Introduction
Materials and Methods
Cannabis sativa Seeds
Seed Germination Assay
In vitro Development of Germinated Seedlings
Data Analysis
Results
Discussion
Introduction
Cannabis sativa L. has long been valued as a source of traditional medicine and fiber. Recently, C. sativa plants are attracting relatively more attention as they represent the most viable source of phytocannabinoids, specifically C21 terpenophenolic or C22 for carboxylated metabolites (Appendino et al., 2008; Andre et al., 2016). Cannabidiol (CBD) and ∆9-tetrahydrocannabinol (∆9-THC) are the two most abundant phytocannabinoids in C. sativa plants. In addition, different minor cannabinoids such as cannabinol (CBN), cannabigerol (CBG), cannabichromene (CBC), tetrahydrocannabivarin (THCV), cannabidivarin (CBDV), cannabinodiol (CBND), and cannabinidiol (CBDL) are present in C. sativa. They may also have some medical benefits. In humans, phytocannabinoids are known predominantly to target cannabinoid receptor types 1 (CB1) and 2 (CB2); however, recent studies have suggested that phytocannabinoids have multiple targets to exert various in vivo activities (Yeasmin and Choi, 2020). Increasing reports suggest that cannabinoids are promising tonic, analgesic, antipyretic, antiemetic, anti-inflammatory, anti-epileptic, and anticancer agents (Salami et al., 2020; Yeasmin and Choi, 2020). Thus, a growing number of researchers and others now see C. sativa as a significant source of phytocannabinoids that can be used to treat multiple diseases in humans (Salami et al., 2020).
In vitro plant culture systems play a key role in the development of disease-free rootstock and can be used for the quick multiplication of exotic plants as well as for genetic transformation and regeneration of commercially and medicinally important plants (Debnath et al., 2006; Altpeter et al., 2016; Gwon et al., 2019; Obsuwan et al., 2019; Vanegas-Espinoza et al., 2020; Adhikary et al., 2021; Kim et al., 2022). In addition, the culturing of plant cells and tissues is an important technique for producing secondary metabolites, examining internal phytohormone metabolism signaling, and generating polyploidy in many plants (Niazian, 2019). Tissue culturing (e.g., callus and cell culturing, de novo regeneration, and hairy root culturing) is not only the starting point of micro-propagation but is also important for producing designed biomolecules and secondary metabolites through synthetic biology approaches in C. sativa (Wróbel et al., 2018; Schachtsiek et al., 2018).
The primary step in a successful tissue culture experiment is the preparation of pathogen- or microorganism-free explants. Besides pathogenic microorganisms, plants and their seeds can have different microbial communities as endophytes without showing any disease symptoms or adversely affecting plants/seeds. Microbial contamination is a serious problem during in vitro tissue culturing due to the high nutrient availability in the almost universally used Murashige and Skoog basal medium or its variants (Cassells, 2012). Thus, a reliable sterile method during germination and in vitro healthy plantlet preparation are critical to ensure a successful tissue culture.
Recently, it has been reported that a 1% hydrogen peroxide (H2O2) solution can significantly enhance the germination rate of C. sativa seeds (Sorokin et al., 2021). H2O2 is not only a well-established antimicrobial agent (Weston, 2000) but is also a signaling molecule for many physiological, developmental, and stress-tolerance processes of plants (Wojtyla et al., 2016). H2O2 has strong oxidizing capacities (Ku et al., 2021). It can interact with most biomolecules, including nucleic acids, proteins, and lipids. Recent evidence has shown that the selective oxidation of proteins and mRNAs can act as a positive regulator of seed germination (Job et al., 2005; Oracz et al., 2007; Barba-Espín et al., 2011; Bazin et al., 2011). Verma et al. (2015) have postulated that H2O2 and ROS can facilitate oxidative modifications of storage proteins to increase the germination potential. In addition, H2O2 can interact with different phytohormones such as abscisic acid (ABA), zeatin-riboside (ZR), salicylic acid (SA), jasmonic acid (JA), and indole acetic acid (IAA) believed to play an important role in signaling processes during development and stress responses (Petrov and Van Breusegem, 2012; Diaz-Vivancos et al., 2013). Better germination performance accompanied by an exogenous H2O2 application can decrease the levels of ABA and ZR in germinated pea seedlings (Barba-Espín et al., 2010; El-Maarouf-Bouteau et al., 2015). Taken together, these findings indicate that H2O2 can be used not only as an antimicrobial agent but also as a signaling molecule within the phytohormonal network involved in the germination process.
Based on these two positive attributes of H2O2 (as a germination inducer and an antimicrobial agent) and results of a previous report by Sorokin et al. (2021), different concentrations of H2O2 as a liquid germination medium against four different cultivars of C. sativa plants, in this case Cheungsam, Cherry Blossom, Queen Dream, and Hot Blonde, were tested in this study. As expected, 1% H2O2 significantly enhanced the seed germination rate while also significantly and drastically reducing contamination rates of all tested C. sativa seeds. Our findings together with previous reports provide a solid foundation for the preparation of contamination-free C. sativa seedlings for multiple uses, including for tissue culture studies.
Materials and Methods
Cannabis sativa Seeds
Cannabis sativa L. seeds were purchased from blue forest farms (https://blueforestfarms.com/), specifically the Queen Dream, Cherry Blossom, and Hot Blonde varieties. Together with these three different CBD hemp cultivars, Cheungsam seeds were also used in this study. The Cheungsam variety was developed by the Rural Development Administration of the Republic of Korea.
Seed Germination Assay
Seeds were soaked overnight in different concentrations (0, 1, 3, 5, and 10% H2O2) of hydrogen peroxide solutions as liquid germination media. For the 0% H2O2 treatment, seeds were surface-sterilized with 70% ethanol for 3 min followed by 6% sodium hypochlorite for 5 min, after which the sterilized seeds were washed with distilled water. For the 1, 3, 5, and 10% H2O2 treatments, seeds were directly treated with the indicated concentrations of H2O2 solutions. With three experimental replicates, 20 seeds of the four different cultivars of C. sativa plants were submerged in different concentrations of H2O2 solutions at room temperature (RT) under a dark condition. The percentage of germinated seeds in each germination solution (appearance of the radicle was considered a germination event) was recorded. After one day, a fresh germination solution in each case was added after the removal of the old solution. Seeds were soaked in the same solution for three more days at room temperature (RT) again in the dark. The percentage of germinated seedlings was then recorded every day.
In vitro Development of Germinated Seedlings
Germinated seeds/seedlings were transferred with or without seed coats from the H2O2 solutions to Murashige and Skoog (MS) agar media to observe their growth. During the transfer, the germinated seeds/seedlings were poured together with the H2O2 solution from the tube into an empty Petri dish. These seedlings were then transferred to the MS media plates using sterilized forceps to remove excess H2O2 solution. Finally, these germinated seedlings/seeds were transferred to a growth chamber (24°C, 18 h light/6 h dark cycle, and light intensity of 200 µmol·m-2·sec-1) for two weeks to check for contamination and to confirm survival rates. For the preparation of the 1 L MS Media, 4.43 g of MS salt (including vitamins) and 20 g of sucrose were dissolved m in 1 L of double-distilled water by stirring using a magnetic stirrer. After adjusting the media pH to 5.7 with KOH, 8 g agar was added as a gelling agent. The media were then autoclaved at 121°C for 15 min.
Data Analysis
The mean seed germination percentage in different liquid germination media, the contamination rate, and the survival percentage on MS media were calculated. Data are shown as the mean ± standard error (SE). Experiments were repeated three times with similar results. In each replicate, 60 seeds were used. All statistical analyses were carried out using the R (ver. 4.2.1) and R studio (ver. 3, Build 554) programs (RStudio Team, 2020). A one-way analysis of variance (ANOVA) was used to determine significant differences at p < 0.05 between the samples. For each significant variable, differences between the means were detected using Tukey's Honest Significant Difference (HSD) test.
Results
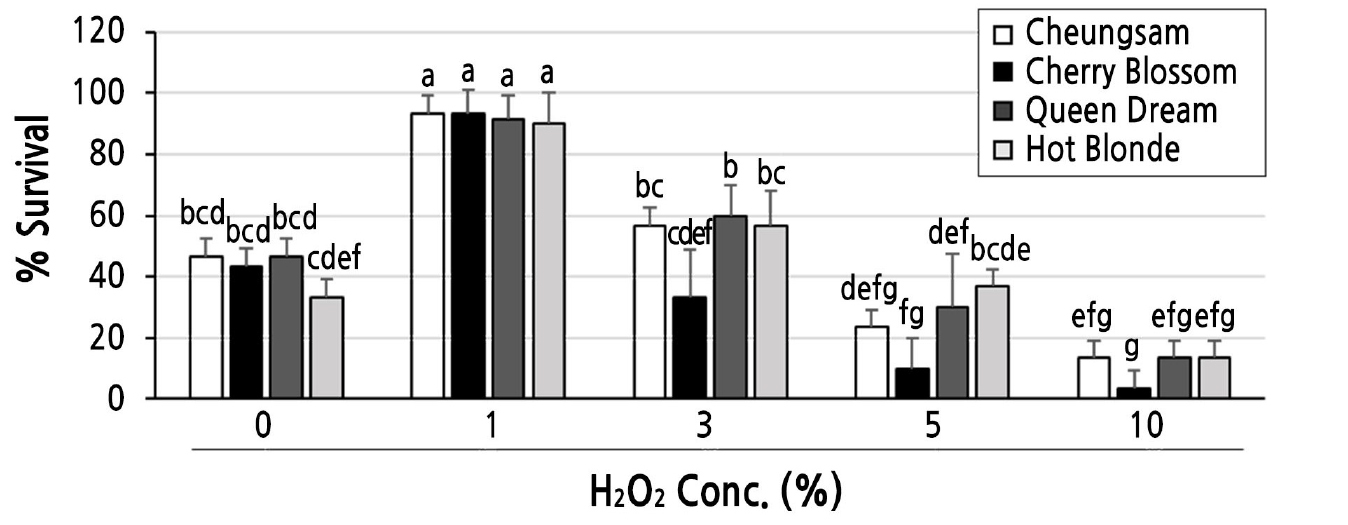
To examine the optimal concentration of H2O2 solution for seed germination of C. sativa plants, seeds were incubated with five different concentrations (0, 1, 3, 5, and 10%) of H2O2. As shown in Fig. 1, C. sativa seeds incubated with 1% H2O2 showed higher germination rates than the seeds in all other tested conditions, i.e., 0, 3, 5, or 10% H2O2. In the 0% H2O2-treated control group, seedlings appeared to be healthier than those in the 1, 3, 5, or 10% H2O2-treated cases, as they developed longer roots and green cotyledons at four days post germination (dpg) (Fig. 1). For the 1, 3, 5, and 10% H2O2-treated seeds/seedlings, shorter roots developed and pale green-colored cotyledons were observed at 4 dpg, suggesting that prolonged incubation with H2O2 may have toxic effects on C. sativa seeds/seedlings. Importantly, the 1% H2O2 treatment significantly enhanced the germination rates of all tested cultivars of C. sativa seeds (Fig. 2A-2D). At 1, 2, 3, and 4 dpg, the 1% H2O2-treated Cheungsam seeds showed germination rates of 85.0 ± 2.9, 91.7 ± 4.4, 96.7 ± 3.4, and 98.4 ± 1.7%, while the corresponding 0% H2O2-treated seeds showed germination rates of 30.0 ± 2.9, 70.0 ± 5.8, 83.4 ± 6.7, and 88.4 ± 4.4, respectively (Fig. 2A). Although the germination rates of the 1% H2O2-treated Cheungsam seeds were higher than those in the 0% H2O2-treated cases, these differences were not statistically significant at 2, 3, or 4 dpg. The germination rates of the 3, 5, and 10% H2O2-treated Cheungsam seeds were significantly lower than those of the 0% or 1% H2O2-treated seeds for up to four days, suggesting that a high concentration of H2O2 could inhibit Cheungsam seed germination. Similar patterns were observed for other C. sativa seeds, specifically those of the Cherry Blossom (Fig. 2B), Queen Dream (Fig. 2C), and Hot Blonde cultivars (Fig. 2D). Taken together, these results indicate that 1% H2O2 can enhance the germination rates of C. sativa seeds, although prolonged incubation can induce a toxic effect.
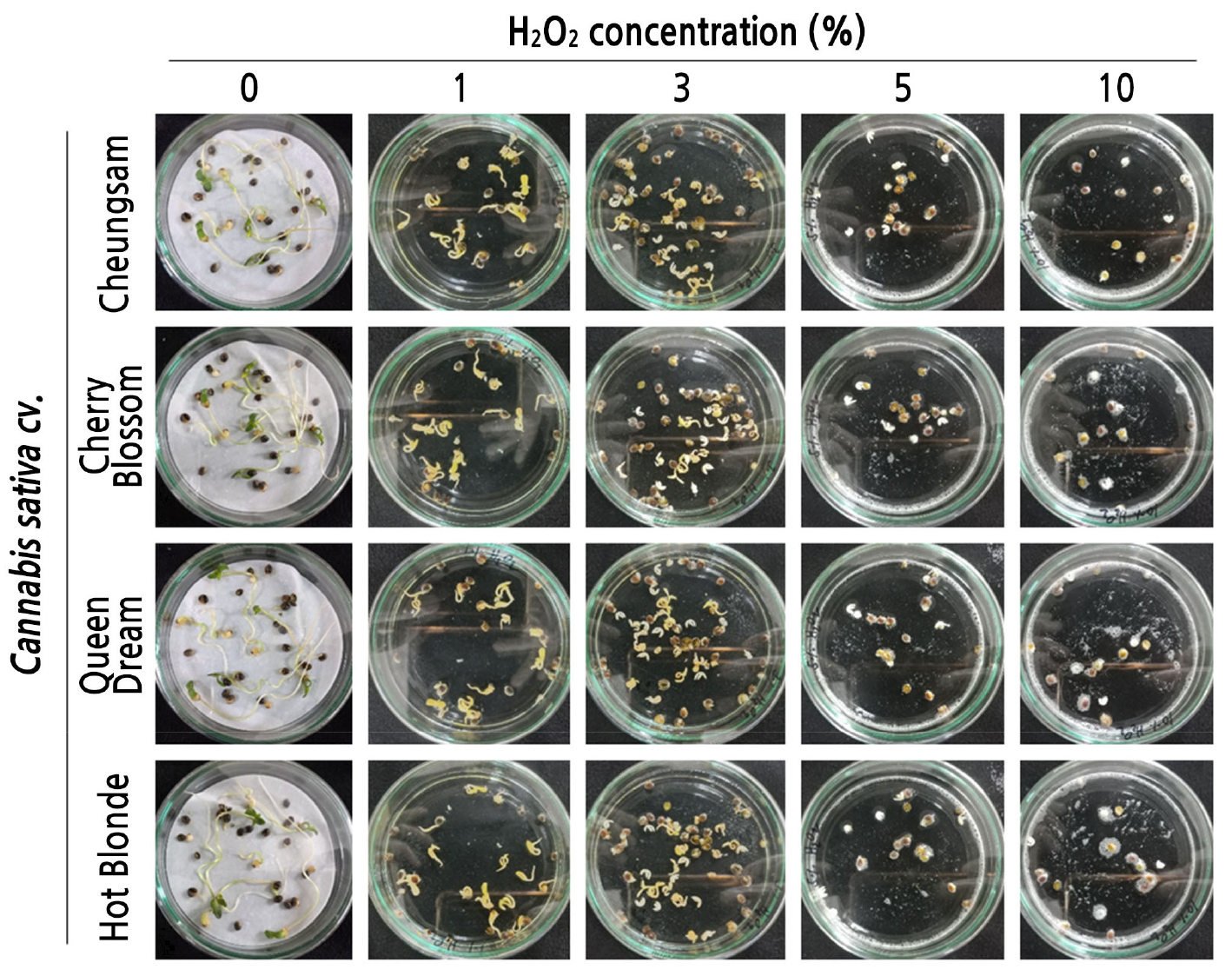
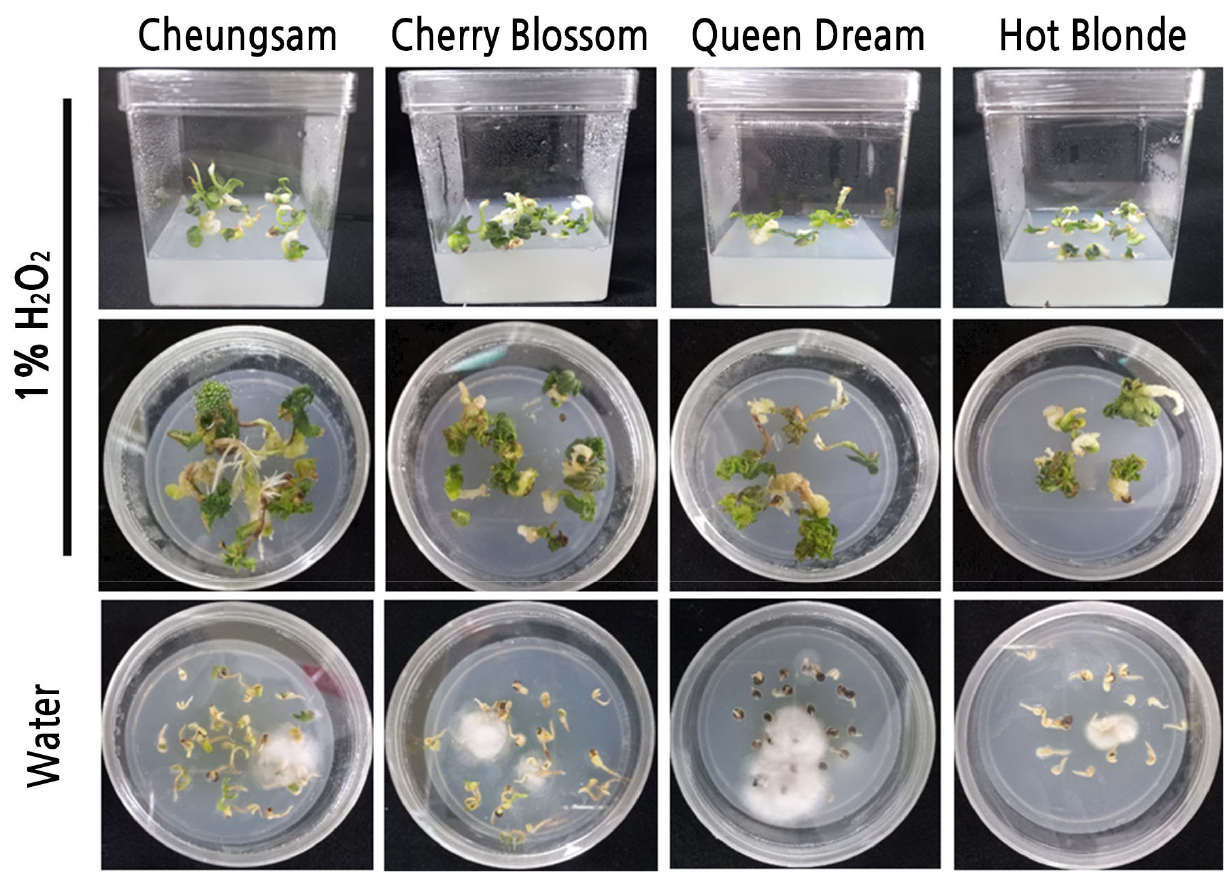
Fig. 1.
Effects of different concentrations of H2O2 on the germination rates of Cannabis sativa seeds. Representative pictures of seed germination of four different cultivars (Cheungsam, Cherry Blossom, Queen Dream, and Hot Blonde) of C. sativa in the presence of different concentrations (0, 1, 3, 5, and 10%) of H2O2.
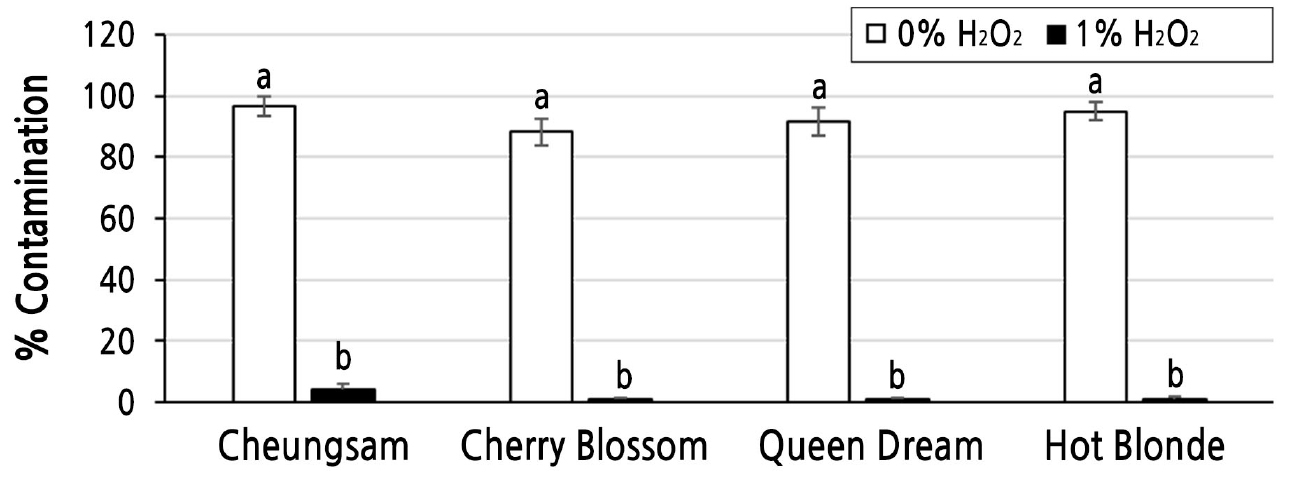
As 1% H2O2 showed the best effect on the germination of C. sativa seeds, we tested whether this condition could also reduce contamination rates during the aseptic growth of C. sativa seedlings on MS media. As shown in Fig. 3, all tested C. sativa seedlings prepared with 0% H2O2 as the germination media were heavily contaminated by unknown fungal and bacterial microorganisms, while almost no contamination was observed with those treated with 1% H2O2. The contamination rates of the Cheungsam, Cherry Blossom, Queen Dream, and Hot Blonde seedlings prepared with 0% H2O2 as the germination media were 96.7 ± 3.4, 88.4 ± 4.4, 91.7 ± 4.4, and 95.0 ± 2.9, respectively (Fig. 4). To assess the survival rates further, C. sativa seeds of the four different cultivars were incubated at five different concentrations (0, 1, 3, 5, and 10%) of H2O2 and then transferred to fresh MS media as soon as the seeds germinated. As shown in Fig. 5, all tested C. sativa seeds showed the highest survival rate when treated with 1% H2O2. Although the 0% H2O2-treated seeds showed germination rates comparable to those of the 1% H2O2-treated seeds, their survival rates were significantly lower because severe contamination resulted in the death of some of the seedlings. Considering all available evidence from this study and from previous reports together, 1% H2O2 is indeed suitable as liquid germination media for C. sativa seeds. It not only enhances germination and survival rates but also reduces contamination rates.
Discussion
Recently, C. sativa plants have attracted increasing levels of attention due to their multipurpose utility, including a number of medicinal uses (Debnath et al., 2006; Altpeter et al., 2016; Niazian, 2019; Hall et al., 2019). Biotechnological studies such as those focusing on plant tissue cultures may find additional feasible uses for C. sativa plants. For example, gene editing using CRISPR-Cas9 enables the selective expression of cannabinoid biosynthesis genes, which can make it possible to produce specific sets of cannabinoids without psychotropic THC or with enriched levels of minor cannabinoids such as CBN, CBG, CBC, CBDV, CBND, and CBDL (Adhikary et al., 2021). The tissue culturing technique enables the aseptic propagation and generation of disease-free root stock with the safe production of useful cannabinoids and other secondary metabolites form C. sativa plants. In our experiments, 1% H2O2 acts best as liquid germination media, as it significantly enhances germination (Figs. 1 and 2) and survival rates (Fig. 5) while also reducing contamination rates (Figs. 3 and 4). Thus, our studies will provide the first step for those seeking a successful tissue culture technique of C. sativa plants, thus enabling aseptic propagation, the generation of disease-free root stock, and the genetic manipulation of C. sativa plants in further studies.
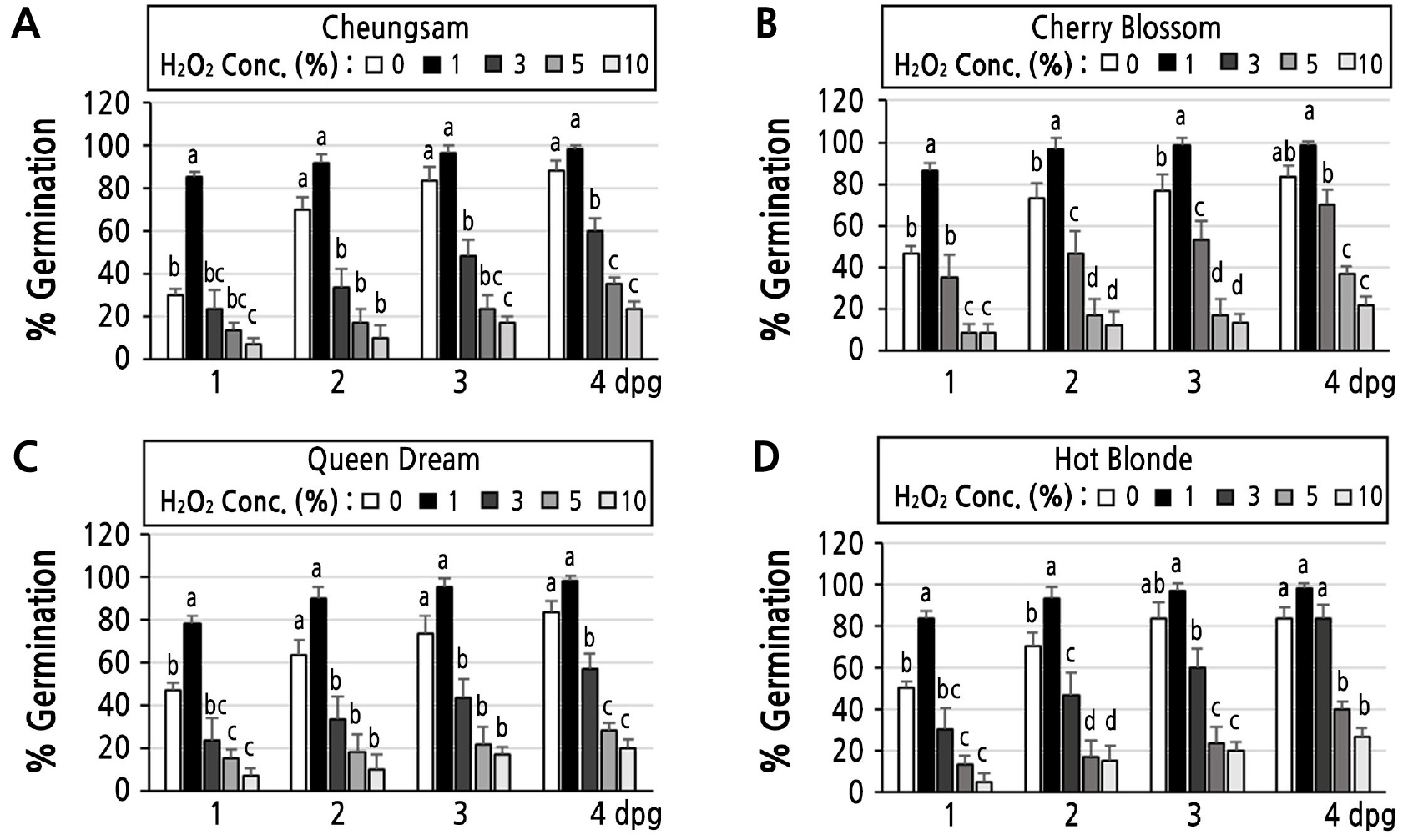
Fig. 2.
Effects of different concentrations of H2O2 on the germination rates of Cannabis sativa seeds: (A-D) Germination rates of Cheungsam (A), Cherry Blossom (B), Queen Dream (C), and Hot Blonde (D) seedlings treated with different concentrations (0, 1, 3, 5, and 10%) of H2O2 at 1, 2, 3, and 4 days post germination (dpg). Different letters represent groups with significant differences as determined by Tukey's HSD tests at p < 0.05 with a one-way ANOVA.
For biotechnological studies of C. sativa plants such as plant tissue culturing studies, in vitro aseptic seed germination is very important. However, this is a highly challenging procedure, as C. sativa seeds have a very thick coat, which can decrease their germination rates. In addition, seed germination can easily become contaminated by various microorganisms (Pepe et al., 2021). Thus, it is crucial to know how to enhance germination rates and reduce contamination as caused by different microorganisms. In this study, we tested the germination and contamination rates of four different varieties of C. sativa plants and found that 1% H2O2 can be used as a liquid germination medium for aseptic in vitro germination, consistent with previous findings (Sorokin et al. 2021).
Although a higher disinfectant concentration can reduce contamination rates during the disinfection process, it can also reduce seed viability (Barampuram et al., 2014; Uhl et al., 2015; Mahaseth and Kuzminov, 2017; Hesami et al., 2019; Cuba-Díaz et al., 2020). As expected, H2O2 at concentrations higher than 1% reduced both germination (Figs. 1 and 2) and survival (Fig. 5) rates of C. sativa seeds despite its disinfection effect. This issue must be carefully addressed to avoid any adverse effects when an H2O2 solution is used as liquid germination media. H2O2 is a toxic reactive molecule. Thus, it is challenging clearly to distinguish between its beneficial (signaling) and deleterious (causing damage as a disinfectant) roles. Two distinct mechanisms for H2O2-driven seed germination have been proposed (Barba-Espín et al., 2011; Barba-Espín et al., 2012). First, exogenous H2O2 can induce a MAPK-signaling-dependent decrease in the ABA content in seeds. Second, H2O2 has direct or indirect negative effects on ABA transport from the cotyledon to the embryonic axis, resulting in a decrease of the ABA content in seeds (Wojtyla et al., 2016; Černý et al., 2018). However, little is known about ABA signaling in C. sativa plants and/or seeds. Thus, it will be interesting in the future to study whether the H2O2-induced increase of seed germination indeed depends on altered ABA signaling.
In this study, it was confirmed that 1% H2O2 can be used as a successful liquid germination medium, as previously reported. Four different cultivars of C. sativa seeds were tested for their germination, contamination, and survival rates in the presence of different concentrations of H2O2. This study provides information about the effective preparation aseptic C. sativa plants for further biotechnological studies on such topics as tissue culturing, cell suspension culturing, hairy root culturing, and other types.