Introduction
Materials and Methods
Plant materials
Growth conditions
Total phenolic content analysis
Total flavonoid content analysis
Antioxidant capacity analysis
Chromatographic analysis
Liquid Chromatography-Mass Spectrometry (LC-MS/MS) analyses of 20-Hydroxyecdysone
Statistical analysis
Results and Discussion
Growth characteristics
Bioactive compounds and antioxidant capacities
Metabolome analysis and comparison of the 20-Hydroxyecdysone content between mature AJN and microgreens
Conclusion
Introduction
Achyranthes japonica Nakai (AJN) is a perennial herb of the Achyranthes genus and Amaranthus family, distributed in Korea, Japan, and China (Um, 2021). It is widely used in Korea to control pain and improve osteoarthritis (Hong et al., 2017). It contains many effective ingredients that suppress osteoarthritis and inflammation, such as inokosterone, oleanolic acid, saponin, and ecdysone (Lee et al., 2012). Excluding major crops such as ginseng, medicinal crops have long relied on wild collection practices (Lee et al., 2020). AJN grows wild in fields and roadsides across the country and is strong enough to grow as a weed, meaning that it grows well anywhere (Kim et al., 2006). Many medicinal plants such as AJN are mainly grown in open fields, so their productivity and quality are not uniform (Jeong et al., 2022).
Plant factories can produce crops throughout the year by controlling and regulating environmental factors such as the temperature, humidity, light, and carbon dioxide levels (Cha et al., 2013). However, economically cultivating crops such as corn and rice is not easy due to high production costs (Lubna et al., 2022). Therefore, it is essential for vertical farms to select crops that grow quickly and have high added value (Wicharuck et al., 2023). High yields and qualities can be obtained by cultivating medicinal crops that are in high demand under customized environmental conditions (Park et al., 2019). In this way, optimal environmental management can shorten the cultivation period and enable sustainable cultivation (Park et al., 2019). Based on these advantages, the cultivation of medicinal plants as functional foods on vertical farms is emerging as a new method (Goto et al., 2016). Because production costs increase when these high-value crops are grown using various technologies, the cultivation of microgreen-type plants that are not only small but also rich in nutrients is increasing (Rajan et al., 2019).
Microgreens are immature vegetable greens similar to sprouts, but grow a few days longer (Janovská et al., 2010), and 1‒3 weeks old young seedlings have two fully developed leaves with their first true leaves (Xiao et al., 2012). According to various studies, phytonutrients such as vitamins and minerals exist in much higher amounts in young seedlings and microgreens than in mature leaves (Xiao et al., 2012). Owing to their small size and compact cultivation strategies, microgreens are typically grown on vertical farms or plant factories using artificial lighting to maximize the yield per unit of land area (Graamans et al., 2018). However, research on vertical farm and microgreen-type cultivation using AJN has not yet been conducted.
Since the production of bioactive compounds from plants varies depending on harvest time and cultivation conditions, it is necessary to investigate the optimal time and cultivation conditions for mass production (Kang et al., 2023). Increasing the planting density within a specific range can maximize the effect of the planting density on the crop yield by increasing the photosynthetic index and the light-use efficiency of crops (Diepenbrock, 2000). Optimizing the seeding density improves sprout yield and quality levels and reduces investment costs by reducing seed rates and fertilizer usage without reducing yields (Zhi et al., 2016). High planting densities can lead to uneven growth and reduced biomass and nutritional quality owing to limitations associated with substrate nutrients and space (Reda et al., 2021; Li et al., 2022). However, an appropriate planting density was shown to increase the content of soluble sugars, proteins, chlorophyll, carotenoids, total phenols, and flavonoids in cucumbers (Ding et al., 2022). Therefore, because the planting density of crops grown in plant factories is an essential factor for economic efficiency, research on the planting density according to the crop type is necessary to increase production (Uoon and Cho, 2019).
Therefore, this study sought to determine the sowing amount that most appropriately affects growth and bioactive compound levels during the growing of AJN microgreens on vertical farms and to analyze the metabolites of commercially used AJN and microgreens.
Materials and Methods
Plant materials
Seeds of Achyranthes japonica Nakai (AJN) (Aram Seeds Co., Ltd., Seoul, Korea) were used in this study, with the weight of a thousand seeds determined to be approximately 3.0 g. Before sowing, the seeds were soaked in distilled water for three days. After three days of soaking, they were soaked in 70% ethanol for 30 s and rinsed thrice with distilled water. Subsequently, they were sterilized with 20% NaOCl (Duksan Science Co., Ltd., Seoul, Korea) for 15 min and rinsed four times with distilled water. The roots of mature plants (Jecheon Agricultural Association Corporation, Jecheon, Korea) and the stems and leaves (Misan Herb Farm Co., Ltd., Daegu, Korea) were purchased and analyzed to compare the secondary metabolite contents between commercial materials and the microgreens grown in this study.
Growth conditions
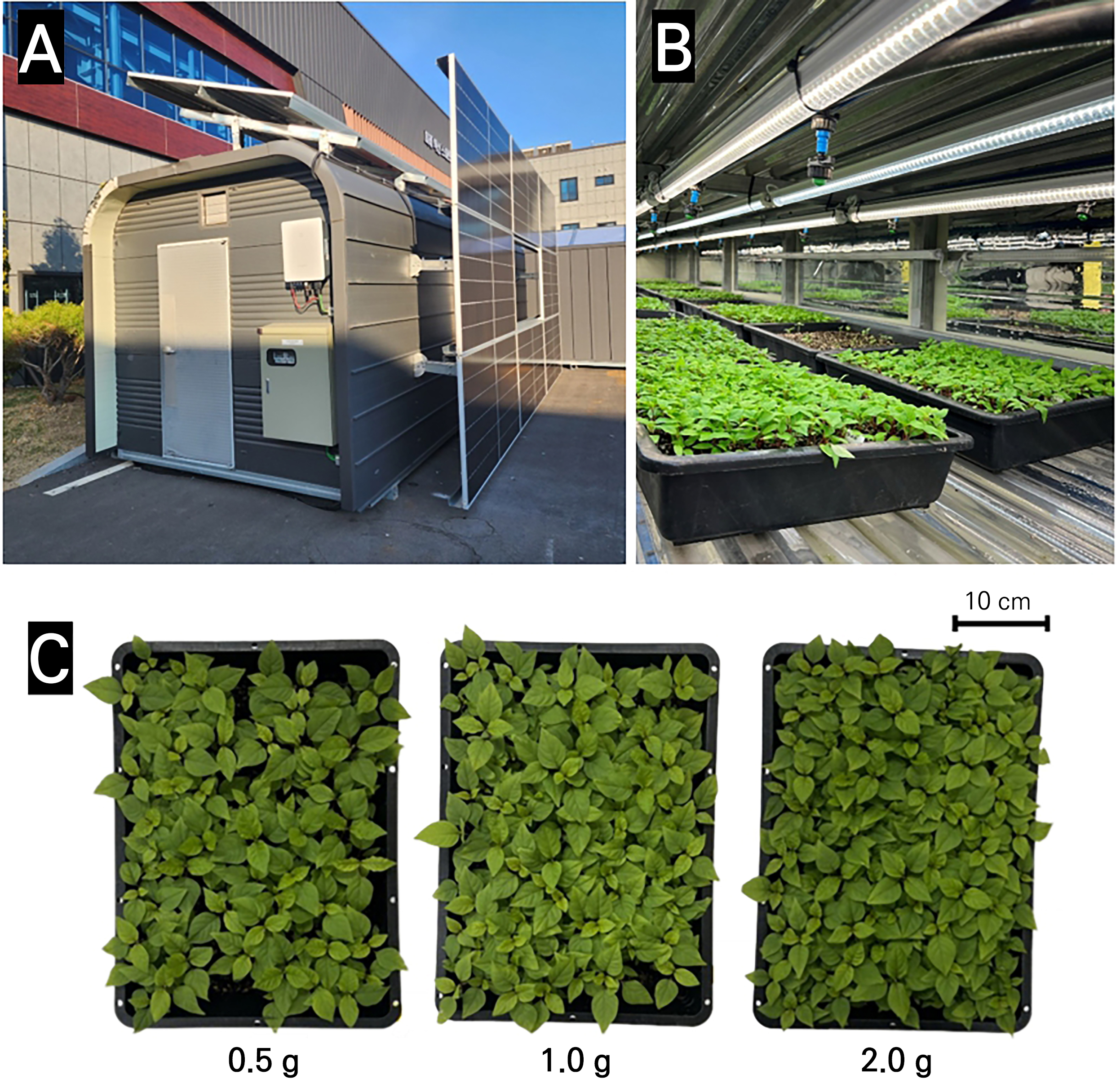
The AJN seeds were sown into a tray with drain holes (31.5 × 23 × 7.5 cm, L × W × H) filled with a commercial ginseng soil mix medium (Myeongpum-Insamsangto, Shinsung Mineral Co., Ltd., Goesan, Korea) in an environmentally controlled vertical farm-type (Fig. 1A) cultivation room. After sowing, germination was conducted in the dark for four days, after which light conditions were used. The environmental conditions of the cultivation room were maintained at a temperature of 23 ± 2°C, relative humidity of 50 ± 10%, and 170 ± 15 µmol·m-2·s-1 [photosynthetic photon flux density (PPFD); white LEDs, 12 h photoperiod] (Fig. 1B). The plants were irrigated using with 200 mL bottom watering using Hoagland’s nutrient solution (pH 6.0, EC 1.0 dS·m-1). The sowing amounts were set to 0.5 g (low density), 1.0 g (middle density), and 2.0 g (high density) to compare the growth efficiency levels. To compare the growth of AJN according to the sowing amount, the fresh weight (FW), dry weight (DW), and growth efficiency were measured 27 days after sowing. The plant height and stem diameter were measured using ImageJ software. A digital scale (PAG214C, Ohaus Corp., Parsippany, NJ, USA) was used to measure the fresh and dry weights. Samples were dried in an oven dryer (EP-20, KOTES, Co., Ltd., Seoul, Korea) at 55°C for 72 h for dry weight determination. Growth efficiency was calculated based on FW and DW and was compared with the sowing amount.
Growth efficiency (g) = FW (g) or DW (g) / Sowing amount (g)
Total phenolic content analysis
The phenolic content was measured using a slightly modified version of the Folin-Denis method (Ainsworth and Gillespie, 2007). The dry powder sample (0.015 g) contained 1.5 mL of 80% acetone and was reacted with a sonicator for 15 min and stored at 4°C for 12 h. The supernatant obtained after centrifugation (WiseSpin, CF-10, Daihan, Korea) for 2 min was used for the analysis. In a 2 mL-microcentrifuge tube, 0.135 mL of distilled water, 0.75 mL of 10% Folin-Ciocalteu reagent, 0.05 mL of the analysis sample, and 0.6 mL of 7.5% Na2CO3 were added in that order and mixed. Also at this time, a blank was prepared with 0.05 mL of 80% acetone instead of the extract of the sample. The analyzed samples were vortexed for 10 s. The completed sample was placed in a 45°C water bath for 15 min. After sufficient cooling, the sample was used for the measurement. The absorbance of the sample was set to 765 nm and analyzed (Libra S32, Biochrom, France). The total phenolic concentration of AJN is expressed as milligrams of gallic acid equivalent (GAE) per gram of DW.
Total flavonoid content analysis
To measure the total flavonoid content, a slightly modified version of the Pękal and Pyrznska method was used (Pękal and Pyrznska, 2014). Flavonoids were extracted from 50 mg of dried AJN plant with 1 mL of 70% ethanol, followed by a sonication for 15 min. The supernatant obtained by centrifuging the extract at 10,000 rpm (WiseSpin, CF-10, Daihan, Korea) for 2 min was used for the analysis. After mixing 750 µL of distilled water, 45 µL of 5% NaNO2, and 150 µL of the analysis sample in a 1.5 mL-microcentrifuge tube, it was placed for 6 min. Then, 90 µL of 10% AlCl3 was added and the sample left for 5 min. NaOH 300 µL of 1 M and 165 µL of distilled water were added and vortexed, with this followed by waiting for 5 min. At this time, a blank was prepared with 150 µL of 70 ethanol of the extract of the sample. The absorbance of each sample was measured at 510 nm (Libra S32, Biochrom, France).
Antioxidant capacity analysis
The antioxidant capacity is measured using ABTS (aminobenzotriazole; 2,2′-azino-bis[3-ethyl benzothiazoline 6-sulfonic acid] diammonium salt), a substance with antioxidant activity and one of the reagents used to measure antioxidant capacities (Miller and Rice-Evans, 1996). The dry powder sample (0.015 g) contained 1.5 mL of 80% acetone and was reacted with a sonicator for 15 min, after which it was stored at ‒4°C for 12 h. The supernatant obtained after centrifugation (WiseSpin, CF-10, Daihan, Korea) for 2 min was used for the analysis. The absorbance was measured at 730 nm (Libra S32, Biochrom, France). The antioxidant capacity of AJN is expressed as mM trolox/g DW per dry weight (g).
Chromatographic analysis
The dried sample (1 g) was extracted using methanol (50 mL) by sonication for 180 min, with 10 μL of the extract injected into an XBridge C18 column (5 μm 4.6 mm × 150 mm, Waters, Milford, MA, USA) connected to the diode array detector of an HPLC device (Agilent 1260 Infinity II, Agilent Technologies). The elution solvent was composed of A (water containing 0.1% acetic acid) and B (acetonitrile containing 0.1% acetic acid) using the gradient method. The running time changed the ratio of solvents A and B from 0% for A at 0 min to 100% for B at 60 min, with a flow rate of 1 mL/min. The detection wavelength was 254 nm. The HPLC chromatograms were monitored using an Agilent ChemStation.
Liquid Chromatography-Mass Spectrometry (LC-MS/MS) analyses of 20-Hydroxyecdysone
The dried sample (0.1 g) was extracted using 50% methanol (15 mL) by sonication for 10 min, with 2 μL of the extract injected into a LIChrospher®100 RP-18 5 μm end-capped (Merck Eurolab, Darmstadt, Germany) column connected to a Shimadzu Nexera X2 UHPLC system (Shimadzu, Kyoto, Japan). The liquid chromatography system was coupled to a QTRAP 4500 mass spectrometer (SCIEX, Framingham, MA, USA) equipped with a Turbo V Ion Source and a Turbo Ion Spray probe for electrospray ionization at the High-Tech Materials Analysis Core Facility (Gyeongsang National University, Jinju, Korea). The elution solvent was composed of A (water containing 0.1% formic acid) and B (acetonitrile containing 0.1% formic acid) using the gradient method. The running time changed the ratio of solvents A and B from 20% for A at 2 min to 90% for B at 6 min with a flow rate of 0.5 mL/min. The MS/MS conditions were as follows: an ESI source ion spray voltage of 5500 V (positive ion mode), a source temperature of 550℃, 50 psi of ion source gas (GS1), 60 psi of ion source gas (GS2), and 30 psi of curtain gas (CUR).
Statistical analysis
Data were analyzed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA) with a variance analysis. Duncan’s multiple range test was used to verify significant differences among all treatments at p < 0.05. All graphs were generated using SigmaPlot 12.5 (Systat Software Inc., San Jose, CA, USA). All figures are presented as the mean ± standard error (S.E.).
Results and Discussion
Growth characteristics
Achyranthes japonica Nakai (AJN) was grown in a vertical farm under controlled environmental conditions (Fig. 1A and 1B). AJN grown with different sowing amounts (Fig. 1C) exhibited different growth characteristics (Fig. 2). The fresh weight (FW) (Fig. 2A) and dry weight (DW) (Fig. 2B) per tray increased with an increase in the sowing amounts. However, there were no significant differences in the FW per plant depending on the sowing amount (Fig. 2C), while the DW per plant increased significantly when the sowing amount was 0.5 g (Fig. 2D). The plant height increased as the sowing amount was increased (Fig. 2E), and the stem diameter decreased as the sowing amount was increased (Fig. 2F). The growth efficiency of the fresh weight (g FW/g sowing amount) relative to the sowing amount was about 1.6 and 1.4 times higher in the 0.5 g and 1.0 g cases than in the 2.0 g case (Fig. 3A). The growth efficiency of the dry weight (g DW/g sowing amount) decreased in the 2.0 g treatment compared to that in the 0.5 g treatment (Fig. 3B).
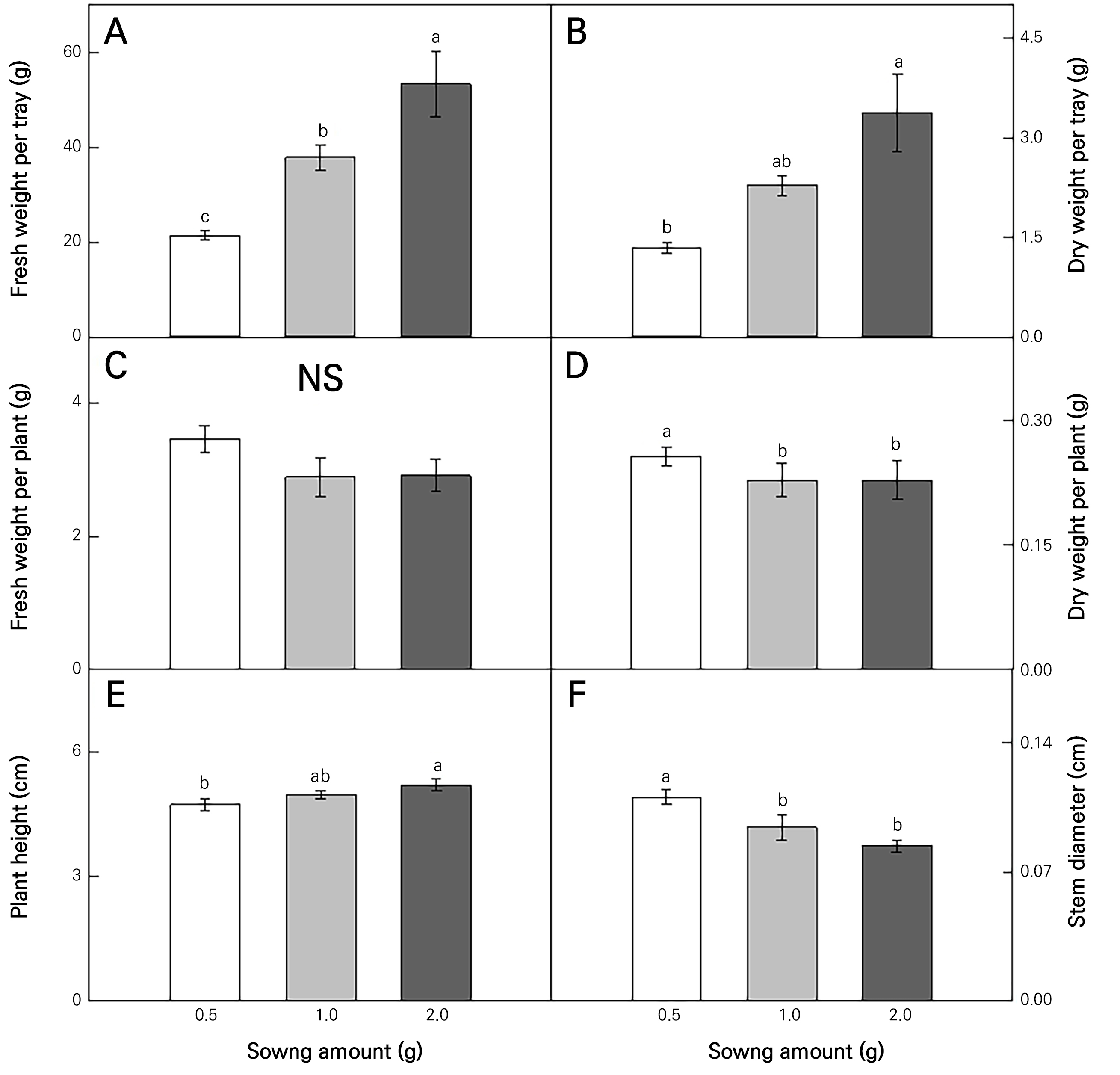
Fig. 2.
Growth of Achyranthes japonica Nakai under different sowing amounts. Fresh (A) and dry weights (B) per tray, and fresh (C) and dry weights (D) per plant. Plant height (E) and stem diameter (F) per plant. The different letters above the bars indicate significant differences among the means (p < 0.05). Error bars represent the standard error of four (A-D) or five (E, F) replicates.
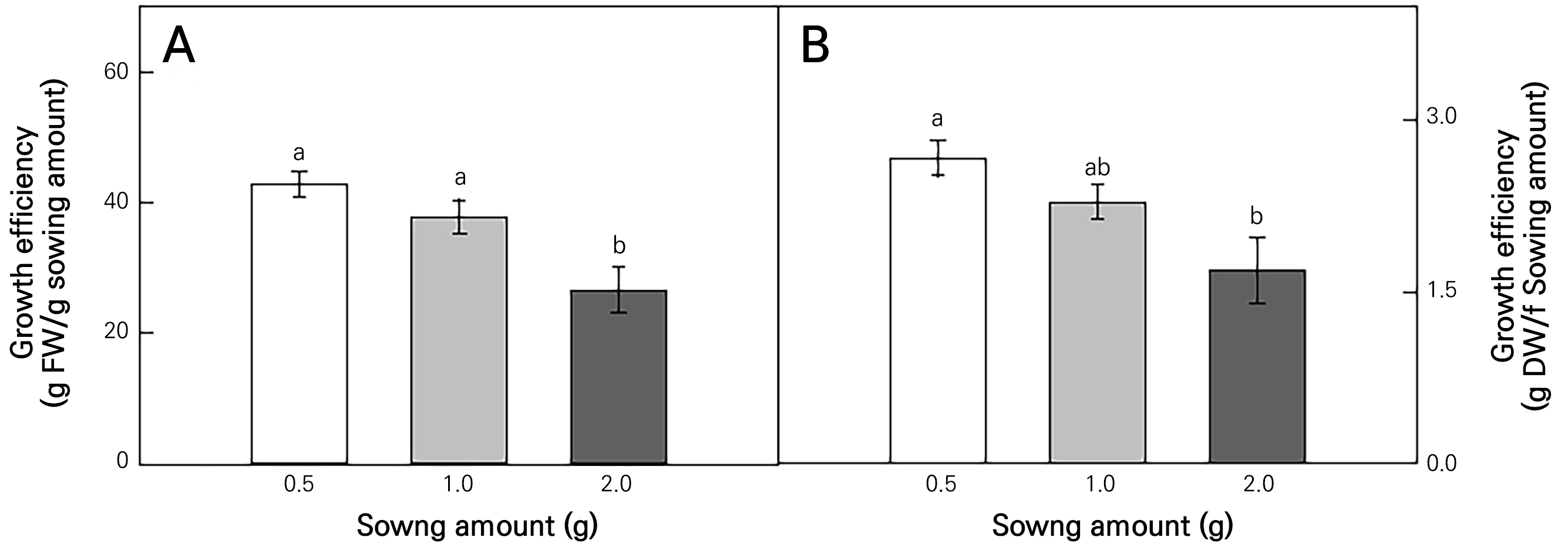
Fig. 3.
Growth efficiency (g/g) based on the fresh (A) and dry weights (B) of Achyranthes japonica Nakai according to different sowing amounts. The different letters above the bars indicate the significant differences among the means (p < 0.05). Error bars represent the standard error of four replicates.
The seeding density and sowing amount affect plant growth and development outcomes by changing the canopy light environment and the competition between plants for water and nutrients (Ricaurte et al., 2016). As can be seen from the results of this study, the sowing amount tended to increase the biomass; however and more importantly, finding the appropriate sowing amount is essential with regard to seed costs (Singh et al., 2010). In this study, as the amount of sowing was increased, the growth efficiency decreased, as low competition between plants took place under low-density conditions, and the leaf growth decreases compared to plants grown alone as the sowing amount or interference between plants increases. These results are similar to the increase in the number of flowers in ballerina plants grown at a low density (Morteza et al., 2009). The height of the plant increases to use more solar energy during the competition between plants at a high density (Güllüoğlu et al., 2016). Therefore, as the plant spacing increases, the heights of planted plants decrease (Ladaniya et al., 2021). The stem diameter was identical to those in other studies, decreasing as the density was increased (Asmamaw, 2017). As the density increases, reduced diameters can be found due to serious competition for plant nutrients, solar energy, and/or space. Consequently, a lower supply of light creates a thinner stem (Asmamaw, 2017). In plant cultivation, it is known that the sowing density is an important factor that affects plant growth and development (Turbin et al., 2014). Various studies have shown that growing at an appropriate density can increase the yields of multiple crops, such as Brussels sprouts (Turbin et al., 2014) and beet roots (Li et al., 2022). Therefore, it is essential to determine the appropriate sowing amount and plant density.
Bioactive compounds and antioxidant capacities
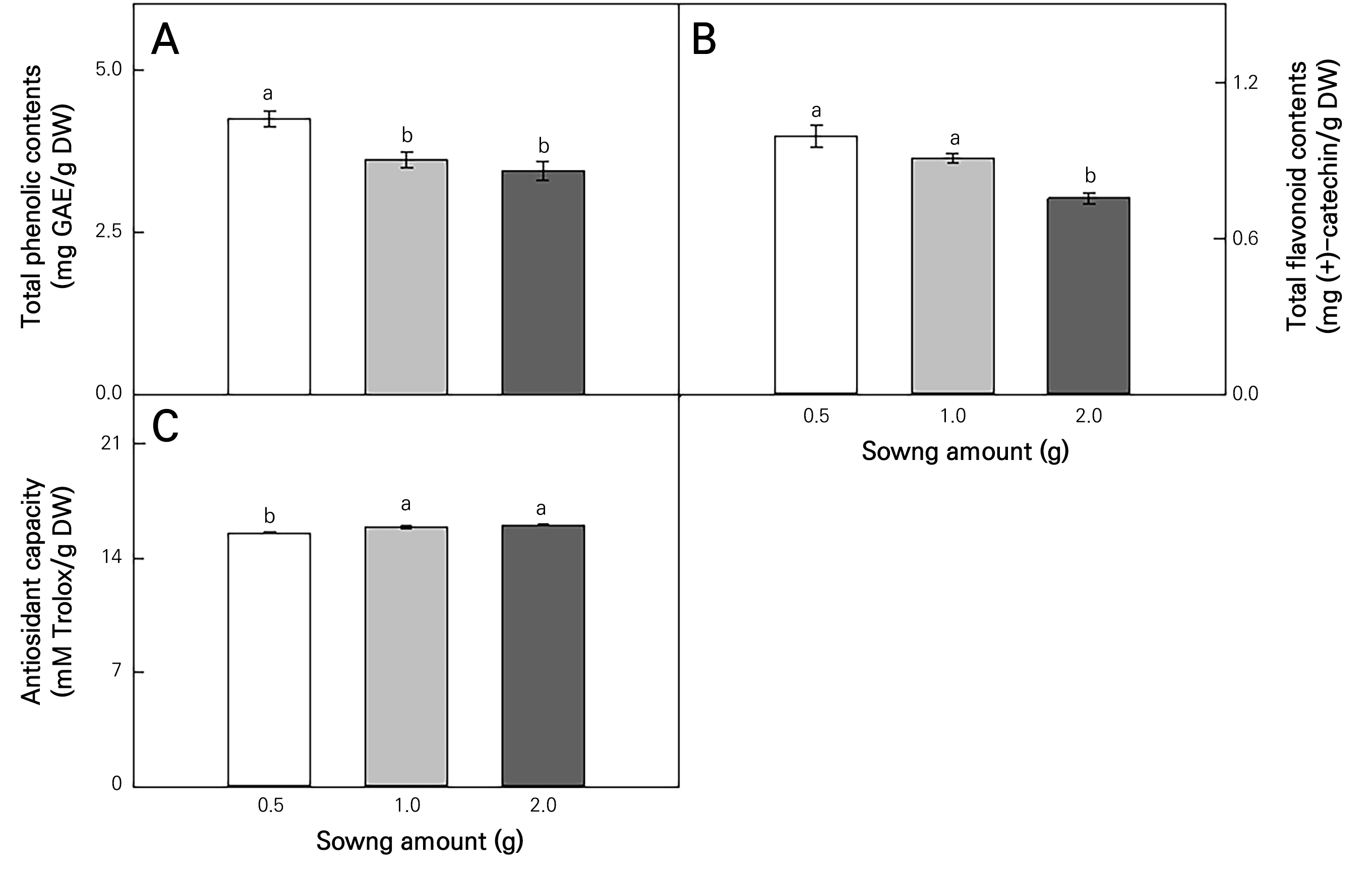
Total phenolic contents were highest at 0.5 g (Fig. 4A), and total flavonoid contents were highest at 0.5 g and 1.0 g (Fig. 4B). Meanwhile, the antioxidant capacities were highest at 1.0 g and 2.0 g (Fig. 4C).
It is known that each crop and plant genotype has a preferred density (Pobereżny et al., 2023). The phenolic content and composition of foods are often related to their potential as functional foods (Świeca et al., 2013). In addition, the synthesis of phenols and flavonoids in plants is influenced by certain environmental factors (Ghasemzadeh et al., 2010). In the present study, the phenolic and flavonoid contents were highest at 0.5 g, and at 0.5 and 1.0 g, respectively. This is consistent with a study showing increased cucumber total phenolic and flavonoid contents at low planting densities (Ding et al., 2022). Phenolic biosynthesis requires or is enhanced by light, and flavonoid formation is dependent on light (Liu et al., 2002; Hemm et al., 2004). Because light interception significantly increases with an increase in the plant density (Mao et al., 2014), phenols and flavonoids were presumed to decrease at 2.0 g compared to the 0.5 and 1.0 g sowing amounts in this study. Therefore, an appropriately low plant density is essential for improving plant yield and quality outcomes (Rezaei et al., 2014; Ding et al., 2018).
On the other hand, the antioxidant capacity was highest at 1.0 and 2.0 g. DPPH is a well-known free radical commonly used to test the pre-radical scavenging activities of compounds or plant extracts (Ghasemzadeh et al., 2010). Plant density affects plant antioxidant systems. Under normal circumstances, there is a balance between ROS production and removal in plants; however, when this balance is disrupted, ROS can accumulate (Wu et al., 2020). Therefore, competition for nutrients and space increases as the planting density increases, thereby increasing the antioxidant capacity due to abiotic stress (Machado et al., 2018).
Various studies have reported significant correlations between the antioxidant activity and phenolic and flavonoid contents (Li et al., 2009). However, this study found no significant correlation between the total antioxidant activity and total phenolic content. Polyphenols are the main plant compounds with antioxidant activity, but this is not always the case (Moure et al., 2001). Additionally, even in the presence of similar concentrations of total phenols, the antioxidant activity can vary significantly owing to synergistic and antagonistic interactions between the antioxidants (Terpinc et al., 2012). The lack of a positive correlation can be explained by differences in the phenolic compositions between plant extracts. An earlier report found that there is no significant correlation between the total phenolic content and antioxidant activity in various plants (Kähkönen et al., 1999).
Metabolome analysis and comparison of the 20-Hydroxyecdysone content between mature AJN and microgreens
The metabolite patterns in the leaves, stems, and roots of mature AJN and microgreens were similar (Fig. 5A-5D). This indicates that AJN can be used in the microgreen form instead of the mature form. Ecdysterone and 20-hydroxyecdysone (20E) affect skeletal muscle and have preventive effects, such as anabolic effects (Cheng et al., 2013), obesity prevention (Agoun et al., 2021), and diabetes prevention (Buniam et al., 2020). The 20E content was highest in mature roots, followed by microgreens (Fig. 5E). The levels of 20E in the microgreens of AJN grown in the vertical farm were approximately 1.2 and 1.6 times higher than those in mature leaves and stems grown in the open field (Fig. 5E). However, the mature roots showed levels that were approximately 2.1 times higher than those of the AJN microgreens. This indicates that microgreens contain more nutrients than mature crops of the same type (Xiao et al., 2012). This is consistent with earlier results showing that all parts of AJN plants contain 20E, the content of which varies depending on the growth stage and harvest time of the AJN (Boo et al., 2010). It takes approximately two years or more from sowing to harvest to obtain the roots of AJN. However, AJN grown as microgreens in a vertical farm environment can be harvested within a month. 20E, a representative substance of AJN, was confirmed to be present in the microgreen form. Therefore, the possibility of cultivating AJN as a microgreen plant within a vertical farm to obtain 20E effectively in a short period was confirmed in this study.
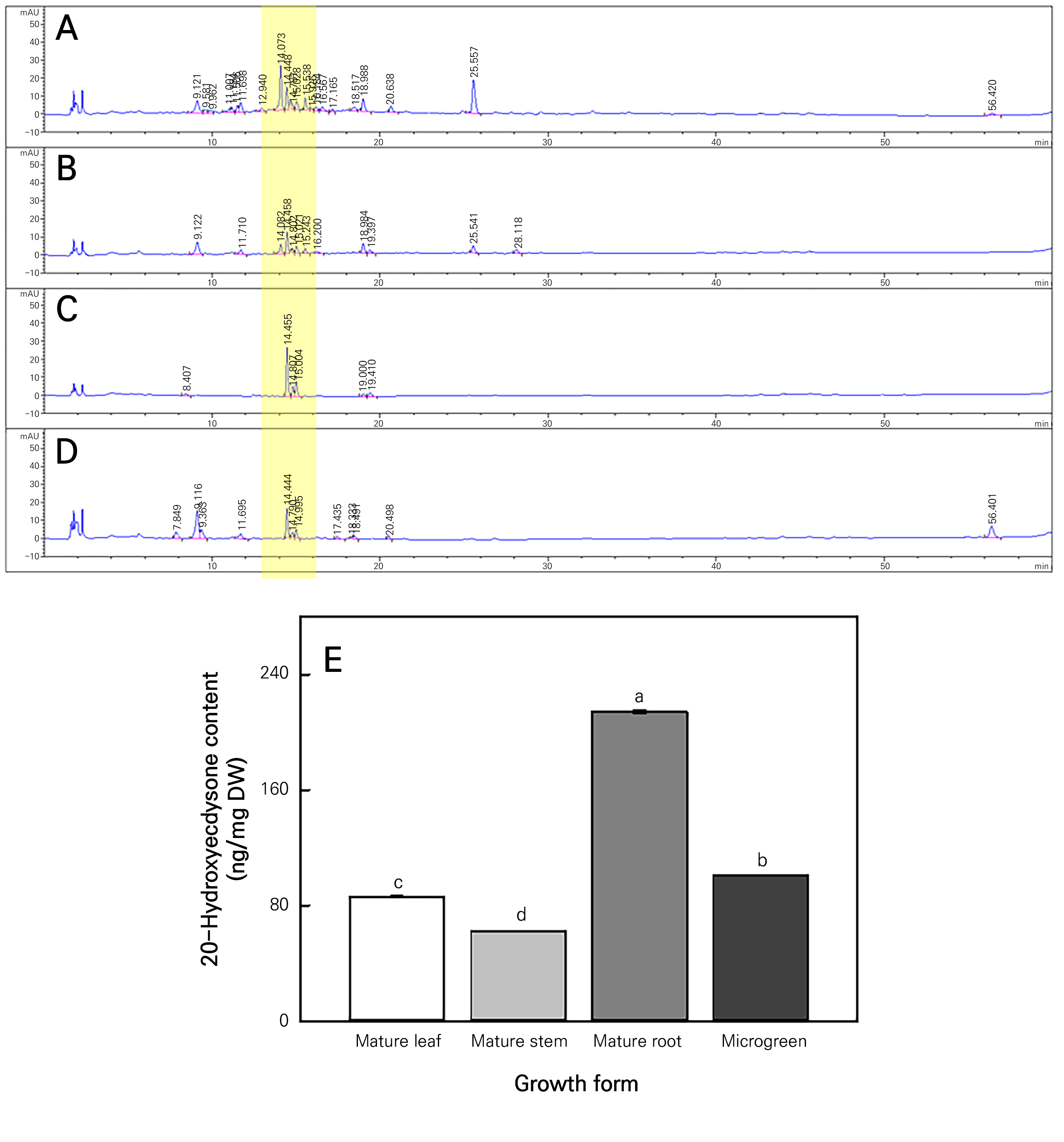
Fig. 5.
LC chromatogram of Achyranthes japonica Nakai extracts. Leaf (A), stem (B), root (C) and microgreen (D) of Achyranthes japonica Nakai. 20-Hydroxyecdysone content of AJN (E) in the form of mature leaves, stems, roots, and microgreens. The different letters above the bars indicate significant differences among the means (p < 0.05). Error bars represent the standard error of three replicates.
Conclusion
Achyranthes japonica Nakai (AJN) is a medicinal plant cultivated in the warm regions. AJN contains various substances, such as saponin and ecdysterone. However, approximately two years must pass from sowing to harvest. Therefore, this study aimed to develop a new AJN cultivation technology in the form of microgreens in vertical farms. In this study, the fresh and dry weights compared to the sowing amounts were good at 1.0 g or less among the 0.5, 1.0 and 2.0 g cases. The phenol content was also highest at 0.5 g, and the flavonoid content was highest in the 0.5 and 1.0 g cases. The antioxidant capacity was highest in the 1.0 and 2.0 g cases. Based on previous results, it was appropriate to sow at 1.0 g or less in terms of growth considering economic feasibility. Therefore, a sowing amount of 14 g, or 4600 per m2, was judged to be appropriate. The metabolite patterns of the mature roots, stems, leaves, and microgreens of the AJN were also similar. The level of 20-hydroxyecdysone (20E) in AJN microgreens grown in the vertical farm was approximately 1.2 and 1.6 times higher than that in mature leaves and stems grown in the open field respectively. The levels of mature roots were approximately 2.1 times higher than that of the microgreens of AJN. The 20E content of microgreens was higher than those of the mature stems and leaves and lower than that of the roots. However, the microgreen form is expected to be more efficient because it can be produced continuously and stably in a short cycle in a vertical farm. Therefore, this study is expected to enable the cultivation of AJN microgreens in vertical farms, and it is judged to be valuable as a plant material for food, medicine, and cosmetics.