Introduction
Materials and Methods
Plant Material and Environmental Conditions
Experimental Treatments
Measurements of Seedling Growth and Morphology
Physical Properties of the Root Medium
Data Collection and Statistical Analysis
Results and Discussion
Bulk Density and Porosity as Affected by Cell Height and Diameter
Growth of Ginseng Seedlings as Affected by Cell Height and Cell Diameter
Conclusions
Introduction
Ginseng cultivation has been conducted mainly in the field and is associated with problems such as exposure to soil-borne diseases, shortage of the preparation field, and low labor efficiency (Kim et al., 1990; Suh et al., 2018). Direct seeding, a conventional method, has been preferred over transplanting mainly due to lower labor costs (Li et al., 2010). To solve some of these problems, research on ginseng seedling production using an efficient transplant production system that is well developed in the horticultural industry should be conducted.
Plug seedling production systems are well developed, especially for ornamental plants and vegetables (Nelson, 1991; Hartmann and Kester, 1963). In these types of systems, only a small rooting volume is required per plant to produce transplants with good quality in a short period. However, the method has not been fully developed for application to ginseng seedlings with a long taproot. A few studies have reported the effects of seeding depth (Proctor and Sullivan, 2013), planting density (Mo et al., 2015), media composition (Park et al., 2014), and nutrient solution concentration (Park et al., 2020) on seedling growth. Container design is one of the most crucial factors in plug seedling production systems as it determines the morphological and physiological characteristics of the seedlings, especially their root systems (Aphalo and Rikala, 2003; Dominguez-Lerena et al., 2006). However, no plug tray has been developed for ginseng seedling production, and the growth of ginseng in cultivation trays or vessels has seldom been studied.
Container size is the first thing to consider for producing transplants of good quality in an optimized space (Styer and Koranski, 1997). Cell diameter, one of the cell dimensions, determines planting density and number of cells per unit area (Vavrina et al., 1993). It also determines the amount of growing media or root volume with cell height, which affects the water content of media and amounts of water and nutrients absorbed by plants. Determining the cell dimensions is important in seedling production using plug trays because it is directly related to production cost (Dufault and Waters, 1985; Marsh and Paul, 1988). In addition, the cultivation period and root system should be considered to determine the container dimensions most suitable for production (Styer and Koranski, 1997; Gallegos et al., 2020). Ginseng seedlings, which have the characteristics of a long cultivation time and a deep, vertical tap root system with a small number of lateral roots, have some specific requirements regarding container dimensions. Therefore, to achieve the optimal growth of ginseng seedlings, especially that of their tap roots, it is necessary to develop a new type of cultivation container for ginseng seedling production systems.
In this study, we investigated the effects of cell height and diameter on the growth and morphology of ginseng seedlings using polyvinyl chloride (PVC) pipes in a plant factory with artificial light to determine the optimal cell dimensions and develop a cultivation vessel prototype for ginseng seedling production.
Materials and Methods
Plant Material and Environmental Conditions
Stratified ginseng seeds (Panax ginseng CA Meyer ‘Chunpoong’) were sown at a depth of 30 mm into cylindrical PVC pipes filled with peat moss and perlite (7:3, v/v) growing media (Golden Root, Nongkyung Co., Ltd., Korea) and placed in a plant factory with artificial light (PFAL). The plants were cultivated under a light intensity of 130 µmol·m-2·s-1 using mint-white LED lamps (T5, Parlux, Incheon, Korea) with a 10-h photoperiod under an average air temperature of 23.7/22.1°C (light-/dark period) and a relative humidity of 67.1 ± 7.2%. After confirming that all sets of true leaves had fully unfolded, the seedlings were fertigated by an ebb-and-flow subirrigation (EFS) method using a nutrient solution for ginseng (NIHHS, 2015) comprising NO3-N 12.0, NH4-N 1.0, H2PO4-P 0.6, K 7.6, Ca 4.0, Mg 2.0, SO4-S 2.0 me·L-1, Fe-EDTA 0.6, Mn 0.5, B 0.5, Cu 0.02, Mo 0.03, and Zn 0.05 mg·L-1 (pH 6.5, EC 1.0). The nutrient solution was diluted to EC 0.85 for use in the experiments. With an irrigation interval of 12 days, the water level reached to two-thirds of each cell height, was maintained for 20 minutes, and then drained.
Experimental Treatments
Some plants were sown and grown for 20 weeks in PVC pipes of four different heights (150, 200, 250, and 300 mm) with the same diameter of 50 mm corresponding to total cell volumes of 0.294, 0.393, 0.491, and 0.589 L. The treatments were denoted H150, H200, H250, and H300, corresponding to the cell heights of 150, 200, 250, and 300 mm, respectively. Other plants were grown for 20 weeks in PVC pipes of five different diameters (15, 20, 30, 50, and 75 mm) with the same height of 200 mm, yielding total cell volumes of 0.032, 0.0628, 0.141, 0.393, and 0.883 L. The treatments were denoted D15, D20, D30, D50, and D75, corresponding to cell diameters of 15, 20, 30, 50, and 75 mm, respectively.
Measurements of Seedling Growth and Morphology
Shoot length, root length, root diameter, fresh and dry weights of shoot and root, and leaf area of the 10 ginseng seedlings for each treatment were measured at 20 weeks after sowing. Root length was measured as the distance from the rhizome to the end of the tap root, and root diameter was measured at the thickest part of the tap root. The total leaf area of each plant was measured with a leaf area meter (Li-3100; LI-COR, Lincoln, NE, USA). Dry weights were measured after drying at 80°C for 3 days.
Physical Properties of the Root Medium
After harvest, four pipes from each treatment were used to measure the bulk density and porosity of the root medium according to the method suggested by NIAST (2000).
Data Collection and Statistical Analysis
The experiment was arranged as a completely randomized block design with three replications. The Statistical Analysis System (SAS) for Windows version 9.4 (SAS Institute Inc., Cary, NC, USA) was used to perform Duncan’s multiple range tests, with differences among treatments evaluated at p < 0.05. Regression analyses were performed with hyperbola equation [y = ax / (b + x)] using Sigma Plot (version 10.0; Systat Software, Chicago, IL, USA).
Results and Discussion
Bulk Density and Porosity as Affected by Cell Height and Diameter
The bulk density was highest in H300 and decreased as cell height decreased. Accordingly, the porosity was lowest in H300 and increased with decreased cell height (Table 1). On the other hand, no significant differences in bulk density and porosity were found among the cell diameter treatments (Table 2). These results demonstrate the compaction of the growing medium by weight, which increased with the height of the medium column. Bulk density and porosity affect a variety of soil characteristics, including water capacity and availability, aeration, and root penetrability, all of which can significantly impact root growth. Corti et al. (1998) reported that a high bulk density had the negative effect of reducing porosity and consequently reducing the air available for root respiration. However, the distribution of bulk density and porosity within the container should also be considered, especially under different cell heights. Milks et al. (1989) reported a water content gradient along the height of a 170-mm-tall pot, with water content ranging between 32% at the top and 59% at the bottom. As a result of the water content gradient, the air-filled porosity of the soil near the surface of a container is much higher than that at the bottom, and the difference over a 160-mm height can range from 4% to 28% depending on the type of soil (Evans et al., 2009). In this study, we watered with an interval of 12 days using EFS, which was a much longer interval than that for most vegetable crops. As the watering interval increases, the water content gradient of media between the surface and the bottom of media may become greater. Furthermore, changes in physical properties of media by gravity compression may also affect the capillary rise within the container. Therefore, the cell dimensions of plug trays should be considered along with growing medium to provide high efficiency of capillary rise and adequate water-holding capacity (Evans et al., 1992).
Table 1.
Bulk density and porosity of the growing media at 20 weeks after sowing in each cell-height treatment
|
Treatment code |
Bulk density (mg·L-1) |
Porosity (%) |
| H150 | 0.148 abz | 91.8 ab |
| H200 | 0.127 b | 93.0 a |
| H250 | 0.157 ab | 91.3 ab |
| H300 | 0.179 a | 90.1 b |
Table 2.
Bulk density and porosity of the growing medium at 20 weeks after sowing in each cell-diameter treatment
|
Treatment code |
Bulk density (mg·L-1) |
Porosity (%) |
| D15 | 0.200 az | 89.0 a |
| D20 | 0.192 a | 89.4 a |
| D30 | 0.172 a | 90.5 a |
| D50 | 0.185 a | 89.8 a |
| D75 | 0.179 a | 90.1 a |
Mineral soils with organic matter are generally reported to exhibit approximately 39.5 to 77.6% porosity (Clapp and Hornberger, 1978). In contrast, a peat-moss-based root medium has 85 to 93% porosity (Blom, 1983; Fonteno, 1988). In this study, the commercial medium was peat moss-based, with a perlite composition of 30%, and the porosity range was 89 to 93% (Tables 1 and 2). Wisdom et al. (2017) reported that a porosity of 50–80% per volume in container cultivation of crops is ideal for maintaining an oxygen level above 12%. In a previous study of ginseng cultivation, seedlings grown in soilless root media of 80.65% porosity, 13.14% air-filled porosity, and 0.115 mg·L-1 bulk density resulted in the optimal growth of seedlings (Choi et al., 2011).
Growth of Ginseng Seedlings as Affected by Cell Height and Cell Diameter
An increase in cell height promoted the root growth, including root length, diameter, and fresh and dry weights, of ginseng seedlings. Root length and fresh and dry weights were highly correlated with cell height, but root diameter showed no significant differences among the cell-height treatments (Fig. 1). In shoot growth, leaf area tended to increase with increasing cell height, but other parameters, such as shoot length, shoot fresh and dry weights showed no significant differences among the cell-height treatments (Table 3). In various woody plants, tap root and shoot growths have been found to increase with container height (Pemán et al., 2006; Chirino et al., 2008; Gallegos et al., 2020).
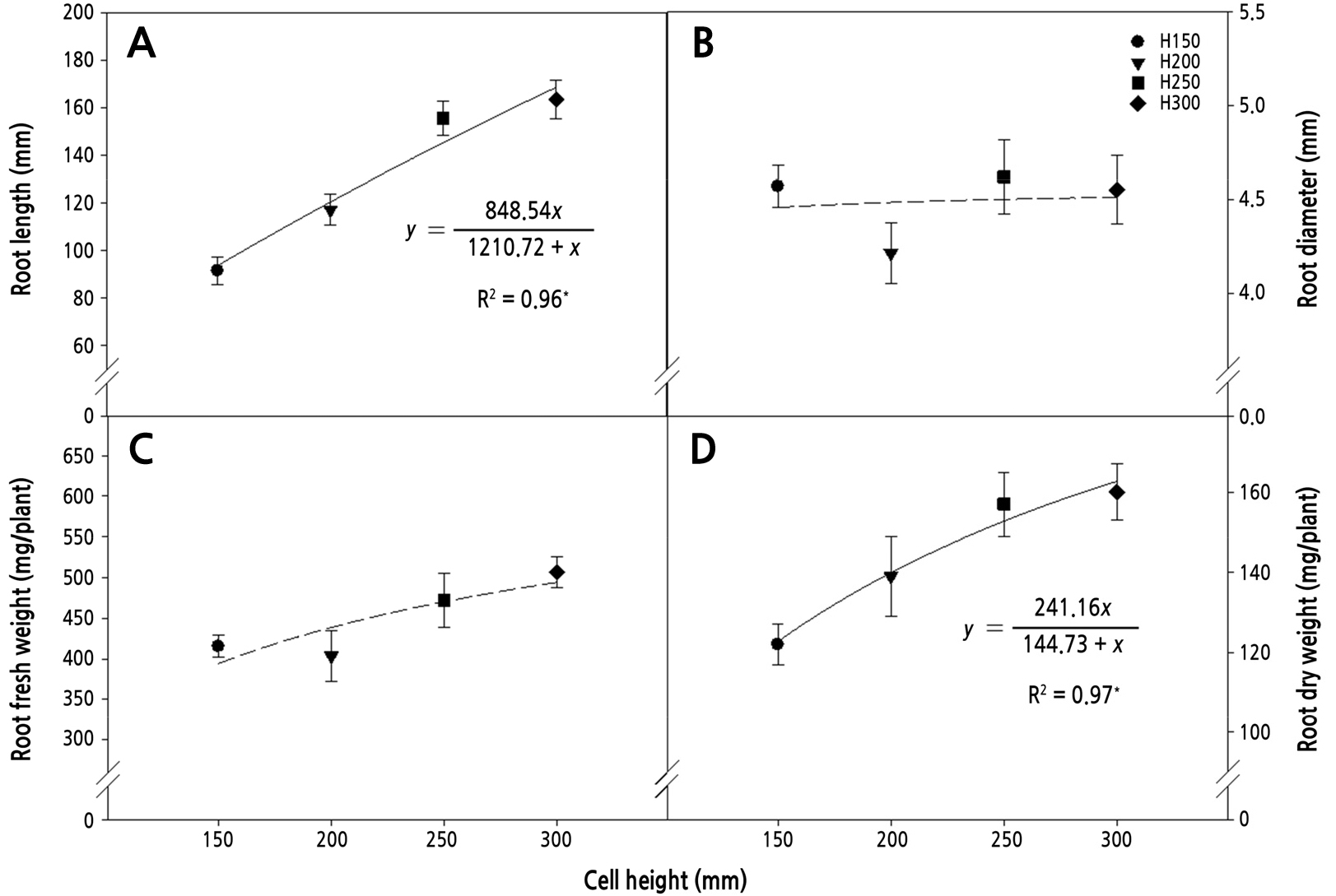
Fig. 1.
Relationships between cell height and root length (A), root diameter (B), root fresh weight (C), and root dry weight (D). Bars represent standard deviation (n = 10). Equations of the regression curve and R2 coefficients are presented only when the correlation was statistically significant (solid line) but not when the correlation was not significant (dashed line). * indicates significance at p < 0.05.
Table 3.
Shoot length, leaf area, and shoot fresh and dry weights of ginseng seedlings at 20 weeks after sowing in each cell-height treatment
|
Treatment code |
Shoot length (mm) |
Leaf area (cm2) |
Shoot fresh weight (mg/plant) |
Shoot dry weight (mg/plant) |
| H150 | 59.5 az | 8.28 b | 182.1 a | 30.0 b |
| H200 | 64.3 a | 9.48 ab | 184.3 a | 34.9 a |
| H250 | 62.4 a | 10.60 a | 196.1 a | 35.9 a |
| H300 | 61.0 a | 10.43 a | 199.3 a | 35.3 a |
The bulk density of the root media increased as cell height increased due to the compaction of the soil at the bottom of the cell. When roots have limited space to expand in a container that restricts their growth, they compete for available oxygen (Peterson et al., 1991). In previous studies of seedling production, the roots of barley (Goss, 1977) and broccoli (Montagu et al., 2001) showed compensatory growth in soil in which the main axes were mechanically compacted. Furthermore, the root growth of ginseng seedlings has been found to show a decreasing trend with increasing soil compaction (Roy et al., 2008), consistent with the finding of decreased yield in plants grown under low soil porosity (Lee et al., 1995).
Cell diameter greatly affected the shoot (Table 4) and root growth (Fig. 2) of ginseng seedlings. Root length, diameter, and fresh and dry weights improved as cell diameter increased. The effects of an increase in cell diameter were strongest between 15 and 30 mm, but further increases had only small effects on root length, diameter, and fresh and dry weights. As cell diameter decreased, the restriction of tap root growth became evident, and the lateral roots might have had to compete for space in cell. These results are in line with the hypothesis that decreases in shoot length, leaf area, and shoot fresh and dry weights occur as cell diameter decreases (Table 4).
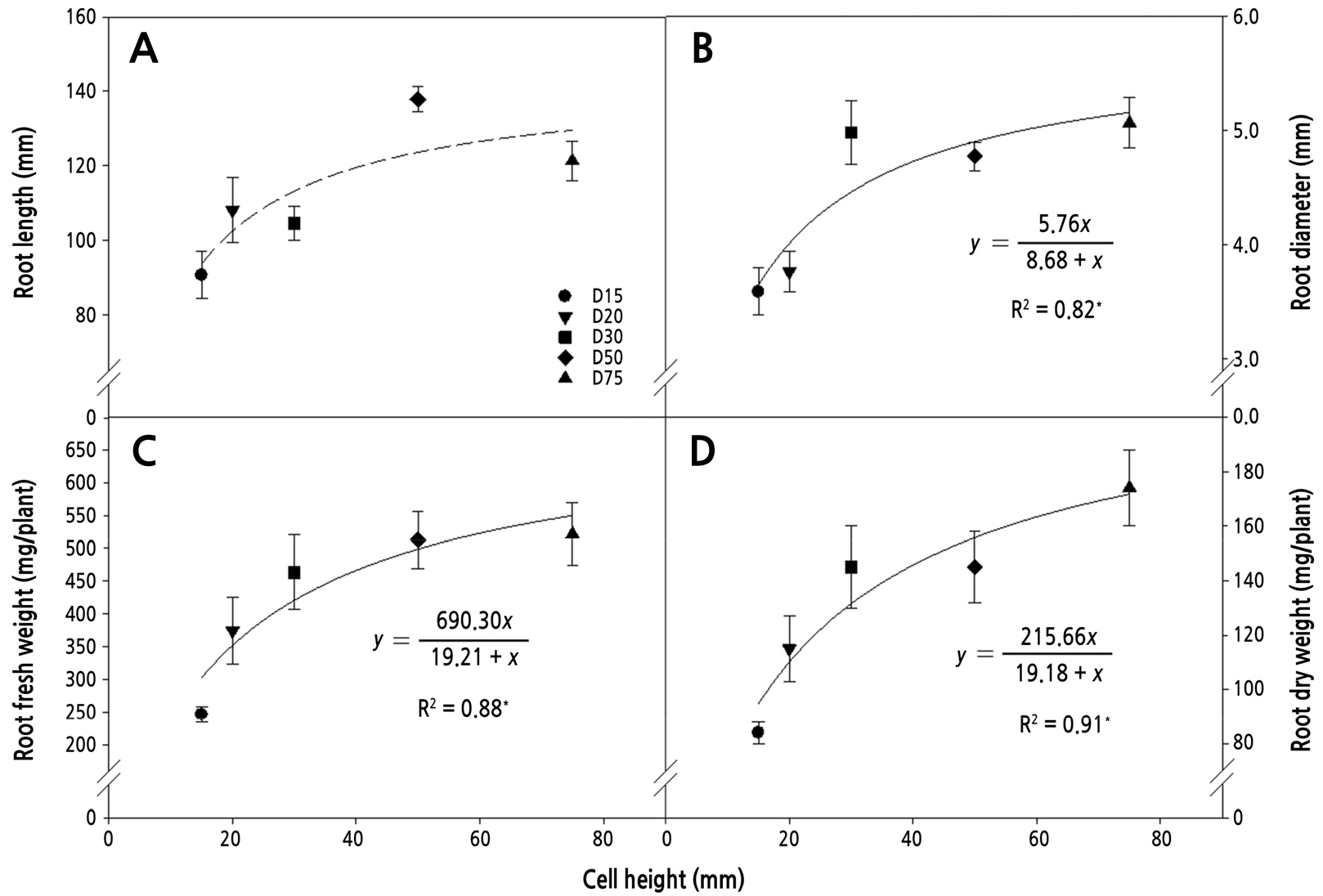
Fig. 2.
Relationships between cell diameter and root length (A), root diameter (B), root fresh weight (C), and root dry weight (D). Bars represent standard deviation (n = 10). Equations of the regression curve and R2 coefficients are presented only when the correlation was statistically significant (solid line) but not when the correlation was not significant (dashed line). * indicates significance at p < 0.05.
Table 4.
Shoot length, leaf area, and shoot fresh and dry weights of ginseng seedlings at 20 weeks after sowing in each cell-diameter treatment
Ginseng seedlings grown in the deeper and wider cells had higher root growth and weight, leaf area, and shoot growth than those grown in smaller cells. In general, as container size increases, plant leaf area, shoot fresh weight, and root fresh weight increase (Cantliffe, 1993). Larger and longer roots allow for faster and deeper root system development after transplanting, which can lead to rapid plant growth (Grossnickle, 2005). For this reason, the initial size of a seedling may have important consequences for the ultimate size of the adult plant (Roach, 1987). However, in seedling production, the use of small containers, allowing the production of more plants in less production space, may be beneficial (Vavrina et al., 1993). Therefore, to reduce the cost of seedling production, it is necessary to identify the optimal container size that allows the production of large seedlings for uniform root yields. Our results showed that the cell diameter of 30 mm was the most efficient in terms of promoting root and shoot growth and that further increases in cell diameter (up to 75 mm) provided little benefit (Fig. 2). Therefore, the cell diameter of 30 mm with the cell height of 200 mm can be considered to be the most suitable cell dimension for growing ginseng seedlings.
However, for the practical application, cell dimension also affects other aspects of the EFS system, such as irrigation efficiency and the weight of the irrigation water. As the cell height increases, the water level increases for uniform water distribution of the growing media, resulting in increased nutrient solution runoff and consequently decreasing irrigation efficiency. In addition, as the amount of water increases, the weight of the irrigation water increases, which can lead to an overweight EFS system. Therefore, our results showed optimal cell dimensions for growing ginseng seedlings, but for application, growers should consider other practical aspects and refer to the regression curve between the growth and cell dimensions (Figs. 1 and 2) to select the cell size that offers acceptable growth with reasonable cost.
Conclusions
In this study, cell height and diameter affected the shoot and root growth of ginseng seedlings. Root growth increased with increasing cell height, and root fresh weight and shoot growth increased linearly with increasing cell height. Furthermore, root and shoot growth improved as cell diameter increased. However, increases in cell diameter to beyond 30 mm had minor effects on seedling growth. We identified the proper cell height (200 mm) and diameter (30 mm) suitable for cultivation of ginseng seedlings. We propose a novel prototype of a plug tray with a root volume of 141.37 mL that can produce ginseng seedlings with a planting density of at least 1,156 seedlings/m2 (9 cm2/plants). The results of this study may improve space utilization in greenhouse cultivation and plant factories, allowing the planting density of ginseng seedlings to be optimized, thereby reducing cost. Thus, the results can lead to cost and labor savings in ginseng seedling production.


