Introduction
Materials and Methods
Plant Materials and DNA Extraction
SNP Calling and Genetic Diversity Analysis
Selecting Polymorphic SNPs on Sex Chromosome
CAPS Marker Design and Application
Results
GBS-SNP Calling and Genetic Diversity
Polymorphic SNP Selection in Sex Chromosome and CAPS Marker Design
Sex Discriminating CAPS Marker Selection
Discussion
Introduction
Kiwifruit (Actinidia spp.) is functionally dioecious (Ferguson and Huang, 2007). Flowers of female vines have functional ovaries and stamens but do not produce pollen. Flowers of male vines have fertile pollen and development of the pistil is suppressed. For breeding of kiwifruit, breeders select female or male vines according to the breeding objective. However, female and male vines cannot be distinguished during the long juvenile period. Therefore, a method for early sex discrimination is needed to increase the efficiency of kiwifruit breeding.
The sex-determination system of kiwifruit is an active Y system and the sex chromosome has been identified to be chromosome 25 (Charlesworth and Charlesworth, 1978; Testolin et al., 1995; Harvey et al., 1997; Gill et al., 1998; Zhang et al., 2015; Akagi et al., 2018). The segregation ratio of female to male of kiwifruit is 1:1 and is not affected by ploidy level (Testolin et al., 1995; Harvey et al., 1997). Seal et al. (2012) reported that fertilization with 2n gametes can affect the sex ratio of Actinidia species. However, the sex of kiwifruits is determined by the presence or absence of the Y chromosome (Testolin et al., 1999).
Sex discriminating markers for kiwifruits have been developed. Harvey et al. (1997) developed the random amplified polymorphic DNA (RAPD) markers SmX and SmY, which are associated with the X and Y chromosomes, respectively, in A. chinensis. Subsequently, SmX and SmY have been converted to sequence characterized amplified region (SCAR) markers (Gill et al., 1998). Shirkot et al. (2002) have also developed RAPD markers for discriminating the sex of A. deliciosa. Zhang et al. (2015) developed three sex-specific simple sequence repeat (SSR) markers in sex chromosomes of A. chinensis and A. rufa. Hale et al. (2018) developed an allele-specific PCR marker associated with male A. arguta. However, these molecular markers have low transferability across Actinidia species.
A. arguta, one of wild kiwifruits in Korea, has valuable traits as breeding material. Fruits of A. arguta have high nutritional and pharmacological values (Latocha et al., 2015; Leontowicz et al., 2016) and are hairless. In addition, freezing tolerance of A. arguta is higher than that of commercial kiwifruits such as A. chinensis and A. deliciosa (Sun et al., 2020). Thus, A. arguta could be a valuable material in kiwifruit breeding programs. However, the large genetic distance between commercially cultivated kiwifruits (A. chinensis and A. deliciosa) and A. arguta (Chat et al., 2004; Oh et al., 2019), and the low transferability of molecular markers across Actinidia species (Harvey et al., 1997; Gill et al., 1998), suggest that the development of A. arguta-specific molecular markers will be required to discriminate sex.
Cleaved amplified polymorphic sequence (CAPS) marker can identify polymorphisms based on sequence variations at restriction sites. A CAPS marker is a co-dominant marker with high reproducibility (Shavrukov, 2016). Polymorphisms between individuals could be easily confirmed on agarose gel using CAPS markers (Caranta et al., 1999). Sex-discriminating CAPS markers have been developed for various dioecious plants, including jojoba, yam, and fig (Parrish et al., 2004; Ince and Karaca, 2011; Tamiru et al., 2017).
Previously, genetic diversity analysis for kiwifruits including A. arguta native to Korea has been performed using single nucleotide polymorphisms (SNPs) derived from genotyping-by-sequencing (GBS) (Oh et al., 2019). GBS-SNPs could be converted into CAPS markers (Jaganathan et al., 2014; Naresh et al., 2018). Therefore, the objective of this study was to develop a sex-discriminating CAPS marker in A. arguta using previously obtained GBS data that could be used to facilitate early sex discrimination during kiwifruit breeding.
Materials and Methods
Plant Materials and DNA Extraction
Young leaves of 33 A. arguta (12 female and 21 male accessions) were collected from Namhae Branch, National Institute of Horticultural and Herbal Science, Rural Development Administration, Korea (34°48'59.8"N, 127°55'36.6"E) (Table 1).
Table 1.
Thirty-three Actinidia arguta accessions used for sex discrimination
The collected young leaves were washed with distilled water and stored at –70°C for DNA extraction. Genomic DNA was extracted from young leaves using a DNeasy Plant Mini Kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. DNA samples were quantified using a Denovix DS-11 spectrophotometer (Denovix, Wilmington, DE, USA) and diluted to 10 ng·µL-1.
SNP Calling and Genetic Diversity Analysis
Based on previously obtained GBS data (Oh et al., 2019), trimmed reads of 33 A. arguta accessions were re-aligned to reference genome sequence of A. chinensis genotype Red5 (Pilkington et al., 2018) using Burrows-Wheeler Alignment with default options (Li and Durbin, 2009). After that, reads mapped to the Red5 genome were processed using SAMtools (Li et al., 2009). SNP calling was performed using genome analysis toolkit (GATK) (McKenna et al., 2010). SNPs with a missing rate of less than 10% and minor allele frequency greater than 5% were filtered using VCFtools (Danecek et al., 2011).
Filtered SNPs were used for phylogenetic tree construction in MEGA7. The neighbor-joining method was used and 1,000 times bootstrapping were performed for data reliability.
Selecting Polymorphic SNPs on Sex Chromosome
SNPs located on chromosome 25 were selected among filtered SNPs. Polymorphic SNPs with different alleles for female and male A. arguta accessions were then selected. These polymorphic SNPs are major alleles, accounting for more than 50% of total alleles in female or male accessions.
CAPS Marker Design and Application
CAPS markers were designed using flanking sequences of polymorphic SNPs. A total of 601 bp flanking sequences, including polymorphic SNP and its 300 bp upstream and downstream sequences, were extracted. These 601 bp flanking sequences were loaded into NEBcutter (https://nc2.neb.com/NEBcutter2/). Restriction enzymes that recognized restriction sites containing the polymorphic SNPs were selected. After that, primers were designed using Primer 3. All primer sequences were designed to be 20 bp in length with GC content of 50% and annealing temperature of 60°C. In addition, forward and reverse primers were located 99 bp from the restriction site. The expected amplicon size was set to be 300 to 600 bp. The distance between digested DNA fragments was at least 20 bp.
PCR was carried out using candidate CAPS markers for discriminating sex. Each PCR amplification (20 µL) consisted of 10 µL HS Prime Taq Plus Premix (2×) (GeNetBio, Daejeon, Korea), 4 µL diluted genomic DNA, 2 µL forward primer, 2 µL reverse primer, and 2 µL tri-distilled water. PCR was carried out three times to obtain 60 µL PCR product. PCR conditions were as follows: pre-denaturation at 95°C for 5 min; 34 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec, and extension at 72°C for 1 min; and final extension step at 72°C for 5 min.
The PCR product (60 µL) was purified using Accuprep PCR/Gel Purification Kit (BIONEER, Korea). Subsequently, purified amplicons were digested according to the protocols of each restriction enzyme. The DNA fragment length was then confirmed by 2% agarose gel electrophoresis.
Results
GBS-SNP Calling and Genetic Diversity
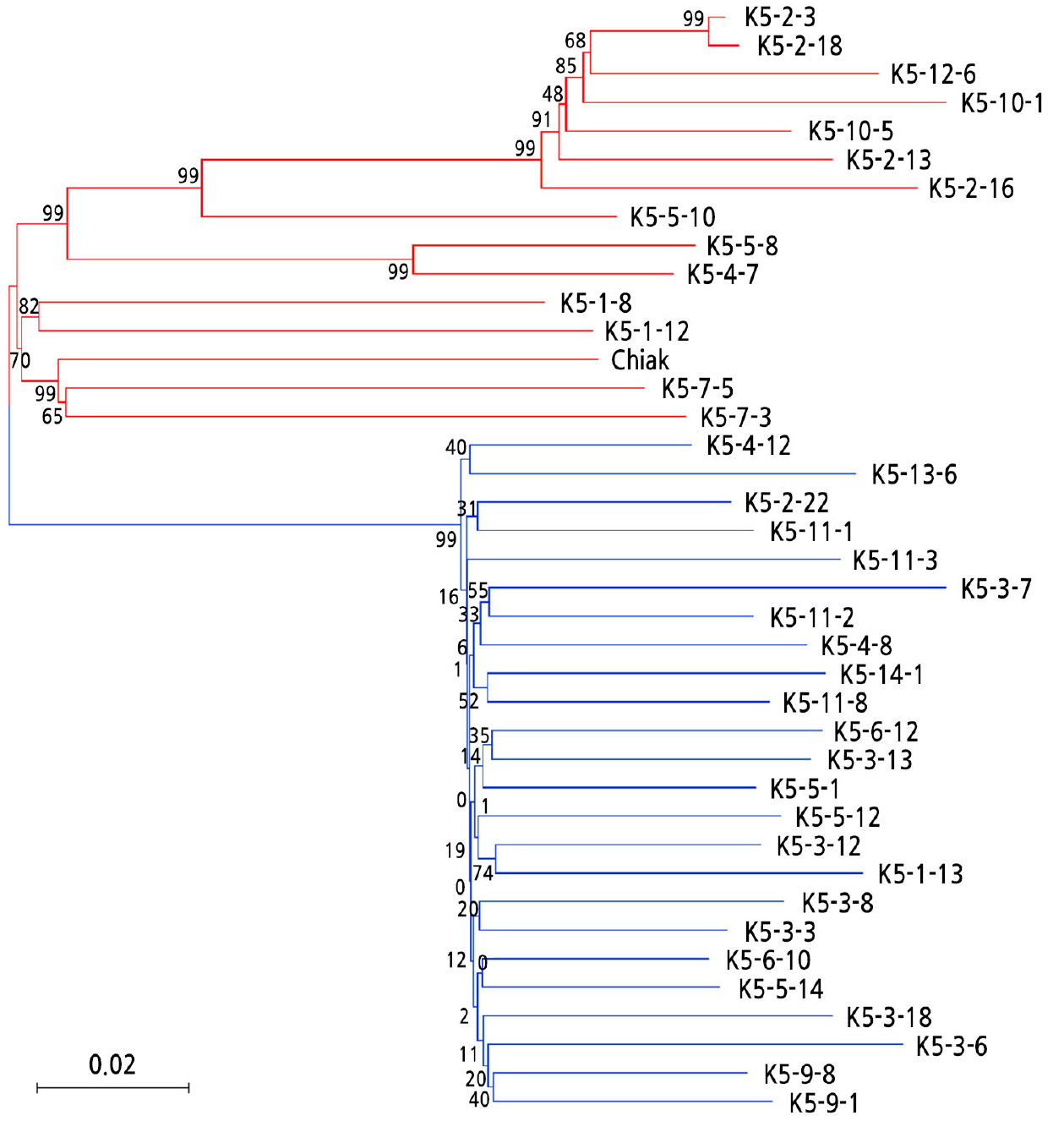
A total of 101,301 raw GBS-SNPs were detected in 33 A. arguta accessions by re-aligning GBS reads to genotype Red5 reference genome. Among them, 40,099 SNPs passed the filtering criteria. They were used to construct a phylogenetic tree. Fig. 1 shows the genetic relationship of 33 A. arguta accessions. Female and male accessions were clearly divided into two groups.
Polymorphic SNP Selection in Sex Chromosome and CAPS Marker Design
Among 40,099 SNPs, 1,239 SNPs were located on chromosome 25. Only 21 SNPs had different alleles in female and male accessions. Their major alleles accounted for more than 50% of SNPs in female or male accessions. Eight of these 21 SNPs were discarded because their reference alleles were incorrectly called. Four of the remaining 13 SNPs were also excluded from the CAPS marker design because their SNP loci were not included in restriction sites. Finally, 16 CAPS markers were designed based on flanking sequences of the finally selected 9 SNPs (Table 2). These 9 SNPs were distributed at 4,653,739 to 16,300,632 bp of chromosome 25.
Table 2.
Locations, major alleles, and average read depth of 9 polymorphic SNPs selected on chromosome 25 for sex discrimination
| Position (bp) | Major allele | Average read depth (×) | |||
| Female | Percentage (%)z | Male | Percentage (%) | ||
| 4,653,739 | G | 60 | A | 96 | 3.3 |
| 4,653,752 | C | 73 | A | 96 | 3.5 |
| 4,653,754 | T | 73 | A | 96 | 3.5 |
| 9,428,396 | A | 73 | T | 33 | 4.3 |
| 10,236,410 | A | 60 | G | 100 | 7.5 |
| 10,664,994 | A | 40 | T | 96 | 1.6 |
| 13,189,178 | C | 67 | T | 46 | 3.9 |
| 13,216,990 | A / C | 60 / 7 | T | 75 | 2.9 |
| 16,300,632 | C | 53 | T | 100 | 4.1 |
Sex Discriminating CAPS Marker Selection
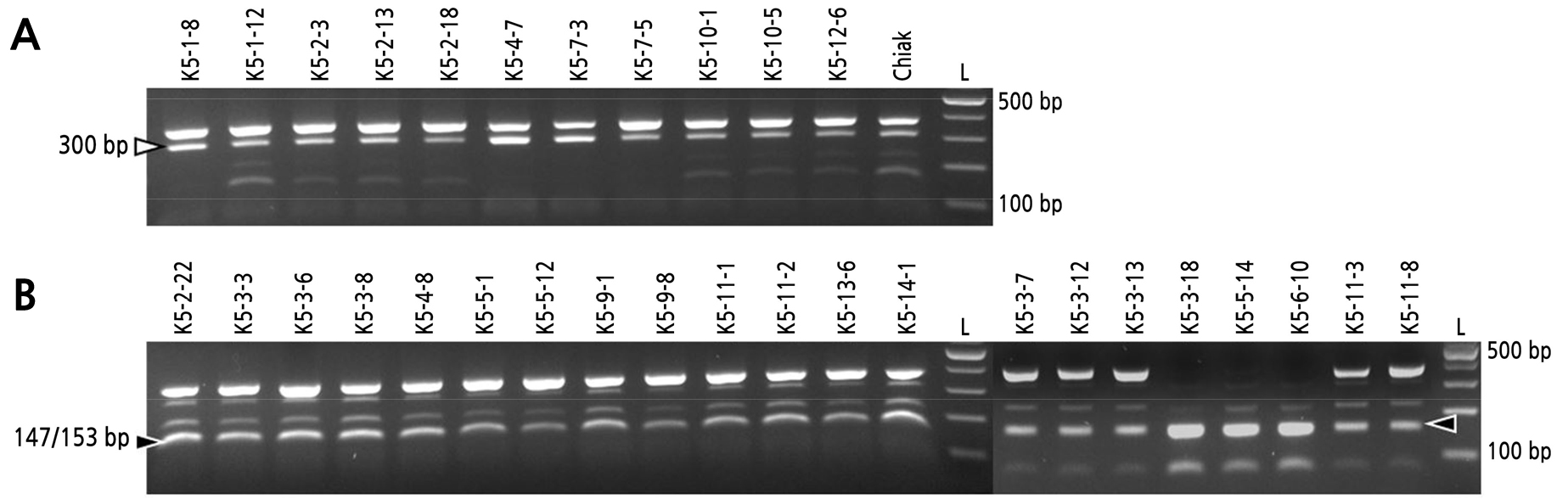
After 16 candidate CAPS markers for sex discrimination were applied to 33 A. arguta accessions, fifteen out of these 16 candidate markers could not distinguish sex. Only one marker designed based on SNP located at 10,236,410 bp of chromosome 25 and the restriction enzyme MboI could differentiate between female and male accessions (Fig. 2 and Table 3). It was named CBk25ca01. The restriction enzyme MboI recognized the 5'-GATC-3' site and digested sequence in front of the 5'-G. The CBk25ca01 marker was used to generate a 372 bp amplicon by amplifying the genomic region from 10,236,257 to 10,236,628 bp of chromosome 25 where the polymorphic SNP is located. The amplified genomic region contained two MboI restriction sites. Therefore, females possessing A allele on 10,236,410 bp of chromosome 25 were expected to generate two bands: 300 and 72 bp. For males possessing the G allele at position 10,236,410 of chromosome 25, CBk25ca01 was expected to generate bands of 147 and 153 bp, which appeared as one band, and 72 bp (Fig. 2). Female and male A. arguta accessions showed sex-specific bands (300 and 147/153 bp, respectively) (Fig. 3). Since differences of 6 bp between the 147 and 153 bp amplicons were indistinguishable on an agarose gel, these two amplicons appeared as one band.
Discussion
For the development of sex discriminating CAPS markers in A. arguta, previously obtained GBS data were reanalyzed using the second kiwifruit reference genome of genotype Red5 (A. chinensis, Pilkington et al., 2018). As a result, the number of raw SNPs was about 7.7 times lower compared to the results of Oh et al. (2019). The number of raw SNPs could be affected by genetic diversity among Actinidia species. Oh et al. (2019) performed GBS using 89 accessions including 8 Actinidia species and 4 interspecific hybrids. However, we only used GBS data of 33 A. arguta accessions. Despite using the same SNP filtering criteria, the number of filtered SNPs (40,099 SNPs) in the present study was higher than that in the previous study (34,570 SNPs) and 33 A. arguta accessions were clearly divided into two groups corresponding to the two sexes (Fig. 1). It could be speculated that there were sex-discriminating SNPs among those 40,099 SNPs. Oh et al. (2019) have also suggested that there are sex discriminating SNPs among their 34,570 SNPs. However, chromosomal localization for 34,570 SNPs was not possible because significant parts of the draft genome of A. chinensis cv. Hongyang (Huang et al., 2013) used as a reference genome were not assigned to pseudo-chromosomes. Xue et al. (2018) have suggested that chromosomal level reference genome assembly could serve as an important foundation for genomics studies. The genome assembly of genotype Red5 was assigned to pseudo-chromosomes (Pilkington et al., 2018), allowing the selection of SNPs located on the sex chromosome.
Since chromosome 25 has been known as a sex chromosome (Zhang et al., 2015), 1,239 SNPs located on chromosome 25 were investigated to develop CAPS markers. Twenty-one SNPs representing ~1.7% of those 1,239 SNPs were selected as candidate sex-discriminating SNPs. Akagi et al. (2018) have reported that X allelic sequences can be utilized for genetic diversity analysis between Actinidia species and that Y-specific sequences are conserved in Actinidia species. Therefore, it appeared that only 21 polymorphic SNPs were selected as sex-discriminating SNPs due to the low polymorphism of X allelic sequences in A. arguta.
The 9 selected SNPs were distributed in 4.6 to 16.3 Mb of chromosome 25 (Table 2). The proportion of major alleles was lower in female accessions than in male accessions. This might be affected by X allelic sequence variation between female A. arguta accessions. On the other hand, the major allele proportion of these 9 SNPs in male A. arguta accessions was 96 to 100% except for 3 SNPs. Since these 3 SNPs had a bi-allelic genotype, their major allele proportion was lower than the other 6 SNPs. The high major allele proportion of the 6 SNPs might be attributed to low genetic diversity among 21 male accessions (Fig. 1).
Using the 9 SNPs (Table 2), 16 CAPS markers were designed. The number of CAPS markers was greater than the number of SNPs used in CAPS marker design because restriction sites containing 9 SNPs could be recognized by multiple restriction enzymes. Only one marker designed using the SNP located at 10,236,410 bp on chromosome 25 was able to distinguish sex for 33 A. arguta accessions (Table 3 and Fig. 2). The CAPS marker named CBk25ca01 could discriminate sex of A. arguta accessions. Interestingly, the average depth of the SNP located at 10,236,410 bp in chromosome 25 was higher than that of the other 8 SNPs with an average depth of 1.6 to 4.3× (Table 2). Read depth is known to determine the sequencing accuracy, with lower read depth resulting in more genotyping errors (Crawford and Lazzaro, 2012). Because 15 CAPS markers based on the remaining 8 SNPs were monomorphic in both female and male A. arguta accessions, it was though that the 8 SNPs might have genotyping errors due to their low read depth.
Although CBk25ca01 produced sex-specific DNA bands of 300 bp for females and 147/153 bp for males, it also produced unexpected DNA bands in both female and male accessions (Fig. 2). DNA bands greater than 300 bp are speculated to be the original DNA bands amplified with the primers for CBk25ca01. They could have a size of 372 bp. DNA bands estimated at 372 bp and the DNA band located above the male-specific DNA band of 147/153 bp were thought to be produced by unknown sequence variations or DNA methylation of the second restriction site (Fig. 3). Because MboI is a methylation-sensitive restriction enzyme, cytosine-guanine dinucleotide (CpG) methylation may block digestion by the restriction endonuclease. Another reason for production of unexpected DNA bands was thought to be due to allele dosage between alleles. All the 33 A. arguta accessions used for experimental materials are autotetraploid, and allele dosage in autopolyploids often leads to unexpected PCR results (Luo et al., 2000). Because there was no association between unexpected DNA bands and genetic diversity of A. arguta accessions, the aformentioned reasons may influence the generation of the unexpected DNA bands. Nevertheless, CBk25ca01 could discriminate sex for A. arguta accessions.
Several sex-discriminating molecular markers have been developed for Actinidia species, including A. chinensis, A. deliciosa, A. rufa, and A. arguta (Gill et al., 1998; Shirkot et al., 2002; Zhang et al., 2015; Hale et al., 2018). However, those sex-discriminating molecular markers have low transferability across Actinidia species. Therefore, species-specific molecular markers are needed to discriminate sex in Actinidia. Hale et al. (2018) have developed aC36306, an allele-specific PCR marker, for sex discrimination in A. arguta. However, the genomic location of aC36306 is unknown. The CBk25ca01 marker was developed in this study using sequence variation in sex chromosomes. It could be used to facilitate early sex discrimination and improve the efficiency of A. arguta breeding programs.