes were RhAP1 (A-function gene in the ABC model), RhAP3, RhTM6, and RhPI (B-function gene), and RhAG and RhSHP (C-function gene). The primers used are listed in Table 1, and four biological replicates were used.
Table 1.
Primers for the qRT-PCR analysis in this study
Statistical Analysis
Statistical significance was analyzed through a one-way analysis of variance (ANOVA), least significant difference (LSD), and an ANOVA using SAS 9.4 (SAS Institute, Cary, NC, USA). Student’s t-test was utilized to determine the relative gene expression levels.
Results
Flowering Responses
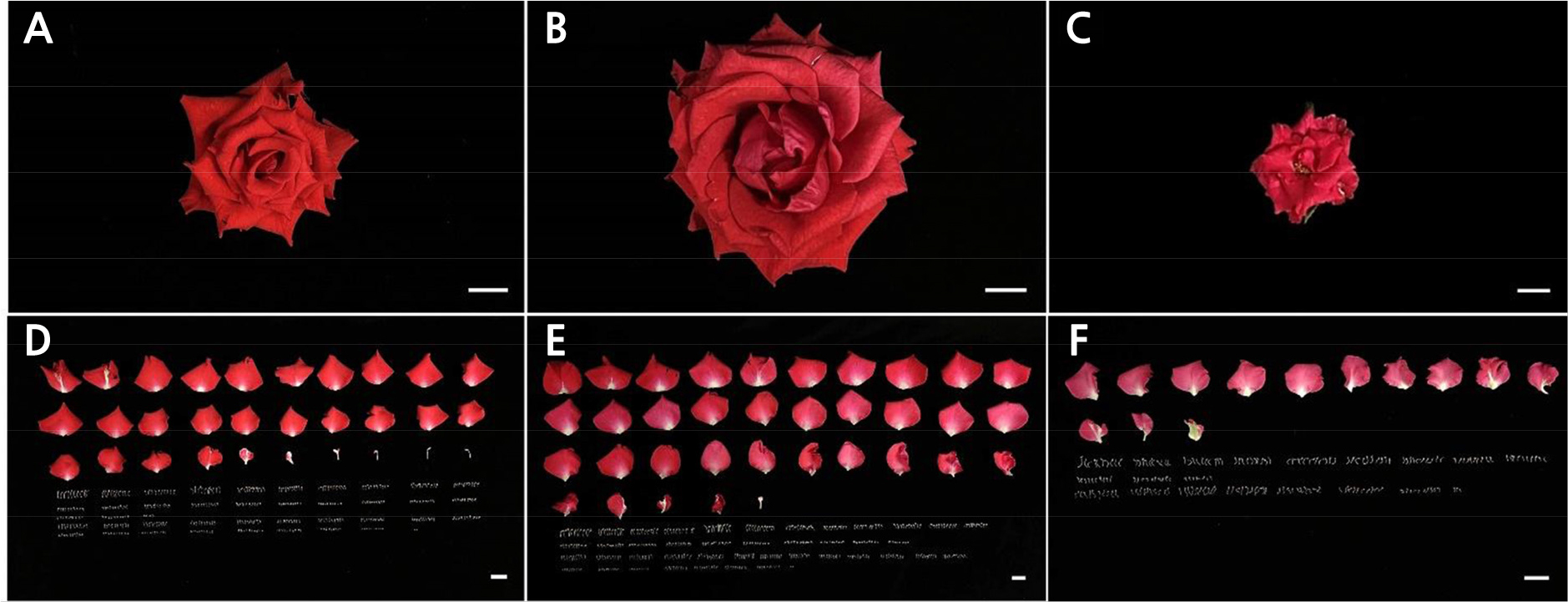
The cut rose ‘Vital’ plants subjected to excessively high- or low-temperature conditions showed significant plasticity in flowering, both quantitatively and qualitatively (Table 2). Compared to OT, the flowering time was delayed by 43.4 days (1.96 times) in LT but accelerated by 14.2 days in HT (0.68 times). However, the flower quality showed responses opposite to those of the temperature condition. The flowers became larger and accumulated additional biomass during the extended period of flowering in LT. Furthermore, LT increased the flowering shoot length, stem diameter, flower height, and petal size by 28.4%, 42.0%, 45.0%, and 41.8%, respectively, compared with OT, but HT decreased these outcomes correspondingly by 67.1%, 33.3%, 35.0%, and 60.6%. The peduncle was shorter in both LT and HT by 17.1% and 47.4%, respectively. In general, LT induced longer shoots and larger flowers with more days to flowering, whereas HT produced smaller shoots and larger flowers in shorter periods (Fig. 1A–1C). Temperature stress also significantly changed the petal color on the adaxial surface of the petals (data not shown).
Table 2.
Flowering responses in cut rose ‘Vital’ grown under different temperature conditions
| Treatment |
Days to flowering (days) |
Flowering shoot length (cm) |
Peduncle length (cm) |
Stem diameter (mm) |
Flower height (cm) |
Flower width (cm) |
Petal sizez (cm2) |
|
OT (25/18°C) | 45.0 by | 51.1 b | 7.6 a | 6.9 b | 4.0 b | 10.2 a | 25.1 b |
|
LT (18/10°C) | 88.4 a | 65.6 a | 6.3 b | 9.8 a | 5.8 a | 11.3 a | 35.6 a |
|
HT (35/25°C) | 30.8 c | 16.8 c | 4.0 c | 4.6 c | 2.6 c | 7.5 b | 9.9 c |
| Significance | *** | *** | *** | *** | *** | *** | *** |
Floral Organ Formation
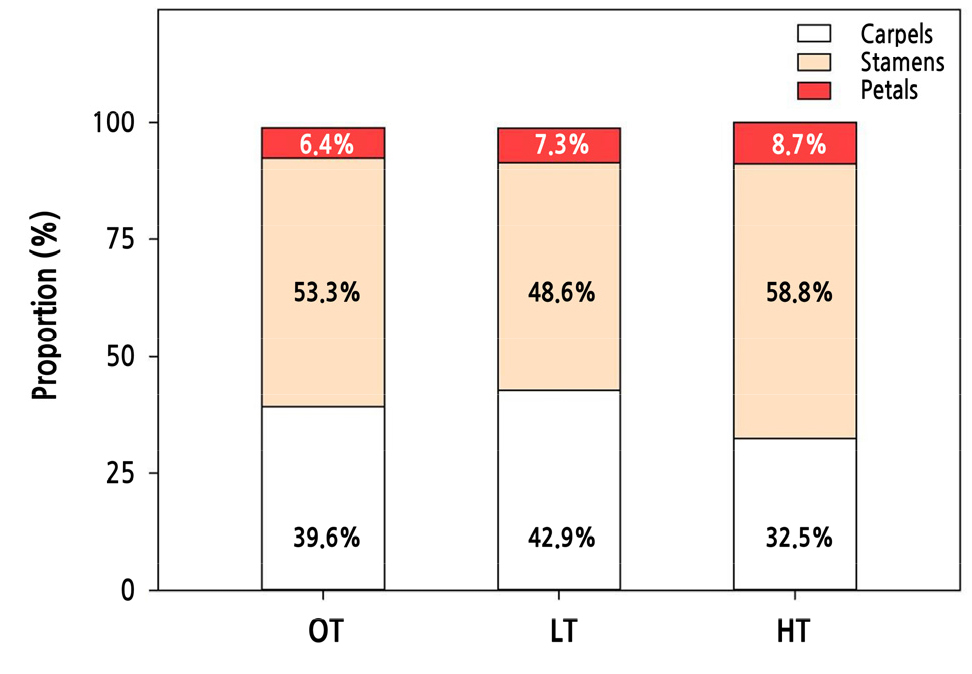
Compared to OT, floral organ differentiation was suppressed under extraordinarily high- or low-temperature conditions (Fig. 1D–1F). This suppression was more severe in HT than in LT. Whole floral organ formation was reduced by 61.4% in HT and 4.5% in LT (Table 3). Based on the OT conditions, the formation of all four floral organs was dramatically decreased in HT, whereas in LT, stamen formation was reduced by 12.9% and petal and carpel formation outcomes were slightly increased by 16% and 5%, respectively. Cut rose ‘Vital’ subjected to extremely high- or low-temperature conditions showed different tendencies in terms of the composition ratio of each floral organ (Fig. 2). LT increased the relative proportion of petals and carpels but reduced the stamen ratio, and HT increased the ratio of petals and stamens but diminished the carpel ratio.
Table 3.
Numbers of floral organs in cut rose ‘Vital’ grown under different temperature conditions
| Treatment | Sepals | Petals | Petaloid stamens | Stamens | Carpels | Total |
| OT (25/18°C) | 5.0 ± 0.0 bz | 23.5 ± 5.2 a | 4.7 ± 2.3 a | 235.3 ± 19.2 a | 173.0 ± 20.3 a | 441.5 ± 39.3 a |
| LT (18/10°C) | 5.0 ± 0.0 b | 27.3 ± 3.5 a | 3.3 ± 1.2 a | 205.0 ± 38.4 a | 181.0 ± 23.4 a | 421.7 ± 51.0 a |
| HT (35/25°C) | 5.6 ± 0.8 a | 10.0 ± 2.4 b | 4.3 ± 7.2 a | 97.0 ± 12.4 b | 53.5 ± 13.7 b | 170.5 ± 19.9 b |
| Significance | * | *** | ns | *** | *** | *** |
The daily differentiated number of floral organs was calculated by dividing the total count by the number of days to flowering (Fig. 3). We found that the daily differentiation of floral organs was restrained significantly by 22.2% to 52.8% under extreme temperature stress conditions. Petal number and biomass outcomes were calculated according to the total integrated input temperature throughout the flowering period (Fig. 4). The petal number per input temperature was maximized at approximately 25°C and on the 45th day from bud-break, while the petal biomass increased in proportion to the input temperature and the days to flowering (Fig. 4).
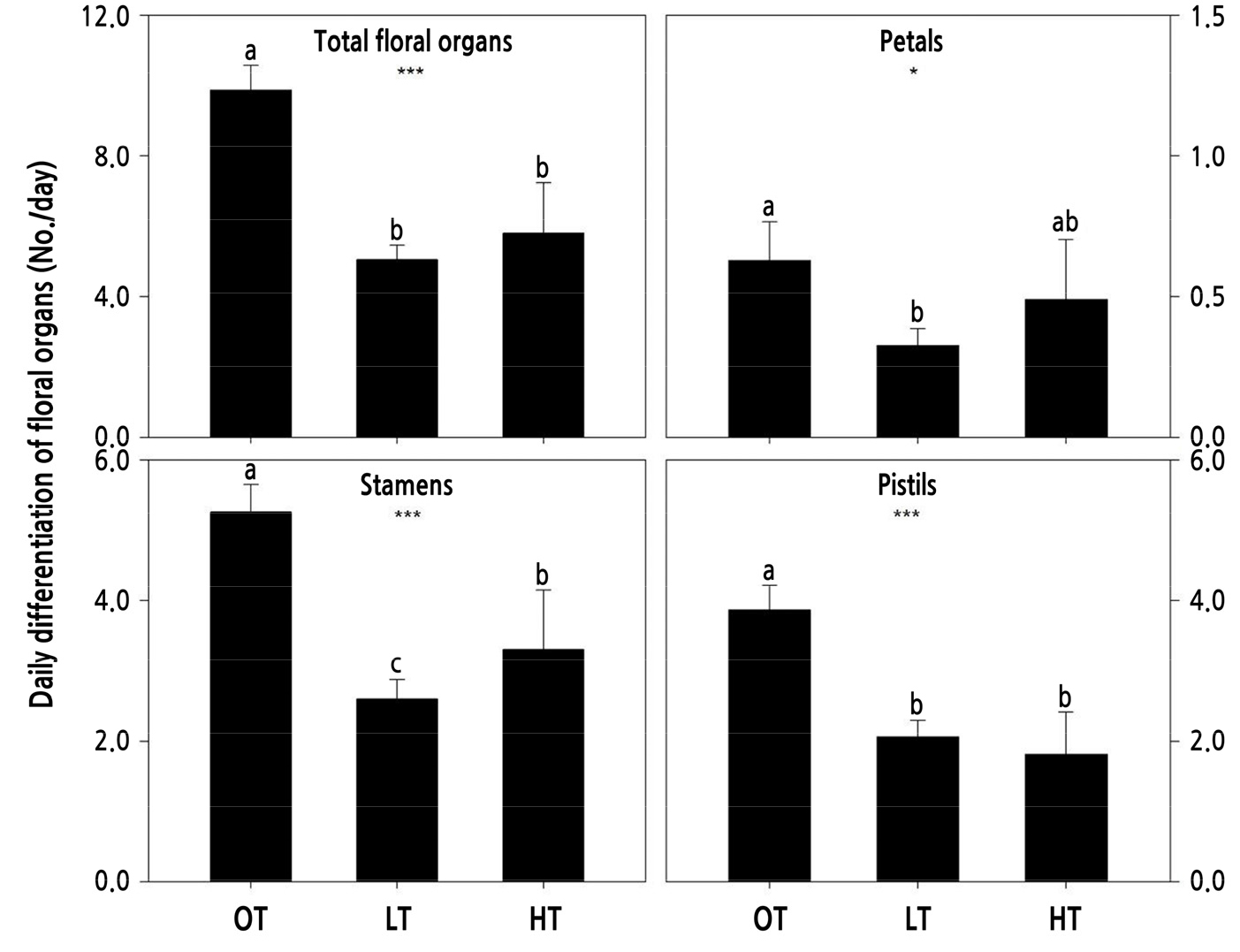
Fig. 3.
Daily differentiation of floral organs in cut rose ‘Vital’ grown under different temperature conditions. OT: day/night 25/18°C; LT: 18/10°C; and HT: 35/25°C. Vertical bars represent standard deviation. Different letters indicate separation within columns at p < 0.05 by LSD. * and ***indicate significance at p < 0.05 and 0.001, respectively, according to ANOVA.
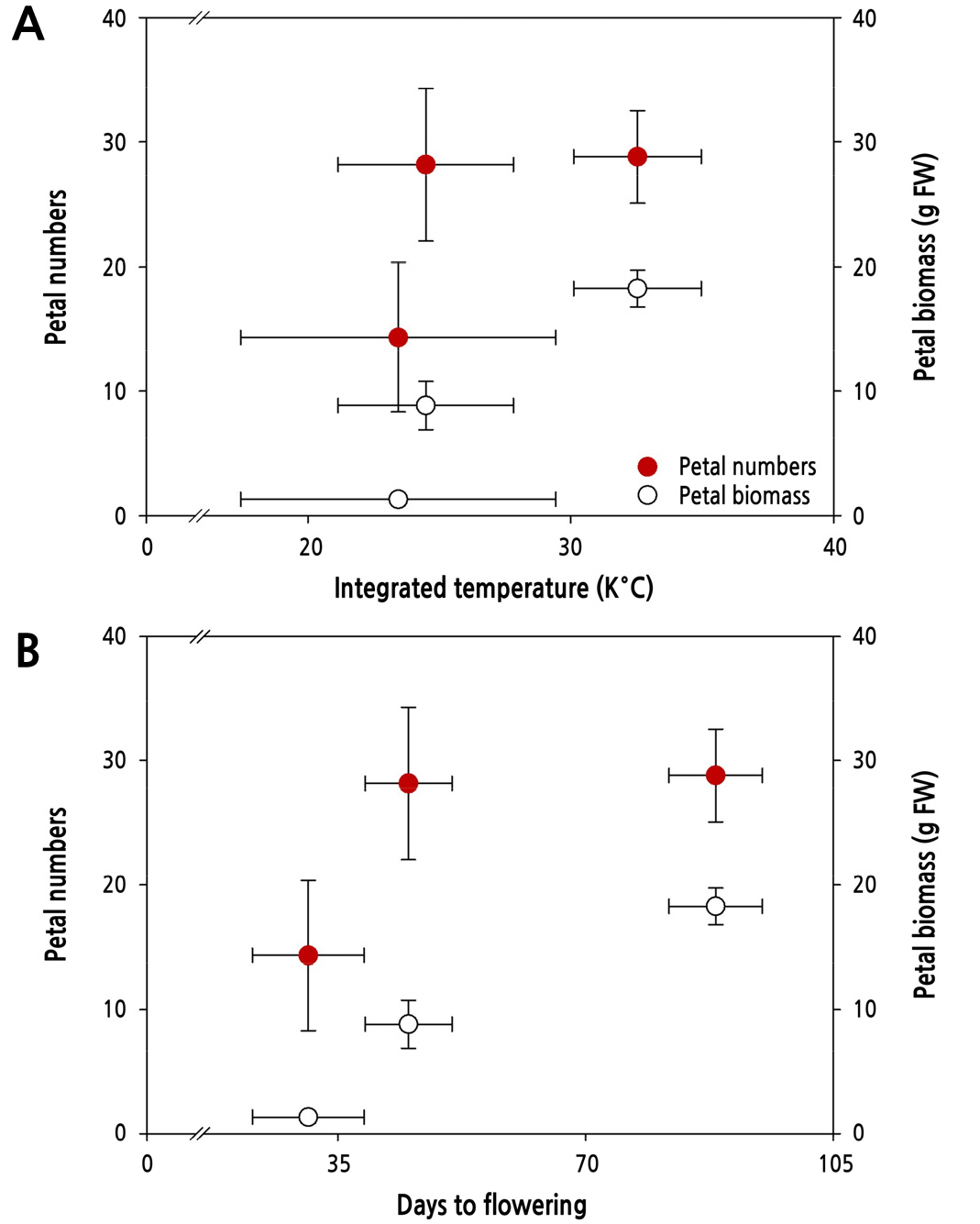
Fig. 4.
Variation in petal number and biomass outcomes by integrated temperature amount (A) and days to flowering (B) in cut rose ‘Vital’ grown under different temperature conditions. The integrated temperature amount was calculated during the days to flowering. Vertical bars represent the standard deviation.
Relative Gene Expression
The effects of extreme temperature conditions on the floral organs in the cut rose ‘Vital’ were determined by the expressions of related genes (Fig. 5). RhAP1, RhAP3, RhAG, and RhSHP were expressed to a significant degree depending on the temperature condition. The relative expression level of the A-function gene, RhAP1, increased by 50% in LT but was reduced by 30% in HT. Only RhAP3 among the B-function genes was less expressed at 32% in HT, though this was significant. RhAG and RhSHP, which are C-function genes, were expressed at considerably high levels, especially the RhAG gene by 60% to 312%, regardless of the temperature condition.
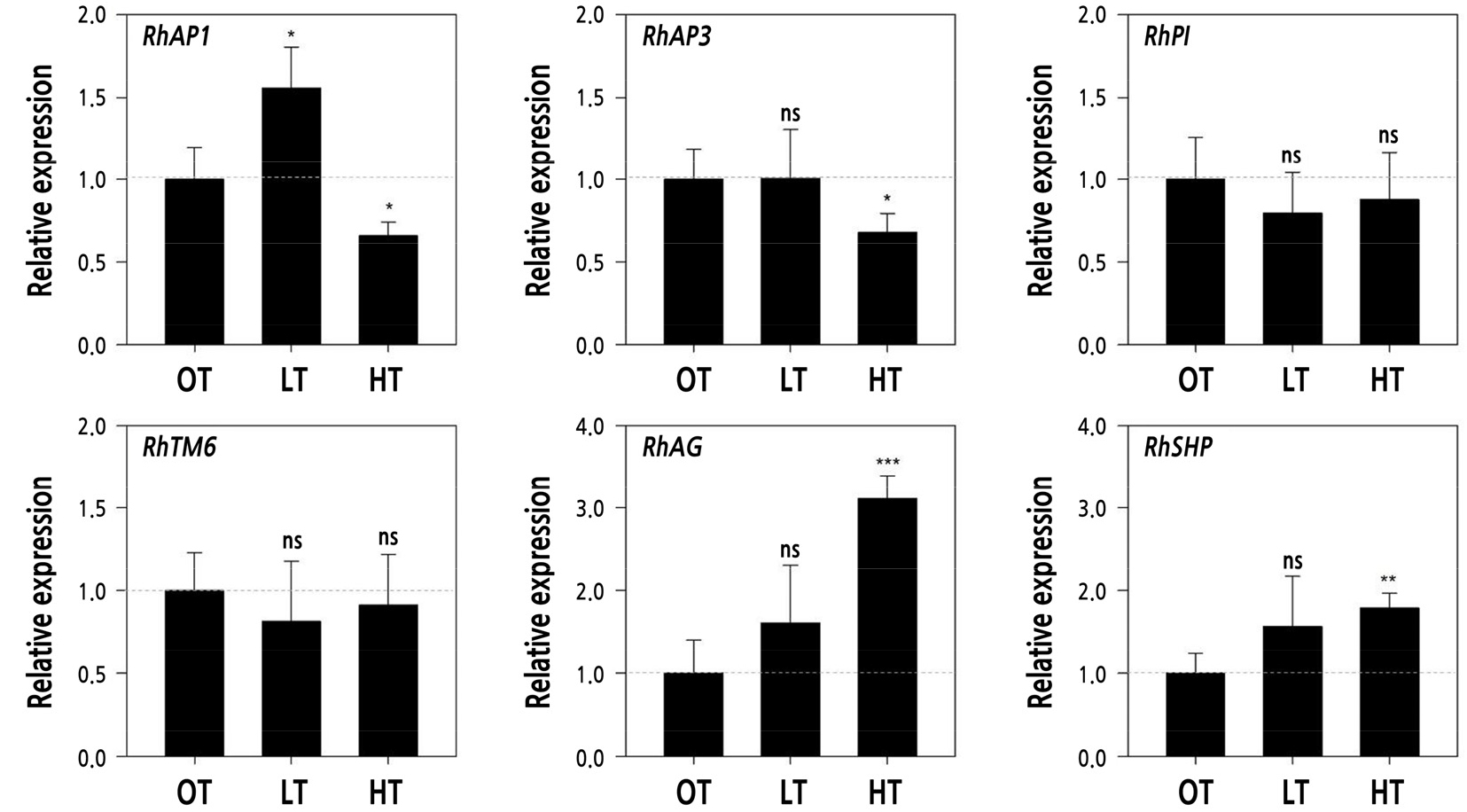
Fig. 5.
Relative expression level of genes related to floral organ formation in cut rose ‘Vital’ grown under different temperature conditions. OT: day/night 25/18°C; LT: 18/10°C; and HT: 35/25°C. Vertical bars represent the standard deviation. ns, *, **, and ***indicate non-significance and significance at p < 0.05, 0.01, and 0.001, respectively, according to Student’s t-tests.
Discussion
Temperature stress is the primary cause of the degradation of flower quality in ornamental crops, particularly in cut rose flowers. Rose plants exhibit various flowering responses under different temperature conditions, especially in high- or low-temperature stress ranges. Previous studies have shown that low temperatures delay flowering but significantly increase petal numbers by promoting stamen petalody (Ma et al., 2015), whereas high temperatures cause poor quality flowers by accelerating the flowering time (Lee and Kim, 2015).
In cut rose flower production, temperature conditions control the production cycle and flower quality, which determine the commercial value of floral crops in the horticultural industry (Yeon and Kim, 2020b). Lower temperatures reduce the respiration rate at night and promote carbon assimilation, resulting in increased biomass in floral shoots (Desta et al., 2022). Younger floral shoots contain fewer carbohydrates translocated toward the base in mother plants under lower temperature conditions (Desta et al., 2022). In the present study, the roses under sub-optimal temperatures (LT 18/10°C) had a more extended vegetative growth period until flowering, and then produced larger flowers and thicker stem diameters than those in OT (Table 2). An extended vegetative growth period in LT increased the integrated air temperature to flowering (Fig. 4), which was positively correlated with petal biomass. High-temperature conditions decrease the distribution rate of carbohydrates to floral shoots, resulting in poor quality flowers (Yeon and Kim, 2020b; Desta et al., 2022). In the present study, the cut rose ‘Vital’ also produced shorter flowering shoots with early flowering under supra-optimal temperature conditions (HT 35/25°C). A characteristic difference resulting from the two non-optimal temperature treatments (LT and HT) was the flowering time of the shoots, which was delayed by 96% in LT (88 days) but accelerated by 31% in HT (31 days) compared to that in OT (45 days). Delayed or accelerated flowering shoot growth directly affected many aspects of the flower quality, including the shoot length, thickness, flower size, and biomass (Table 2 and Fig. 1).
Information on how the flowering shoot growth period affected floral organ differentiation is presented in Table 3 and in Figs. 2 and 3. Floral organ formation exhibited a clear correlation with the temperature. The number of whole floral organs in OT (441.5) was reduced under non-optimal temperature treatments by 61.4% in HT (170.5) and 4.5% in LT (421.7). However, the composition ratio of each floral organ showed distinct differences, as depicted in Fig. 2. Under sub-optimal temperature conditions (LT), rose plants increased their proportions of petals and carpels but reduced their proportion of stamens. Supra-optimal temperature conditions (HT) were more effective in reducing floral organs (Table 2); however, the ratios increased for petals and stamens and decreased for carpels (Fig. 2). The limited carbon availability can explain the reduction in the carpel proportion in HT, which causes carbohydrate shortages owing to poor photosynthesis during rapid growth.
Flowers develop from the apical meristem in concentric whorls and are divided into four parts in relation to MADS-box genes that identify different organs (Kieffer and Davies, 2001; Bendahmane et al., 2013). According to the ABC model of flower development based on concurrent genetic studies in Arabidopsis and Antirrhinum (Coen and Meyerowitz, 1991), sepals, petals, stamens, and carpels can be identified by separate functional genes: sepals with A-function, petals with A- and B-function, stamens with B- and C-function, and carpels with C-function genes (Coen and Meyerowitz, 1991). The A-function gene AP1, mainly expressed in sepals, contributes to the transition from vegetative to reproductive growth and forms floral organs in rose plants (Han et al., 2018). The AP1 ortholog in R. damascena is less expressed in single-type flowers than in double types (Rusanove et al., 2019). C-function genes contribute to the development of reproductive organs, including stamens and carpels (Cheng et al., 2020). The relative expression level of the C-function gene RdAG with numerous stamens in single types was three-fold higher than in doubles (Rusanov et al., 2019). Lower temperature conditions promote increased numbers of petals, resulting from a decrease in the expression level of RhAG through the enhancement of DNA methylation and contributing to the decline in the stamens of roses (Ma et al., 2015).
In the present study, RhAP1 (A-function gene), RhAP3 (B-function gene), and RhAG and RhSHP (C-function gene) were more highly expressed under non-optimal temperature conditions compared to OT conditions. The relative expression level of RhAP1 increased by 50% in LT but was reduced by 30% in HT. This gene appears to play a direct role in increasing or decreasing the petal number in LT or HT (Table 3 and Fig. 5). Meanwhile, the relative gene expression levels of RhAP3, RhAG, and RhSHP were significantly reduced in HT, which could be evidence of a decrease in the number of petals under HT caused by functional interaction with other genes, such as RhAP1. Nevertheless, gene expression responses did not always coincide with floral organ differentiation in cut rose ‘Vital’ subjected to suboptimal temperature conditions. Yan et al. (2016) reported that a higher expression level of the AP1 ortholog led to the formation of more sepals, whereas a lack of this ortholog induced petal development in R. chinensis, in contrast to the results of this study and suggesting that the function of AP1 differs somewhat in certain species (Han et al., 2018). AP1 and LEAFY activate B-function genes of AP3, TM6, and PI by upregulating UNUSUAL FLORAL ORGANS (Kieffer and Davies, 2001; Dubois et al., 2012; Chen et al., 2021). In addition, AP3 and PI develop into petals and stamens, and these mutations can convert petals to sepals or carpels (Müller et al., 2016; Chen et al., 2021). In addition to the six genes used in this study, the interaction with other MADS-box genes, such as AP2, SEPALLATA, and WUSCHEL in rose flowers, should be studied further (Ma et al., 2015; Han et al., 2018). In conclusion, non-optimal temperature stress led to different responses of the quality and organ formation in flowers of R. hybrida ‘Vital’ compared to optimal conditions. Sub-optimal conditions delayed the flowering period with flower quality improvement but decreased the efficiency of floral organ differentiation. Supra-optimal conditions produced flowers rapidly but of poor quality, including reduced floral organs, which are determined based on the gene expression related to organ formations.