Introduction
Materials and Methods
Seed Information
Confirmation of Seed Dormancy and Initial Germination Test
Seed Storage
Saturated Salt Accelerated Aging Test
Germination Index Calculation and Statistical Analysis
Results
Seed Characteristics
Changes in the Germination Index According to Storage Temperature and Period
Saturated Salt Accelerated Aging Test
Discussion
Introduction
Seven species of Pulsatilla belonging to Ranunculaceae are native to South Korea (KPNI, 2021). They have appeared in various tales and poems from the past and have special cultural significance. Among them, Pulsatilla dahurica is morphologically similar to P. cernua var. koreana, but the sepals are shorter and the petals are light pink in color. Pulsatilla species are used as traditional medicine in South Korea and China, and P. dahurica contains triterpenoids, such as hederagenin (Zinova et al., 1992; Kumar et al., 2008; Sun et al., 2009). This compound was confirmed to have anti-tumor, anti-inflammatory, anti-depressant, anti-diabetic, and other activities (Zeng et al., 2018). In addition, P. dahurica has high value as an ornamental plant. P. dahurica is primarily cultured by seed; however, to the best of our knowledge, there have been no studies on the dormancy and longevity of its seeds.
Seed longevity is an important seed trait. In terms of the conservation of genetic resources, seed longevity is used as a reference for long-term storage facilities such as seed banks and is important for the continuity of storage. Seed longevity also provides an appropriate renewal time for seeds to enable continuous conservation. From an industrial point of view, to avoid loss of production efficiency, it is important to cultivate plants before seed longevity is lost.
Seed longevity is affected by genetic and environmental factors. Environmental factors, such as storage temperature and humidity, are crucial for the long-term storage of seeds. Therefore, it is important to consider seed characteristics, such as the moisture content and tolerance to drought and cold, to determine the optimum storage conditions (Vertucci and Roos, 1990; De Vitis et al., 2020). In general, seed longevity doubles for every 1% reduction in seed moisture or a 5°C reduction in storage temperature (Harrington, 1972). These conditions enable long-term storage of seeds and affect their germination ability (Dickie et al., 1990). Conversely, inappropriate storage conditions rapidly reduce seed viability due to accelerated aging (Walters et al., 2010).
Seeds can be classified into orthodox, recalcitrant, and intermediate, depending on their viability. As orthodox seeds tolerate desiccation, viability can be maintained for a long time, even in long-term storage under dry conditions and low temperatures. In contrast, recalcitrant seeds have high moisture content and intolerance to low temperatures, making their long-term storage difficult (De Vitis et al., 2020).
Even though seed longevity can be presumed from other characteristics, the exact longevity and optimal storage conditions must be identified through long-term monitoring. However, this procedure can be lengthy. Alternatively, the accelerated aging (AA) test, which tests viability, can be used to indirectly evaluate the relative longevity of seeds (Delouche and Baskin, 1973). The AA test performed at high temperature and humidity can be used to indirectly confirm seed storability and is primarily used to compare the storability of crop seeds. The saturated salt accelerated aging (SSAA) test is used for small seeds such as those of flower crops (Jianhua and McDonald, 1997; McDonald, 1997). Appropriate storage conditions can be determined based on the data obtained by testing seeds stored under various conditions.
Pulsatilla cernua var. koreana, which is a similar species to P. dahurica, shows a high germination rate when sown immediately after seed harvesting. However, its seeds are short-lived, and their longevity decreases rapidly over time (Sang et al., 1993). Seed longevity can be highly variable within the same taxon (genus, species) (Priestley et al., 1985; Walters et al., 2005), and may vary depending on maturity, growth environment, and individuals (Kochanek et al., 2009; Yuan et al., 2021).
In our study, P. dahurica seeds were stored at various temperatures immediately after collection and then periodically sown. The degree of aging according to the storage period was checked, and the appropriate storage temperature was confirmed using the SSAA test. The characteristics of seeds, such as longevity and optimal storage conditions, can be used for the future propagation and conservation of genetic resources.
Materials and Methods
Seed Information
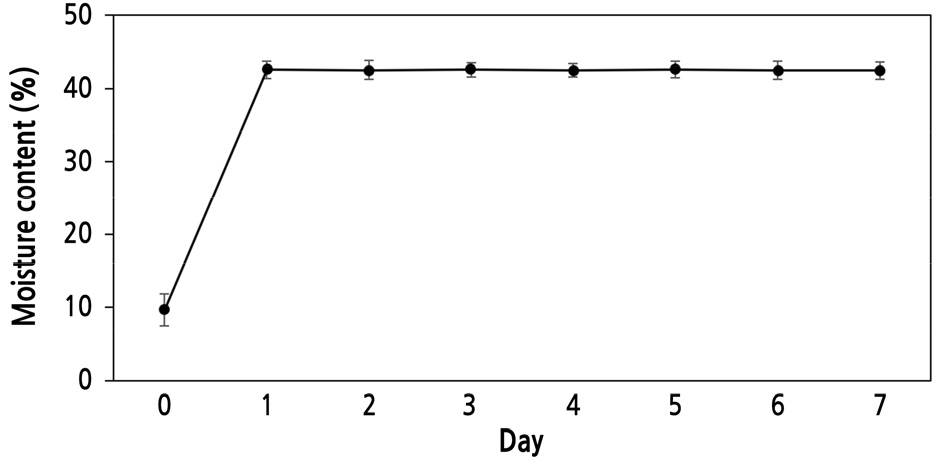
Pulsatilla dahurica seeds used for seed storage were collected in May 2020 in Andong-si, Gyeongsangbuk-do, before seed dispersal. The collected seeds were dried in the shade at room temperature (25 ± 1.0°C) for 7 d, and contaminants (foreign matter including hair-like seed structures) were removed. The size and weight of the seeds were measured to document their morphological characteristics. The moisture content of the seeds was measured at 24 h intervals for 7 d after immersing the seeds in distilled water. Measurements were performed using a microbalance after removing all the moisture from the seed surface. Then, the moisture-absorbed seeds were dried in an oven at 60°C for 48 h to measure the dry weight of seeds, and the moisture content was calculated based on the difference in the weights. Seed viability was confirmed using 1% triphenyl tetrazolium chloride (Sigma-Aldrich, MO, USA) solution, and the seeds were incubated at 30°C for 24 h. Seeds were observed using a stereomicroscope (SZ61; Olympus, Tokyo, Japan), and the embryo length: seed length (E:S) ratio was measured immediately after collection and after germination using a CMOS camera (eXcope F630; Dixi Sci., Daejeon, Korea) and the eXcope software (ver. 3.7.12277).
Confirmation of Seed Dormancy and Initial Germination Test
Seeds were immersed in 1% sodium hypochlorite for 10 min for sterilization and then rinsed with distilled water at least five times. For sowing, two sheets of qualitative filter paper (Whatman no. 1, Bucks, UK) and 4 mL of distilled water were added to a Petri dish (cat. No. 11010; SPL Life Sciences, Pocheon, Korea) and sealed with parafilm to prevent moisture loss after sowing the seeds. Thereafter, it was placed in a chamber at 25/15°C (16/8 h, White-LED, 30 µmol·m−2·s−1 PPFD/Dark), and the germinated seeds were checked every day for 30 d. If the seed root protruded more than 1 mm, it was considered to have germinated and removed from the Petri dish. Fifty seeds with four replicates were used in the experiment.
Seed Storage
After collection, the seeds were placed in a plastic bag with silica gel, double-sealed, and stored in the dark at −20°C (freezer), 4°C (refrigerator), and 25°C (chamber). After 1, 2, 3, 6, and 12 months of storage, a portion of the seeds was removed, and the germination rate was checked. Seeds stored in the freezer were immediately thawed in water at 40°C for 5 min in a plastic bag. Seeds stored at 4°C and 25°C were not thawed. The germination test was carried out in the same way as the initial experiment with the same number of seeds.
Saturated Salt Accelerated Aging Test
For the SSAA test, seeds stored for 1 year were used, and seeds collected at the same location immediately after collection were used as the control (Table 1). Accelerated aging of seeds was performed by modifying the ISTA protocol (ISTA, 2018). A seed testing container (11.0 × 11.0 × 3.5 cm, Hoffman Manufacturing Inc., Oregon, USA) equipped with an accelerated aging tray (60 mesh, Hoffman Manufacturing Inc.) was used, and 40 ml of saturated salt solution (NaCl) was used. The seeds were then artificially aged at 41°C and 47°C for 72 h. Aged seeds were checked for viability using the tetrazolium (TZ) test, and by classifying the degree of staining, seeds with normal staining of embryos and endosperm were classified as viable, partially stained as abnormal, and unstained were classified as dead. In addition, under the same conditions as in the initial germination experiment, 100 seeds were sown in 4 replicates to test the germination.
Table 1.
General information of Pulsatilla dahurica seeds used in this study
Germination Index Calculation and Statistical Analysis
Based on the germination rate of seeds, the final germination rate (percent germination, PG), start germination (SG), mean germination time (MGT), and median germination time (T50) were calculated and compared between the treatment groups. All calculated values were expressed as mean and standard error, and significance was tested at p < 0.05, using ANOVA and Duncan's multiple range test using SAS (ver. 9.4, SAS Institute Inc., Cary, NC, USA).
Results
Seed Characteristics
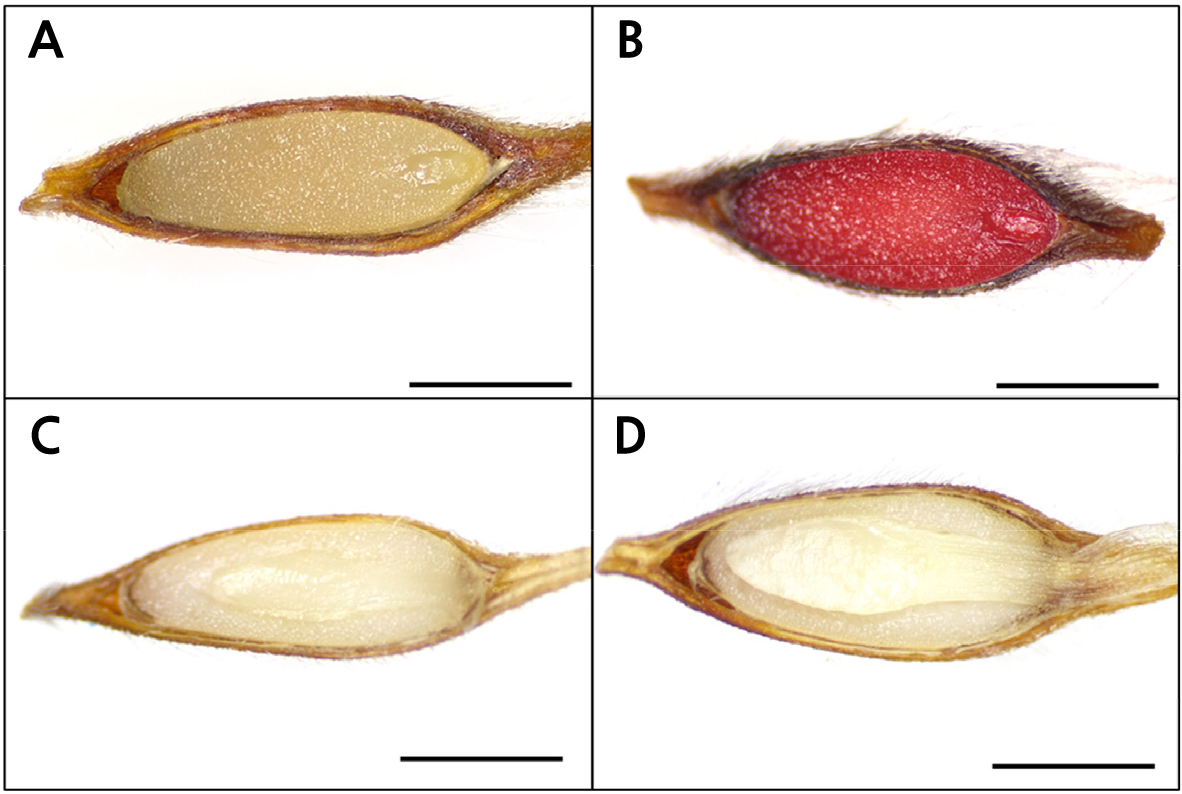
The length and width of P. dahurica seeds were 3.95 ± 0.103 mm and 1.18 ± 0.066 mm, respectively. The weight of 1,000 seeds was 0.88 ± 0.003 g (Table 1). The moisture content of the seeds was 8.16% ± 0.802%, and the maximum moisture content (41.5% ± 0.69%) was reached within 1 d of immersion; the change in moisture content was measured at 24-h intervals (Fig. 1). After collection, the E:S ratio of the seeds with underdeveloped embryos was 0.23 ± 0.012 (Fig. 2A). Seed viability was confirmed to be 100%, and both embryos and endosperms were stained red (Fig. 2B). Therefore, the seed coat or structure did not interfere with water absorption. Seeds collected at the same location in the following year were also confirmed to have similar characteristics to those collected in 2020.
The seeds were sown without any scarification procedure as physical dormancy was absent. Most of the seeds of P. dahurica germinated during the 30 d sowing period (Fig. 2D). Seed germination started from the 15th day of sowing and showed uniform germination. More than 50% of the seeds germinated between the 14th and 18th day after sowing. At the time of germination, the E:S ratio was 0.72 ±0.02, showing a large increase compared to the E:S ratio after collection and characteristics of morphological dormancy (Fig. 2D). Finally, 97.5% ± 0.96% of seeds germinated with an MGT of 18.0 ± 0.65 d and a T50 of 17.5 ± 0.81 d (Fig. 3).
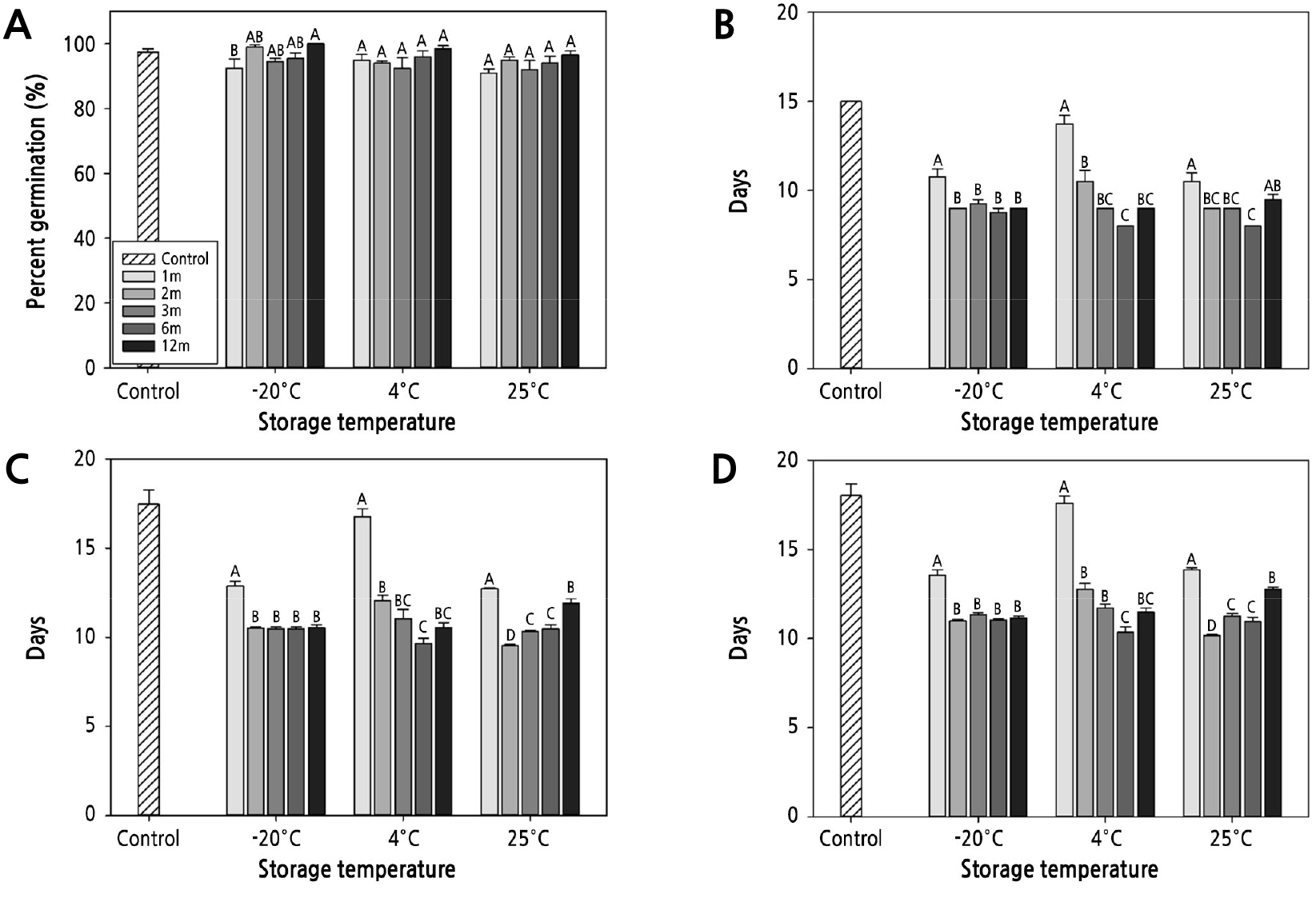
Fig. 3.
Germinability of Pulsatilla dahurica seeds after 1 year of storage. Control group refers to seed properties at collection. (A) Percent germination; (B) start of germination; (C) median germination time; (D) mean germination time; Bars represent standard error. Mean separation was tested by Duncan's multiple range test at p < 0.05.
Changes in the Germination Index According to Storage Temperature and Period
No reduction in PG was observed with respect to the seed storage period. PG after the first month of storage was 92.5% (−20°C), 95.0% (4°C), and 91.0% (25°C), showing a slight reduction compared to the PG observed immediately after collection (Fig. 3A). However, after 3 months of storage, PG was more than 92% for all storage temperatures, showing no significant difference compared to the PG value obtained immediately after collection. Notably, PG did not reduce further even after storage for 12 months at all temperatures.
The SG of the seeds immediately after collection was 15.0 d. However, after 1 month of storage at −20°C, 4°C, and 25°C, germination started at 10.8 d, 13.8 d, and 10.5 d, respectively, 4.5 d earlier compared to SG immediately after collection (Fig. 3B). The amount of reduction in SG was the largest at −20°C and 25°C and was relatively small at 4°C. This tendency was observed even after 2 months of storage, and germination occurred 6 (−20°C), 4.5 (4°C), and 6 (4°C) d earlier than the germination time required when sowed immediately after collection. SG showed an abrupt reduction at the early stage of storage and decreased gradually thereafter. After 6 months of storage, the radicle protruded 8.0 d after sowing at 4°C and 25°C, 7.0 d earlier compared to sowing seeds immediately after collection. However, after 1 year of storage, SG showed a tendency to increase again at 4°C and 25°C.
The SG and germination time were shortened after storage. MGT and T50 were reduced as the storage period increased, and germination proceeded uniformly and rapidly. After 1 month, the T50 was significantly reduced to 12.9 and 12.7 d at −20 and 25°C, respectively. However, it was found to be 16.8 d at 4°C, similar to the T50 of 17.5 d observed immediately after seed collection (Fig. 3C). After 2 months of storage, the T50 reduced significantly at all temperatures. Thereafter, the T50 of the seeds stored at −20°C was maintained without a significant difference until 12 months. In contrast, at 25°C, it tended to increase again as the storage period increased, whereas, at 4°C, it reduced.
MGT, the average number of days required for germination, also showed a pattern similar to that of T50. After 1 month, at −20 and 25°C, the MGT was 13.6 d and 13.9 d, respectively, shortened by more than 4 d compared to the MGT observed immediately after collection (Fig. 3D). For the seeds stored at 4°C, the MGT started to reduce after 2 months of storage. Conversely, at 25°C, MGT showed a tendency to increase during long-term storage.
Among the four germination indices investigated in the experiment, SG, T50, and MGT showed a high correlation with storage temperature and the storage period, and PG was only correlated with storage period (Table 2).
Table 2.
Significance of germination indices according to storage temperature and period
| PG | SG | T50 | MGT | |
| A | NS | *** | *** | *** |
| B | ** | *** | *** | *** |
| A × B | NS | *** | *** | *** |
Saturated Salt Accelerated Aging Test
Seeds artificially aged with the SSAA test under different temperatures showed different patterns of viability and germination according to storage temperature. The viability ratio of the control seeds aged via SSAA at 41°C without storage was 90%, and the stored seeds showed ratios of 96%, 97%, and 58% at −20°C, 4°C, and 25°C, respectively (Fig. 4A). There was no reduction in viability among the stored seeds compared to the control seeds, except at 25°C. In contrast, the germination rate of the control seeds was 80%, similar to the viability, but the germination rates for the seeds stored at −20°C, 4°C, and 25°C were 44%, 66%, and 2%, respectively, indicating that many viable seeds did not germinate. Among the artificially aged seeds, the germination of the seeds stored at 4°C showed the lowest reduction compared to the control. Control seeds aged at 47°C showed similar viability and germination of 27% and 28%, respectively (Fig. 4B), and the effect of aging was higher at 47°C than that at 41°C. The viability ratios of the stored seeds were 6%, 12%, and 0% at −20°C, 4°C, and 25°C, respectively. However, none of the seeds germinated after sowing.

Fig. 4.
Viability and percent germination of stored Pulsatilla dahurica seeds treated with artificial aging by saturated salt accelerated aging at different temperatures. (A) 41°C; (B) 47°C. Filled bars and circles represent the viability and percent germination, respectively. Mean separation was tested by Duncan's multiple range test at p < 0.05.
Discussion
The embryonic shape of P. dahurica seeds was similar to the ‘rudimentary’ shape according to Martin (1946) criteria; it presented morphological dormancy (MD) or morphophysiological dormancy (MPD) due to underdeveloped embryos. In addition, there was no physical dormancy (PY) as the factors that interfere with the absorption of moisture were not observed. Baskin and Baskin (2005) reported that when seeds with morphologically underdeveloped embryos were sown, seeds that germinated within 4 weeks were classified as seeds with MD, and seeds that did not germinate within 4 weeks were classified as seeds with MPD (Song et al., 2019). Seeds of P. dahurica germinated within 4 weeks after sowing at 25/15°C (16/8 h) and were confirmed to be morphologically dormant.
When the seeds were stored at three different temperatures and then sown, contrary to our expectation, no reduction in germination rate was observed based on the storage period; however, SG, T50, and MGT were shortened, and the germination index increased. However, it is presumed that the slight decrease in the germination rate in the early stage of storage is due to seed contamination during the sowing period. In subsequent experiments, fungi-contaminated seeds were immediately removed from the Petri dish, and the filter paper was replaced to prevent further contamination; therefore, seed contamination was prevented.
Sang et al. (1993) reported that the germination rate of P. cernua var. koreana seeds immediately after collection was 63.5% but decreased to 0.3% after storage for 14 weeks at room temperature. However, although the habitat environments were similar, the longevity of P. dahurica seeds was completely different. In our experiment, the germination rate of P. dahurica seeds stored at 25°C, similar to room temperature, did not decrease compared to that after collection, even after 12 months. Moreover, the storage increased germinability, with positive results in various germination indices. Another study reported that P. cernua var. koreana, with a germination rate of 94.8%, showed a reduction in the germination rate by about 40% after 28 weeks of storage at 0°C (Sang et al., 1996). However, in this study, there was no reduction in the germination rate of P. dahurica, even when stored at a temperature close to 4°C for 1 year. In these regards, unlike P. cernua var. koreana with extremely short longevity and storability, the longevity of P. dahurica is not reduced rapidly, and the germinability was maintained for more than 1 year under storage.
In our experiment, no reduction in the germination rate was observed for P. dahurica seeds after storage; however, some germination indices tended to increase after storage compared to the indices observed immediately after initial collection. This increase in the germination indices after storage seems to be closely related to the condition of the seeds at the time of collection. The germination rate of seeds is also affected by the time of collection, and seeds that are dried before full development can lose vitality quickly (Hay and Probert, 1995; Donohue et al., 2005; Probert et al., 2007). Our seeds were not physiologically mature at the time of collection, and it can be assumed that the germination index had been increased after ripening during storage. Yuan et al. (2021) also reported that for P. cernua var. koreana seeds collected at different times, the germination rate, germination time, and fidelity were significantly different, and the germination rate increased as the maturity increased. They suggested physiological dormancy of the seeds according to maturity; however, in our experiment, no physiological dormancy was observed in P. dahurica, and after-ripening was the major contributor that increased the germinability. This suggests that the time of seed collection is a crucial factor for seed storability and germinability.
In our experiments, P. dahurica could not be observed aging even after 1 year of storage. Therefore, the difference in the degree of aging at different storage temperatures was confirmed after artificial aging. Of the two temperatures used for SSAA, the viability and germination rate decreased compared to the control (seeds aged immediately after collection) at 47°C, confirming the cumulative aging caused by storage. Among the two temperatures used for artificial aging, at 41°C, it was possible to compare the aging of seeds stored at three different temperatures. Aged seeds may show a decrease in germinability due to reactions such as a change in soluble carbohydrate and lipid peroxidation (Bernal-Lugo and Leopold, 1992; Narayana Murthy and Sun, 2000). These reactions differ depending on the seed moisture content and storage temperature and may also differ depending on the species (Murthy et al., 2003; Beardmore et al., 2008; Suriyong et al., 2015). Seeds stored at 25°C showed the lowest viability after artificial aging at 41°C, suggesting a rapid decline in the viability of seeds when stored at a high temperature. In contrast, the germination rate after artificial aging was higher in the seeds stored at 4°C than at −20°C, and contrary to our expectations, the longevity of seeds was higher when stored at 4°C compared to −20°C.
Interestingly, after storage, some aged seeds were viable in the TZ test but did not germinate when sown. It is presumed that the seeds are in a viable state but cannot germinate because of low germinability. Therefore, a gap may appear in the TZ test, which is a biochemical test method used to predict the germination rate of seeds and actual germination (radicle protrusion). Based on these results, we can conclude that aging was the slowest at 4°C among the three storage temperatures. Indeed, Pulsatilla seeds are considered orthodox (SID, 2022), and for orthodox seeds, temperatures between 0°C and 5°C are sufficient to maintain viability when stored for less than 18 months (De Vitis et al., 2020). However, while seeds have characteristic longevity such as orthodox, recalcitrant, and intermediate, their relative longevity may vary depending on habitat, seed condition, and storage conditions (Walters et al., 2005).
The storage conditions affect the longevity of the seeds, and among them, the storage temperature and humidity are important factors in determining the longevity of the seeds (Alhamdan et al., 2011; Mbofung et al., 2013). The P. dahurica seeds used in the present study showed the slowest aging when stored at 4°C and could maintain longevity for a long time. However, P. dahurica seeds can be stored in a relatively wide temperature range for a short-term storage of less than 1 year without affecting the germination rate. In addition, a change in longevity through storage for more than 1 year in our experiment is the predicted data obtained by an indirect method (SSAA), and additional verification is required through long-term monitoring during actual storage. The seed traits for dormancy and storage of P. dahurica identified in our study provide a guide for the conservation of resources and can be used as a reference for seedling production in the ornamental plant industry.