Introduction
Materials and Methods
Plant materials and germination conditions
Measurement of contamination rates after the H2O2 treatment
Measurement of contamination rates after the NaClO treatment
Contamination measurement after antibiotics treatment
Estimation of growth conditions
Results and Discussion
Introduction
Cannabis (Cannabis sativa L.), belonging to the family Cannabaceae, has been widely used as a medicine, for fiber, and as food throughout human history (Russo et al. 2008). Recently, Cannabis plants have attracted considerable interest as sources of diverse cannabinoids, terpenes, and phenolic compounds with prominent nutraceutical potential (Schluttenhofer and Yuan 2017). Among these, cannabinoids have been well studied as pharmacologically active ingredients, and 90 cannabinoids have been identified and characterized (Giacoppo et al. 2014; Citti et al. 2018). To date, 177 phyto-cannabinoids have been identified in Cannabis plants (Adamek et al. 2022). The most predominant cannabinoids are cannabidivarin (CBDV), cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC), and cannabichromene (CBC) (Andre et al. 2016; Kim et al. 2022). Although THC is responsible for the psychoactive effects of Cannabis consumption, non-psychoactive CBD has potent neuroprotective, anti-tumor, and antioxidant effects and is being investigated as a treatment for schizophrenia and Alzheimer’s disease (Rajan et al. 2016; Osborne et al. 2017; Watt and Karl 2017; Yeasmin and Choi 2020).
Genetic engineering, such as the clustered regulatory interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9)-mediated gene editing system, provides opportunities in various molecular biology studies. This technology can also be applied to increase the production of targeted cannabinoids in Cannabis plants by knocking out biosynthetic gene functions. To apply the CRISPR/Cas9 system in molecular breeding, the in vitro tissue culturing of protoplasts and explants of diverse plant organs is essential. Avoiding contamination is critical for successful plant tissue cultures and micropropagation (El-Banna et al. 2021; Romadanova et al. 2022). Bacteria that are undetectable in the initial stage can begin to multiply after a long period of culturing. Despite extensive surface sterilization of initial explants and the use of best practices for maintenance, contamination of plant tissue may be a consistent concern during long-term tissue culturing (Gunson and Spencerphillips 1994; Orlikowska et al. 2017).
Several methods have been proposed for reducing contamination in plant tissue cultures (Orlikowska et al. 2017). Hydrogen peroxide (H2O2) and sodium hypochlorite (NaClO) are among the most popular traditional biocides (Sauer and Burroughs 1986; Rowntree 2006; Sorokin et al. 2021). Antibiotics are another method of eliminating contamination during plant tissue culturing (So et al. 2024; Luna et al. 2008). However, understanding the nature of endogenous bacteria to select an appropriate antibiotic and the proper concentration is very important for eliminating bacterial contamination without affecting plant growth (Shehata et al. 2010). Therefore, the objective of this study was to determine the efficacy of H2O2, NaClO, and antibiotics for the elimination of contamination that occurs during Cannabis plant tissue culturing.
Materials and Methods
Plant materials and germination conditions
Cannabis (cheongsam)seeds were purchased from Andong Nonghyup (Gyeongsangbuk-Do, Andong, South Korea). The seeds were grown in a growth room at 25°C under long-day conditions (photoperiod, 16 h: 8 h, light: dark) at a light intensity level of 120 µmol·m-2·S-1. For a seed germination assay, the seeds were soaked in either sterile distilled water (H2O) or 1% H2O2, and Cannabis seeds were also cultured in darkness at 23°C for one, two, three, and four days. The sterile H2O and 1% H2O2 were replaced daily with fresh solutions, and the germination rates of the seeds were measured daily. Three replicates were used, and 30 seeds were considered for each experiment.
Measurement of contamination rates after the H2O2 treatment
Cannabis seeds treated with 1% H2O2 for two days were used to measure the contamination rates. After the 1% H2O2 treatment, seeds with and without a seed coat were prepared. The seed coats for the former set were removed on a clean bench using flame-sterilized forceps. Seeds with and without seed coats were placed on filter paper to remove any residual solution, after which they were transferred to the 1/2 MS (Murashige and Skoog 1962) medium and cultured under long-day conditions at 23°C. Contamination rates were measured on days 2, 4, and 6. Three replicates were used, and 20 seeds were considered for each experiment.
Measurement of contamination rates after the NaClO treatment
Cannabis seeds without a seed coat were prepared by removing seed coats after incubation in a 1% H2O2 solution for two days, with all seeds were treated with either 0.04%, 0.2%, 0.4%, or 0.8% NaClO for 15 min and washed five times with sterile H2O. The seeds were placed on filter paper to remove any residual solution and were transferred to the 1/2 MS medium. The medium was cultured under long-day conditions at 23°C. Contamination rates were measured on days 2, 4, and 6. Three replicates were used, and 20 seeds were considered for each experiment.
Contamination measurement after antibiotics treatment
Cannabis seeds without a seed coat were prepared by removing the seed coat after incubation in a 1% H2O2 solution for two days, followed by treatment with 0.2% NaClO for 15 min and washing five times with sterile H2O. The seeds were placed on filter paper to remove the residual solution and transferred to a 1/2 MS medium solution containing 300 mg/L Augmentin, 300 mg/L Timentin, and 300 mg/L carbenicillin. The medium was cultured under long-day conditions at 23°C. Contamination rates were measured on days 2, 4, and 6. Three replicates were used, and 20 seeds were considered for each experiment.
Estimation of growth conditions
Fresh weights, primary root lengths, and hypocotyl lengths were measured after six days. Primary root and hypocotyl lengths were measured using the Imaging Edge Desktop application (Sony, Japan) and the ImageJ program. Three replicates were used, and 20 seedlings were considered for each experiment.
Results and Discussion
In previous studies, using 1% H2O2 was found to be a reliable and sterile method for the in vitro germination of Cannabis seeds (Sorokin et al. 2021; Ahsan et al. 2022). To examine the effect of a 1% H2O2 solution on the sterilization and germination of Cannabis seeds, seeds were soaked in sterilized H2O and 1% H2O2 and incubated for four days (Fig. 1). The germination rate of the seeds incubated in 1% H2O2 exceeded that of the seeds incubated in H2O (Fig. 1A). The germination rate was 96.7% at two days after soaking (DAS), and a 100% germination rate was observed after 3 DAS in 1% H2O2. Sterilized H2O-treated seeds had germination rates of 31.2, 57.3, 65.5, and 64.4% at 1, 2, 3, and 4 DAS, respectively. These results are similar to those of previous studies that reported that a treatment with 1% H2O2 can enhance the germination rate of Cannabis seeds (Sorokin et al. 2021; Ahsan et al. 2022). Although the germination rates were highest at 3 and 4 DAS in 1% H2O2, the color of the roots turned brown, resulting in the inhibition of hypocotyl and root development (six days after incubation [DAI] after the 1% H2O2 treatment) (Fig. 1A). Taken together, these results suggest that a 1% H2O2 treatment for two days can enhance germination rates and reduce the toxic effects on Cannabis seeds. Nevertheless, contamination was still detectable with 1% H2O2 and two days of treatment despite the better germination rates and reduced toxicity to Cannabis seeds. Next, seeds with or without a seed coat were incubated in 1/2 MS media to eliminate the contaminant source, as contamination was still observed after prolonged incubation with 1% H2O2 and two days of treatment (Fig. 1B). First, the seeds were treated with 1% H2O2 for two days, and seeds without a seed coat were prepared by removing the seed coat. The contamination rates of the seeds with a seed coat were 78.8%, 100%, and 100% at 2, 4, and 6 DAI, respectively, relatively high compared to those of seeds without a seed coat (27.3%, 39.4%, and 63.3%, respectively).
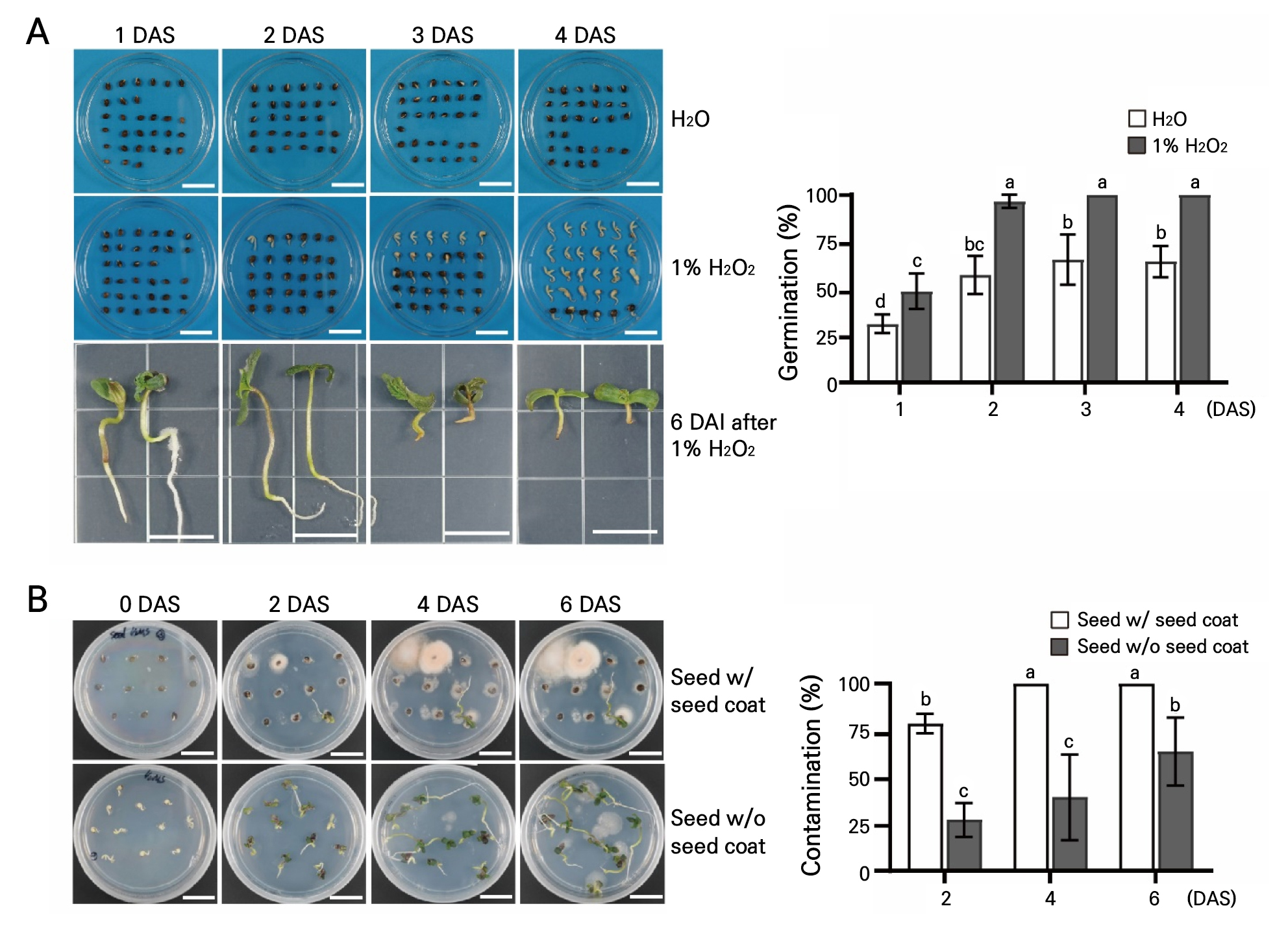
Fig. 1.
Effects of 1% H2O2 on the germination rates of Cannabis seeds: (A) Germinated seeds and germination rates one, two, three, and four days after soaking (DAS). Photographs of the seedlings were taken an additional six days after incubation (DAI) after 1, 2, 3, and 4 DAS (left to right). (B) Contamination that occurred on the seeds with and without a seed coat during culturing on 1/2 MS media with contamination rates at 0, 2, 4, and 6 DAI. Seeds were soaked in a 1% H2O2 solution for one, two, three, and four days. Scale bars represent 2 cm. Different lowercase letters indicate a significant difference (p < 0.05) according to Tukey’s test.
The next section of the survey concerned the effects of additional sterile reagents and antibiotics on the complete suppression of contaminants during prolonged incubation. NaClO is one of the most common reagents used for the surface sterilization of diverse plant seeds (Sorokin et al. 2021; Si et al. 2022; Hong et al. 2023). To determine the optimal concentration of NaClO solution for the suppression of contamination and its effects on the growth of Cannabis seedlings, sample seeds were treated with different NaClO concentrations of 0.04%, 0.2%, 0.4%, and 0.8% for 15 min (Fig. 2 and Fig. 3A). As shown in Figs. 2 and 3, 0.2%, 0.4%, and 0.8% NaClO significantly reduced contamination rates to 5.0%, 0%, and 0% at 6 DAI (Fig. 2), respectively. However, negative effects such as growth inhibition of the hypocotyl (Fig. 3B) and primary root (Fig. 3C) and a loss of fresh weight (Fig. 3D) were observed in the seeds treated with 0.4% and 0.8% NaClO. Taken together, the treatment with the 0.2% NaClO solution showed relatively high suppression of contamination rates without negative effects on the growth of Cannabis seedlings.

Fig. 2.
Concentration effects of NaClO solutions. Contamination rates of Cannabis seedlings at 0, 2, 4, and 6 DAI. Seeds were treated with either a 0.04%, 0.2%, 0.4%, or 0.8% NaClO solution. Contamination rates were measured at 0, 2, 4, 6 DAI. Scale bars represent 2 cm. ND, not determined. Different lowercase letters indicate a significant difference (p < 0.05) according to Tukey’s test.
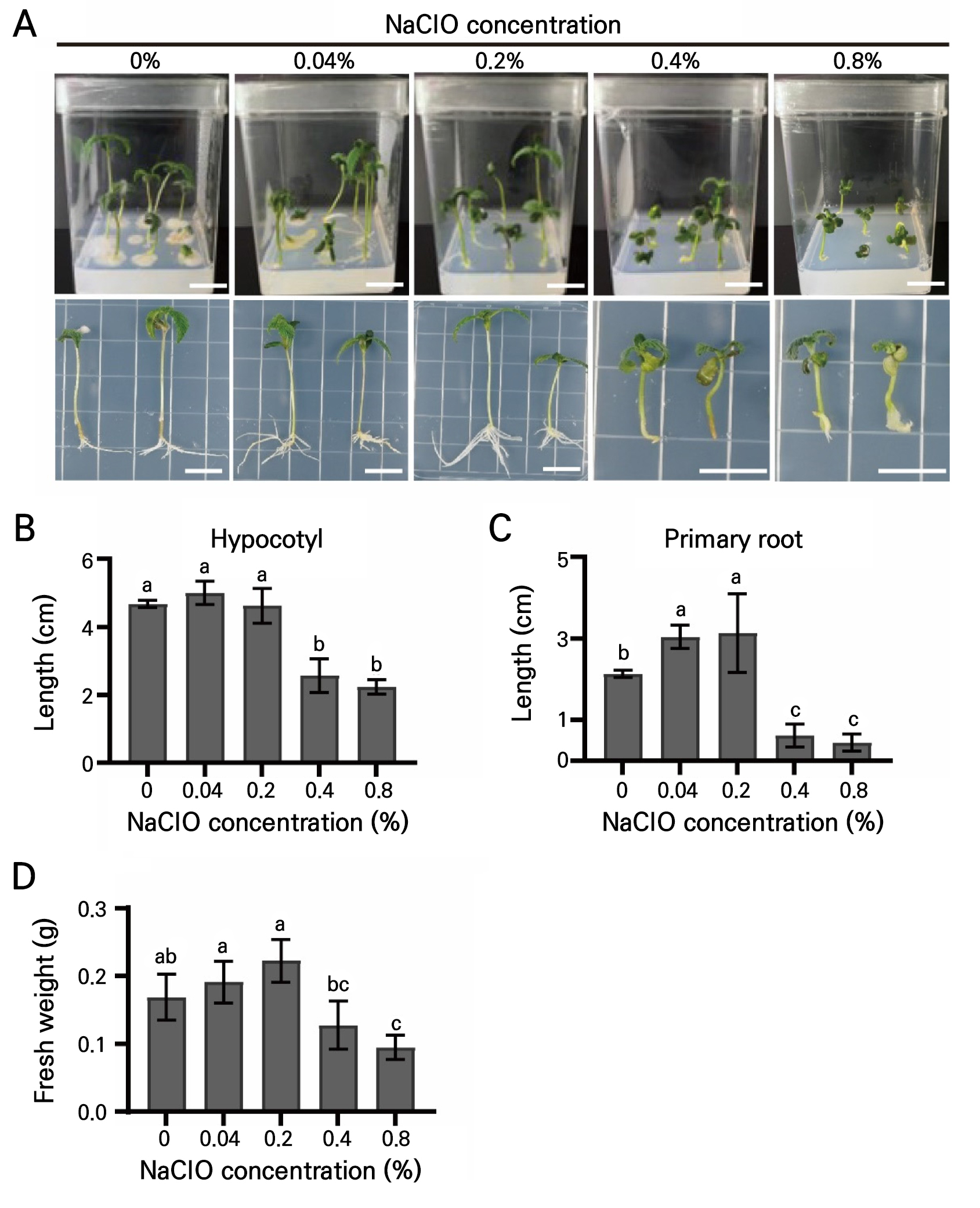
Fig. 3.
Growth conditions of Cannabis seedlings after the NaClO treatment: (A) Growth conditions of Cannabis seeds treated with either a 0.04%, 0.2%, 0.4%, or 0.8% NaClO solution. (B-D) Measurement of morphological traits in Cannabis seedlings. Hypocotyl length (B), primary root length (C), and fresh weight (D) of Cannabis seedlings shown in Fig. 3A. Scale bars represent 2 cm. Different lowercase letters indicate a significant difference (p < 0.05) according to Tukey’s test.
Commonly used antibiotics, carbenicillin, Augmentin, and Timentin (Costa et al. 2000; Alsheikh et al. 2002; White et al. 2004), were added in the final part of the study to the 1/2 MS medium to evaluate their suppressive effects on contamination. Cannabis seeds without a seed coat were treated with 1% H2O2 for two days and 0.2% NaClO for 15 min. The seeds were then transferred to a 1/2 MS medium plates containing either 300 mg/L carbenicillin, 300 mg/L Augmentin, or 300 mg/L Timentin antibiotics, which are known to be effective at suppressing bacterial growth without significant toxicity to plants (Ieamkhang and Chatchawankanphanich 2005). As observed, contamination was not detectable in the medium containing Timentin (0%), and contamination rates of 0.67% and 0.33% were noted in the media samples containing carbenicillin and Augmentin, respectively (Figs. 4 and 5A). There were no significant differences in the hypocotyl (Fig. 5B) and primary root (Fig. 5C) lengths or fresh weights (Fig. 5D) of Cannabis seedlings incubated in the respective carbenicillin, Augmentin, and Timentin media. Taken together, these results suggest that a series of 1% H2O2 treatments for two days, removal of the seed coat, the use of 0.2% NaClO for 15 min, and culturing on a medium containing 300 mg/L Timentin would be an effective method to guarantee contamination-free in vitroCannabis seed germination (Fig. 5E).
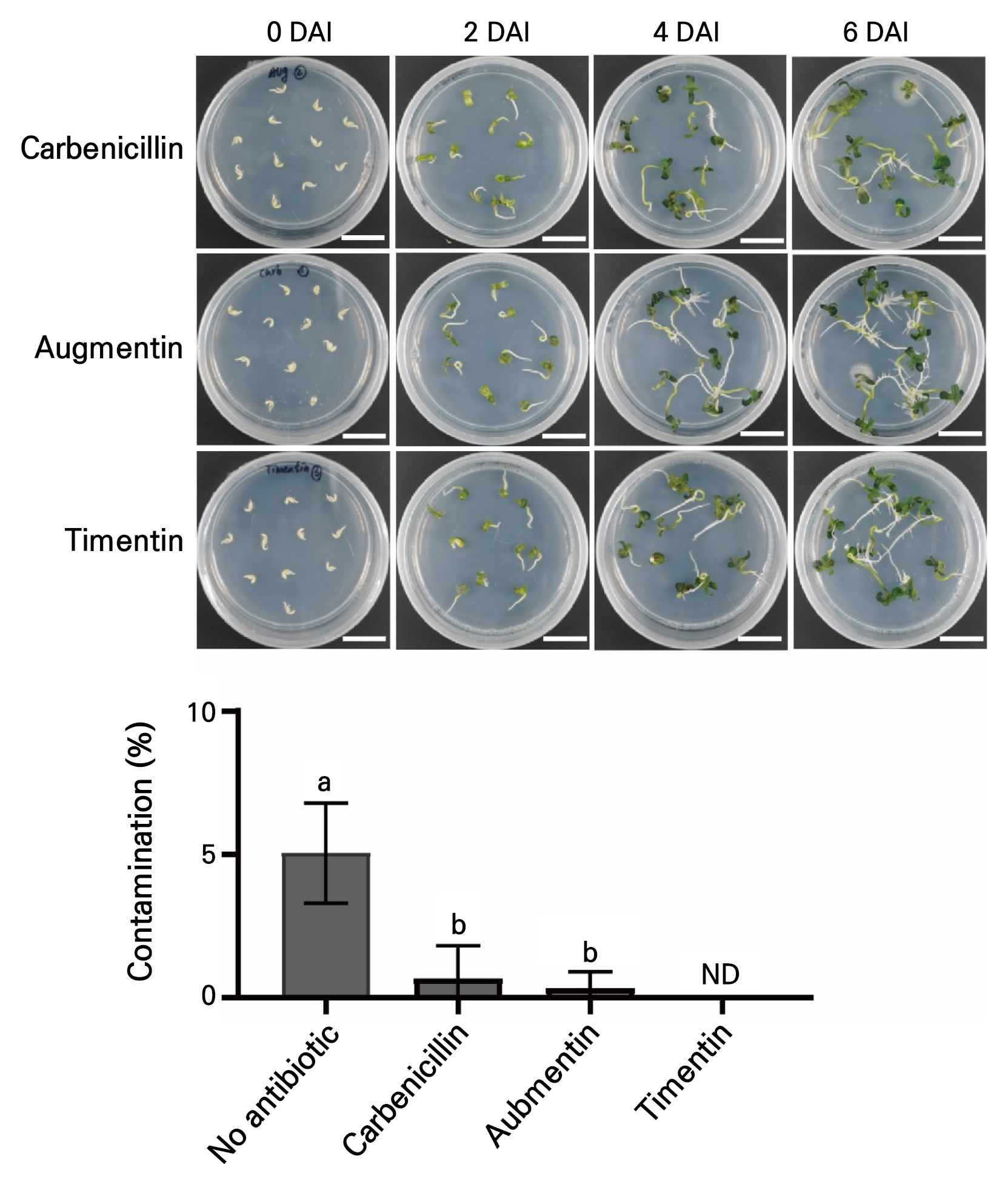
Fig. 4.
Suppression effects of antibiotics on seed contamination. Cannabis seedlings and contamination rates found during culturing on 1/2 MS media containing either 300 mg/L carbenicillin, 300 mg/L Augmentin, or 300 mg/L Timentin. Scale bars represent 2 cm. Contamination rates were measured at 6 DAI. Scale bars represent 2 cm. ND, not determined. Different lowercase letters indicate a significant difference (p < 0.05) according to Tukey’s test.
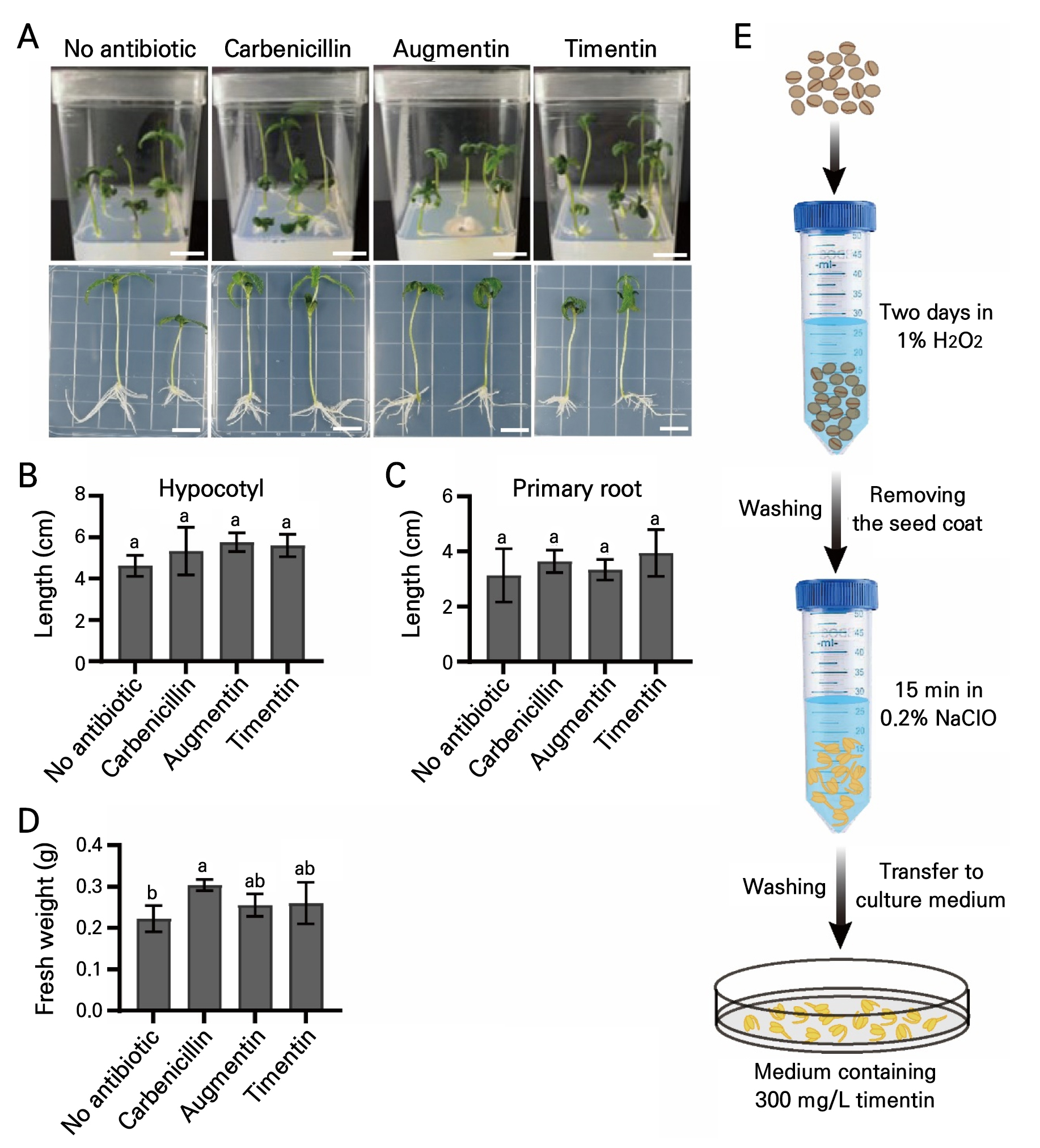
Fig. 5.
Effects of antibiotics on the growth conditions of Cannabis seedlings: (A) Growth conditions of Cannabis seedlings cultured on 1/2 MS media containing either 300 mg/L carbenicillin, 300 mg/L Augmentin, or 300 mg/L Timentin. (B-D) Measurement of morphological traits in Cannabis seedlings. Hypocotyl length (B), primary root length (C), and fresh weight (D) of seedlings shown in Fig. 5A. Scale bars represent 2 cm. Different lowercase letters indicate a significant difference (p < 0.05) according to Tukey’s test. (E) Strategy suggested by this study for the elimination of contamination during Cannabis plant tissue culturing.
Genetic engineering tools such as the CRISP/Cas9-mediated gene editing system can be applied to realize beneficial Cannabis traits by selectively knocking down or eliminating cannabinoid-biosynthesis-related gene functions. However, the application of the CRISPR/Cas9 system for new Cannabis breeding is hindered by its recalcitrant nature, lack of regeneration methods, and the requirement of in vitro tissue culturing of Cannabis protoplasts or explants. In vitro tissue culture systems without microbial contamination are crucial for the success of genetic engineering research. Furthermore, the surface sterilization, process, including effective sterilization and treatment of the concentration and the duration of the sterile reagents, as well as the sequence of their use, is very important (Orlikowska et al. 2017). Another approach used by several researchers to eliminate endogenous bacterial contamination in tissue cultures involves the use of antibiotics (Barrett and Cassells 1994; Tanprasert and Reed 1997; Luna et al. 2008). Therefore, this study aimed to assess the effects of H2O2, NaClO, and various antibiotics, the most commonly used sterile reagents and microbiomes, on contamination-free in vitro Cannabis seedlings. In this study, our results revealed that the Cannabis seed coat was one of the contamination sources, and treatment with a 1% H2O2 solution and a 0.2% NaClO solution followed by subsequent incubation in a culture medium containing 300 mg/L Timentin is an effective protocol for eliminating contaminants without negative effects on the growth of Cannabis seedlings (Fig. 5E). The methods used in this study provide knowledge pertaining to an effective aseptic tissue culturing method for Cannabis plants for use in molecular biotechnological studies that rely on protoplasts, cell suspensions, hairy roots, embryos, and other tissue cultures.


