Introduction
Materials and Methods
Plant Materials and Growth Conditions
Photoperiod and Transfer Treatments
Data Collection and Statistical Analysis
Results
Discussion
Introduction
There are approximately 40 species of Elsholtzia in the family Lamiaceae, which are widely distributed in East Asia, Africa, North America, and Europe. At least 33 species of the genus are distributed in China and Korea. The most aromatic Elsholtzia plants are used for herbal tea, food, perfumeries, and aromatherapies. In folk medicine, the genus Elsholtzia has long been used for the treatment of colds, headache, fever, digestion disorders, and other conditions (Liu et al., 2007; Guo et al., 2012).
Elsholtzia angustifolia (Loes.) Kitag. (narrow-leaf mint) is a Korean native annual plant that has been used medicinally for essential oil components (Korea Biodiversity Information System, 2020). Several phytochemical investigations isolated the major chemical constituents, e.g., flavonoids, in essential oil from Elsholtzia and identified the chemical composition of E. angustifolia essential oil (Guo et al., 2012; Kwon et al., 2017).
E. angustifolia has recently been used as a potted plant due to its ornamental value for its fragrant purple flowers. In 2018, the value of wildflower production in Korea increased by 13% compared with that in the previous year (KFS, 2019). In Korea, research on wild flowers has been actively performed to develop new ornamental crops and meet the new demands in the flower market. The flowering season of E. angustifolia is generally known as autumn, from September to October. However, there are no studies on flowering and environmental factors in the floral induction of this wild species.
For flowering, many herbaceous plants require a cold treatment (vernalization), an inductive photoperiod, or both (Heins et al., 1997). Vernalization is a cold treatment that is required for floral initiation, and many species generally require several weeks of cold (Lang, 1965). E. angustifolia is a monocarpic annual plant that germinates in spring and blooms in autumn when the daylength is shorter than the nighttime. Although some short-day plants (SDPs) need vernalization, cold treatment is usually required for flowering of long-day (LD) perennial plants that overwinter and bloom from spring to early summer (Ha, 2014).
Other studies on the genus Elsholtzia showed that flowering of E. ciliate and E. splendens could be induced by a short-day (SD) photoperiod without vernalization (Chang et al., 2003; Sohn and Kim, 2003). According to Sohn and Kim (2003), E. ciliate and E. splendens can be classified as SDPs. Chang et al. (2003) reported that 16 h of photoperiod suppressed floral induction of E. splendens ‘Jahyang’, whereas there was 100% flowering under a 9-h photoperiod. These obligate responses of the two Elsholtzia species to SDs suggest that E. angustifolia may show the same responses. Therefore, the objectives of this study were to 1) determine whether E. angustifolia is also an obligate SDP and 2) evaluate the influence of transfer date from LD or night interruption (NI) conditions to SD conditions on vegetative growth and flowering.
Materials and Methods
Plant Materials and Growth Conditions
Seeds of Elsholtzia angustifolia were sown in 105-cell plug trays filled with commercial peat-perlite medium (Baroker; Seoul Bio Co., Ltd., Eumseong, Korea) on 26 April 2019. Seedlings were grown in a walk-in chamber at Seoul National University Farm (Suwon, Korea; 37°27'N, 126°99'E). The temperature and relative humidity were 22°C and 60%, respectively.
Seed germination took an average of 14 days. At 6 weeks after sowing, plants were transplanted into 10-cm plastic pots filled with the same commercial medium as above. After transplanting, the base light intensity was kept at 250 µmol·m-2·s-1with white light-emitting diodes (LEDs) for 9h. Plants were irrigated three times a week and fertigated once every two weeks with water-soluble fertilizer (EC 0.8 dS·m-1; HYPONeX professional 20N-20P-20K; HYPONeX Japan Co., Ltd., Osaka, Japan).
Photoperiod and Transfer Treatments
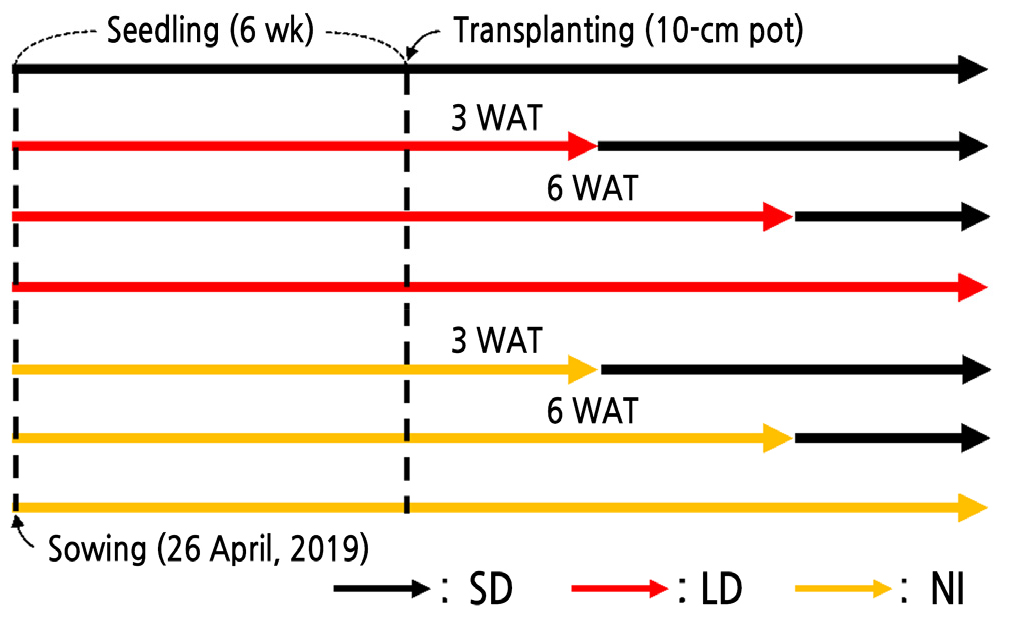
The basic photoperiod was 9/15 h (day/night), and the base light intensity over 9 h was 150 µmol·m-2·s-1 with cool white LEDs (LEDT5-9015-DHE; FOCUS Lighting Co., Ltd., Bucheon, Korea). The photoperiod treatment was immediately applied after sowing. Plants were grouped for each treatment and were assigned randomly to benches. Treatments included short day (SD, 9/15 h), long day (LD, 16/8 h), and night interruption (NI, 4 h around midnight). Plants under continuous SD conditions only received the base light intensity for 9 h, and supplemental lighting was applied for the LD or NI treatments. The light intensity for the LD and NI treatments was 4 µmol·m-2·s-1 cool white LEDs (LEDT5-9015-DHE; FOCUS Lighting Co., Ltd., Bucheon, Korea). Additionally, each LD and NI treatment was divided into three groups: continuous LD or NI, transfer from LD or NI to SD at 3 weeks after transplanting (WAT), or at 6 WAT (Fig. 1).
Data Collection and Statistical Analysis
Over 50 observations for each treatment were used. All vegetative growth parameters, such as the number of leaves and branches, were recorded when plants developed visible floral buds. Plants that did not flower to the end of this experiment and continued in vegetative growth under continuous LD or NI conditions were considered non-flowering and were excluded. Days to the first open flower were counted from the start of the SD treatment, but, in the continuous SD treatment, it was calculated based on the germination time. Data were analyzed using SAS (Version 9.4; SAS Institute, Inc., Cary, NC, USA), and analyses of variance (ANOVA) and mean separation by Duncan’s multiple range test (p < 0.05) were performed for all data. Non-flowering plants were excluded from the analyses.
Results
At 5 weeks after sowing, the first floral bud was observed under the continuous SD condition. Most plants under the SD treatment began developing floral buds during the seedling stage, and the average number of days to the first open flower was 44.8 days (Table 1 and Fig. 2). Plants under the continuous SD condition developed 100% floral buds after 6 weeks and showed 100% flowering at 9 weeks after sowing. Conversely, plants under continuous LD and NI conditions did not show floral bud initiation and continued vegetative growth (Fig. 2). These plants never flowered during this experiment (Fig. 3).
Table 1.
Effects of photoperiod treatments on percent flowering, days to the first open flower, and the number of leaves and branches
| Treatment |
Flowering (%) |
Total days to first open flower |
Days to first open flowerz |
Number of inflorescences |
Number of leaves |
Number of branches |
| Continuous SD | 100 | 44.8 cy | 44.8 a | 16.0 c | 39.7 c | 11.4 d |
| Transfer at 3 WATx | ||||||
| LD to SD | 100 | 56.8 b | 35.8 b | 70.5 b | 522.8 b | 107.0 bc |
| NI to SD | 100 | 56.9 b | 35.9 b | 81.0 b | 561.9 b | 96.7 c |
| Transfer at 6 WAT | ||||||
| LD to SD | 100 | 78.5 a | 36.5 b | 129.4 a | 1095.6 a | 148.1 a |
| NI to SD | 100 | 78.7 a | 36.7 b | 138.2 a | 1036.6 a | 122.9 b |
| Significance | ||||||
| Photoperiodw | – | NS | NS | NS | NS | ⁎ |
| Transfer datev | – | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ | ⁎⁎⁎ |

Fig. 3.
Growth and flowering of Elsholtzia angustifolia. Each photo was taken on 13 August (A) and 27 August (B), 2019. NI, LD: continuous night interruption or long day; NI→SD, LD→SD: transfer treatments from NI to short day or LD to SD conditions. Potted plants were transferred to the SD condition at 3 or 6 weeks after transplanting (WAT).
The plants that were transferred from the LD or NI condition to the SD condition also developed floral buds and showed 100% flowering (Table 1 and Fig. 3). The average number of days to the first open flower of all transfer treatments (from LD or NI to SD) was approximately 36 days, and the time to flowering was significantly (p < 0.001) shorter than that of the continuous SD treatment. However, there was no significant difference between transfer dates (WAT to SD treatment) (Table 1). In addition, although the difference in growth among treatments did not appear in Fig. 3, the numbers of inflorescences and leaves were significantly (p < 0.001) higher at 6 WAT than at 3 WAT. For the number of branches, the transfer date induced a tendency similar to the number of inflorescences and leaves (p < 0.001). The number of branches was more increased by the LD treatment than by the NI treatment (Table 1).
Discussion
The photoperiod, or daylength, is a major environmental factor for successful reproduction of plants. Flowering responses are generally classified into three groups: LDPs, SDPs, and day-neutral plants (Thomas and Vince-Prue, 1997). In this study, the photoperiodic responses of E. angustifolia proved that this species is a typical obligate (qualitative) SDP similar to other Elsholtzia species (Chang et al., 2003; Sohn and Kim, 2003). Continuous SDs caused 100% flowering (Table 1) and this indicates that E. angustifolia is highly sensitive to SD conditions.
The days to the first open flower of early flowering plants under the continuous SD condition were 8 – 9 days longer than those of plants transferred from LD or NI conditions to the SD condition (Table 1). This might be attributed to the insufficient vegetative growth of seedlings, which were forced to flower early under the continuous SD condition. Moreover, since the days to first open flower in the continuous SD treatment were counted based on the germination date, it is possible to explain the difference if considering the time to emerge and develop the first leaf for detecting photoperiod. It implies that this species requires minimal growth for flowering and has little juvenility.
However, the days to the first open flower of all transfer treatments were relatively consistent and not significantly different (Table 1). Sohn and Kim (2003) reported that during more than 30 days of SD conditions, the start date of the SD treatment did not influence the flowering dates of E. ciliata and E. splendens. This implies that, in this experiment, a vegetative period of more than nine weeks (six weeks of the seedling stage and three weeks after transplanting) was sufficient to develop floral buds and flowers in E. angustifolia.
LD and NI conditions allowed E. angustifolia to continue vegetative growth without floral bud initiation and enabled them to develop more leaves and branches, followed by more inflorescences after transfer to SD conditions (Table 1). Moreover, the longer LD or NI conditions were maintained, the more vegetative and reproductive organs E. angustifolia developed, which is an important consideration in commercial production for year-round cultivation. In the cultivation of chrysanthemum, which is a typical SDP, lighting methods such as day extension or NI (or night break) have been used to inhibit floral initiation and maintain a vegetative state until flowering (Kwon et al., 2013; Park and Jeong, 2019). Chrysanthemum growers currently apply NI lighting using LEDs to prevent early flowering and increase their shoot length from autumn to winter (Kwon et al., 2014; Liao et al., 2014).
The number of branches grown under the LD condition was higher than that under the NI treatment, even though the number of inflorescences and leaves did not show significant differences between LD and NI conditions (Table 1, Figs. 2 and 3). One reason is that the light quantities of the LD and NI treatments were fundamentally different. The LD treatment consisted of a 9-h photoperiod + a 7-h day extension, whereas the NI treatment consisted of a 9-h photoperiod + a 4-h NI. Increasing light quantity can promote growth (Lee et al., 2019). Although 4 µmol·m-2·s-1of light intensity was applied to diminish the effect of the daily light integral, plants under the LD condition had received more light energy than those under the NI condition. Low light intensity for day extension or NI promotes growth and flowering in Lysimachia mauritiana and Tecoma stans (Torres and Lopez, 2011; Im et al., 2020).
In addition, shoot branching is closely related to environmental factors or endogenous regulators (e.g., phytohormones). Hormone-metabolizing enzymes or hormone-signaling pathways are generally involved in the effect of light on development (Leduc et al., 2014). Gibberellic acid (GA) is often associated with branching by the inductive effect of light on the hormone. In axillary buds of Rosa sp., even if the light intensity was only 2 µmol·m-2·s-1, the activities of two GA biosynthesis enzymes increased and that of a GA-catabolizing enzyme was downregulated (Choubane et al., 2012). According to the change of the daylength, various molecular and biochemical responses, such as auxin, cytokinin, and phytochromes, would influence the development of branches.
In conclusion, E. angustifolia is an obligate SDP, and flowering can be manipulated by lighting methods. Artificial SD conditions can induce floral bud initiation, and day extension for LD conditions and NI (i.e., exposure to light in the middle of the night) promoted vegetative growth with perfect inhibition of flowering of this species. These results will provide useful information for researchers and growers to understand the flowering manipulation of Elsholtzia species.