Introduction
Materials and Methods
Plant Materials and ABA Treatments
Shelf Life and Quality
Statistical Analyses
Results and Discussion
Shelf Life and Senescence Parameters
Change in Stomatal Size between Day and Night
Chlorophyll Contents, Maximum Quantum Yield of PSII, and Vegetation Indices (NDVI and SR)
Introduction
Hydrangeas (Hydrangea spp.) are popular ornamental plants worldwide (Kitamura et al., 2018) and are often used in floral displays because of the various colors and many flowers they produce (Kitamura et al., 2017; Lee and Lee, 2018). Hydrangeas originated in Asia. Most hydrangeas blossom in the summer and exist in various colors and sizes. Therefore, hydrangeas are frequently used as potted and garden plants (Schiappacasse et al., 2014), making them a popular woody plant species (Reed, 2004). The import and export plant quarantine statistics (2019) indicated that most hydrangea is exported and imported as cut flowers, and imported as the cutting and seedling for cultivation (QIA, 2020). Potted hydrangea distributed throughout Korea are mostly grown domestically (Lee, 2016). Potted flowers can be preserved and may continue to grow during storage and transport. They are shipped as whole plants with the substrate, which can lead to logistic difficulties due to their volume and weight (Yeh and Chiang, 2001). Potted flowers are subjected to increased stress due to exposure to ethylene gas during transport (Jones, 2002). Additionally, potted flowers that require a high amount of moisture are particularly vulnerable to the environmental variations to which they are exposed during distribution and use. Thus, the ornamental value of these flowers can be reduced due to ethylene gases or water stress (Kim and van Iersel, 2011). Evidence in the literature describes the effect of ethylene on the development of water stress response. Water stress increases the concentration of ethylene precursor in the xylem and root of potted flowers (Gómez-Cadenas et al., 1996). Movement of the immediate precursor of ethylene via the xylem to watered plants also inhibits leaf growth, suggesting that ethylene plays an important role in the regulation of leaf growth, such as dry soil (Davies et al., 2005). Therefore, studies have focused on generating ethylene and preventing water loss during the distribution of potted kalanchoe (Serek and Reid, 2000; Son et al., 2002), roses (Buanong et al., 2005), salvia (Kim and van Iersel, 2011), Phalaenopsis spp. (Jeon et al., 2019), and geranium (Oh and Runkle, 2009) in countries including South Korea. Furthermore, an ongoing study is investigating sustainable value chains, aiming to reduce the degradation of quality in potted flowers from production to distribution (Havardi-Burger et al., 2020). Despite increasing consumer demand, few studies have focused on the post-production management of potted hydrangea, with the majority of active studies focusing on the cultivation, quality improvement, and post-harvest control methods of cut hydrangea (Lee et al., 2017; Nam et al., 2017; Lee and Lee, 2018; Lee et al., 2019).
Most available potted hydrangeas are of the Hortensia (Hydrangea macrophylla) type, which produce clusters of small fertile flowers in the center area surrounded by large sterile flowers, giving the appearance that the small flowers bloom (Kitamura et al., 2018). The most commonly displayed hydrangea organ is the sepal, and not the petals. As the leaves are longer and wider than those of other flowering shrub species, hydrangeas require large amounts of water (Uemachi and Nishio, 2005). More than 70% of transpiration occurs through the stomata of plants. Under water stress, stomatal closing and opening effectively control water use efficiency and prevent dehydration (Kim et al., 2016). Sepal stomatal opening during market distribution may enable the entry of pathogens and microbes, resulting in a loss of quality (e.g., tissue browning and necrosis) (Kitamura et al., 2017). Therefore, our study sought to establish a method for inhibiting transpiration and extending the shelf life of potted hydrangea during shipment and transport via the regulation of stomatal closing and opening.
Abscisic acid (ABA) is a hormone that effectively controls water loss from plants by inducing stomatal closure under water stress (Kuromori et al., 2018). When soil lacks moisture, plants react via root-to-shoot signaling, which induces ABA synthesis to mitigate water loss (Kim and van Iersel, 2011). Once ABA accumulates in the leaf, stomatal conductance decreases due to changes in the guard cells, leading to stomatal closure (van Iersel et al., 2009). Kim and van Iersel (2011) reported that, in Salvia, ABA drench treatments induced rapid stomatal closure and that treatment with 250 and 500 mg·L-1 ABA reduced transpiration-induced water loss compared to other treatments, extending the shelf life of the potted flowers by 3 days. Kitamura et al. (2017) found that ABA drench treatments during the antique stage of cut hydrangeas reduced stomatal conductance and changed the transpiration rate, extending the shelf life of cut flowers. Additionally, van Iersel et al. (2009) reported that 1,000 ppm ABA drench treatment prolonged the shelf life of potted hydrangea by approximately 11 days, although low-concentration ABA drench treatment caused yellowing of potted hydrangea leaves. However, those studies were based on ABA drench treatments; few studies have reported the effective use of ABA spray treatments in the field. Thus, to establish a post-production management system for potted hydrangea during distribution, this study compared shelf life and quality under different concentrations of ABA spray treatments. In addition, we investigated the physiological changes caused by the treatments using nondestructive methods, including the maximum quantum yield of PSII (Fv/Fm), normalized difference vegetation index (NDVI), and simple ratio index (SR) measurements.
Materials and Methods
Plant Materials and ABA Treatments
‘Speedy Red’ potted hydrangeas with at least four solitary branches per pot (approximately 45 cm in height and 54 cm wide) were purchased from a farm located in Yongin, Gyeonggi-do, Korea, in April 2019. The plants were acclimated in a glass greenhouse at Dankook University, Cheonan, South Korea, for 2 weeks after purchase. The acclimation conditions were the same as the experimental conditions. When more than 80% of the sepals from two of four branches turned red, the plant was considered ready for marketing and treated with ABA. The spray treatments consisted of 50 mL ABA at concentrations of 0, 1,000, 2,000, and 2,500 mg·L-1 prepared with ABA powder (Sigma-Aldrich, USA). ABA at a concentration of > 98% was added to a small volume of 99.5% ethyl alcohol. The treatments were sprayed onto the surface of sepals and leaves in each pot at 10 a.m., and six replicates were performed per ABA spray treatment. The plants were fully irrigated before the experiment, and no irrigation was performed after ABA treatment. The experiment was performed in a greenhouse from April 17th to the 24th in 2019, and temperature, relative humidity, light intensity, and day length (light period) were 21.2 ± 3.1°C, 52.8 ± 2.8%, 176.4 ± 4.2 µmol·m-2·s-1 PPFD (mean ± SD), and 14 h, respectively.
Shelf Life and Quality
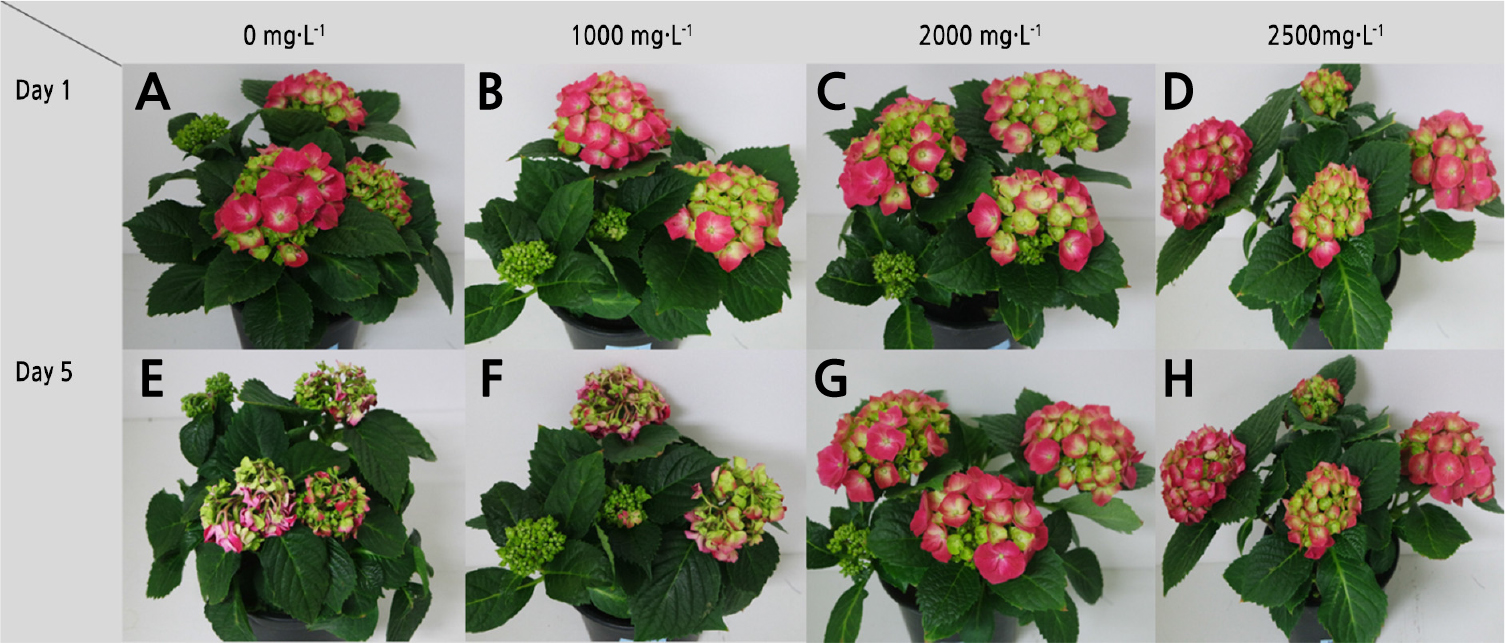
Treatment effects were evaluated based on the shelf life of the potted plants, senescence parameters, changes in stomatal size during the day and night, leaf chlorophyll content, maximum quantum yield of PSII (Fv/Fm), NDVI, and SR. The symptoms of senescence in potted hydrangea were identified in a preliminary experiment and were classified as wilting (> 30% of all sepals) and color change (blue and brown discoloration; > 30% of all sepals) of the sepals and wilting (> 30% of all leaves) of leaves. Symptoms were examined and calculated as percentages of the replicates. The day on which a senescence parameter was observed, resulting in the loss of ornamental value to consumers, was determined as the end of the shelf life. Changes in stomatal size were evaluated using an optical microscope (CX 31, Olympus, Japan) at 400 × magnification with a stoma observation kit (SUMP, Kenis, Japan), with daily observations at 9 p.m. (night) and 9 a.m. (day). Then, the area of the stomatal opening was measured in a ‘+’ shape in micrometers and calculated as an elliptical area. The stomatal area under dark conditions (DSA) and light conditions (LSA) was calculated and used to calculate the change in stomatal size using the following equation:
For non-destructive measurements, leaf chlorophyll content was measured using a chlorophyll meter (SPAD 502, Minolta, Japan), and the Fv/Fm of the leaves was measured using a chlorophyll fluorescence profiler (Fluorpen FP 100, Photon Systems Instruments, Czech Republic). NDVI and SR were measured in the sepals with a vegetation index meter (PolyPen, Photon Systems Instruments, Czech Republic). Chlorophyll content, Fv/Fm, NDVI, and SR were measured using four leaves per plant, which were the uppermost fully expanded leaves immediately below the flower.
Statistical Analyses
This experiment adopted a completely randomized design with six replicates. Statistical analyses were performed using SAS software (SAS 9.0, SAS Institute Inc., USA). Analysis of variance and Duncan’s multiple range test were used to compare means. Comparisons of senescence percentage were performed using the chi-square test. Additionally, Pearson’s correlation analysis was performed using SPSS (SPSS 23.0 Statistics, SPSS Inc., USA) to evaluate the relationships between shelf life, stomatal size change, Fv/Fm, NDVI, and SR.
Results and Discussion
Shelf Life and Senescence Parameters
To determine the optimal concentrations of ABA to extend the shelf life of potted hydrangea, senescence parameters and shelf life were investigated after spray treatments containing 0 (control), 1,000, 2,000, or 2,500 mg·L-1 ABA. The average shelf lives of plants with the control and 1,000 mg·L-1 ABA spray treatments were 5 and 5.2 days, respectively. The 2,000 mg·L-1 ABA spray treatment extended the shelf life by 2 days beyond that of the control treatment, resulting in an average shelf life of 7 days (Table 1 and Fig. 1). However, the average shelf life of plants treated with 2,500 mg·L-1 ABA spray was 6 days, which was not significantly different from that of the other treatment groups. Regarding the senescence parameters 5 days from the initiation of the experiment, 100% of control plants and those subjected to 1,000 mg·L-1 ABA spray treatment exhibited sepal and leaf wilting, which corresponded to the final day of their shelf life, whereas plants subjected to the 2,000 and 2,500 mg·L-1 ABA treatments presented less wilting (Fig. 1E and 1H). Particularly, the 2,000 mg·L-1 ABA spray treatment resulted in a sepal and leaf wilting rate of 33%, which was less than that of other treatments, indicating that this treatment was effective at maintaining the quality of potted hydrangea (‘Speedy Red’). Additionally, the 2,000 mg·L-1 ABA spray treatment ameliorated color changes in the sepals (i.e., the sepals turned brown and blue) compared to the other treatments; thus, the treatment effectively preserved the ornamental value of the potted hydrangea (‘Speedy Red’). Lee and Lee (2018) reported that the quality of cut hydrangea flowers was associated with moisture inside the plants and that the main cause of low marketability was wilting. Our results suggest that ABA spray treatments can prolong the shelf life of potted hydrangea by reducing wilting and that spray treatments containing at least 2,000 mg·L-1 ABA should be effective for preserving the ornamental quality of hydrangea. These results are consistent with those of van Iersel et al. (2009), who found that high-concentration ABA drench treatments could extend the shelf life of potted hydrangea ‘Mini Penny’ to a greater extent than low-concentration ABA treatments. However, the optimal ABA concentration (1,000 mg·L-1) proposed by van Iersel et al. (2009) was lower than the effective concentration found in this study. As the optimal ABA concentration is likely to differ depending on the variety of potted hydrangea or the ABA treatment method (drenching or spraying), the effects of different ABA treatments should be further studied in various hydrangea cultivars to identify optimal concentrations and methods.
Table 1.
Shelf life and senescence parameters at 5 days after treatment with different ABA concentrations sprayed on potted hydrangea
|
ABA concentration (mg·L-1) |
Shelf life (days) | Senescence on day 5 (%) | ||
| Sepal | Leaf | |||
| Wilting | Change of color | Wilting | ||
| 0 | 5.0 bz | 100 | 66.7 | 100 |
| 1,000 | 5.2 b | 100 | 83.3 | 100 |
| 2,000 | 7.0 a | 33.3 | 33.3 | 33.3 |
| 2,500 | 6.0 ab | 66.7 | 66.7 | 50.0 |
| Chi-square test | **y | * | ** | |
Change in Stomatal Size between Day and Night
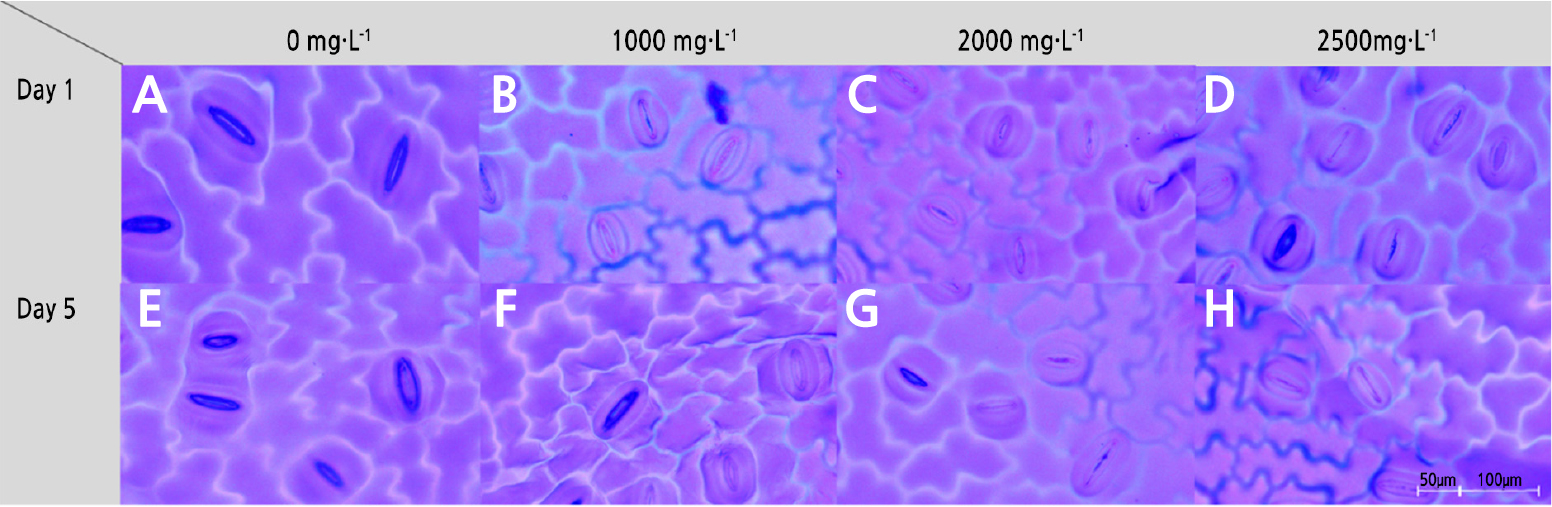
To examine physiological responses related to transpiration, the change in stomatal size between day and night was investigated in potted hydrangea (Figs. 2 and 3). From day 1 of the experiment, there were differences in the change in stomatal size depending on the ABA spray concentration (Fig. 3A–3D). On days 1 and 3 of the experiment, potted hydrangeas sprayed with all ABA treatments exhibited less change in stomatal size than the controls (Fig. 2).
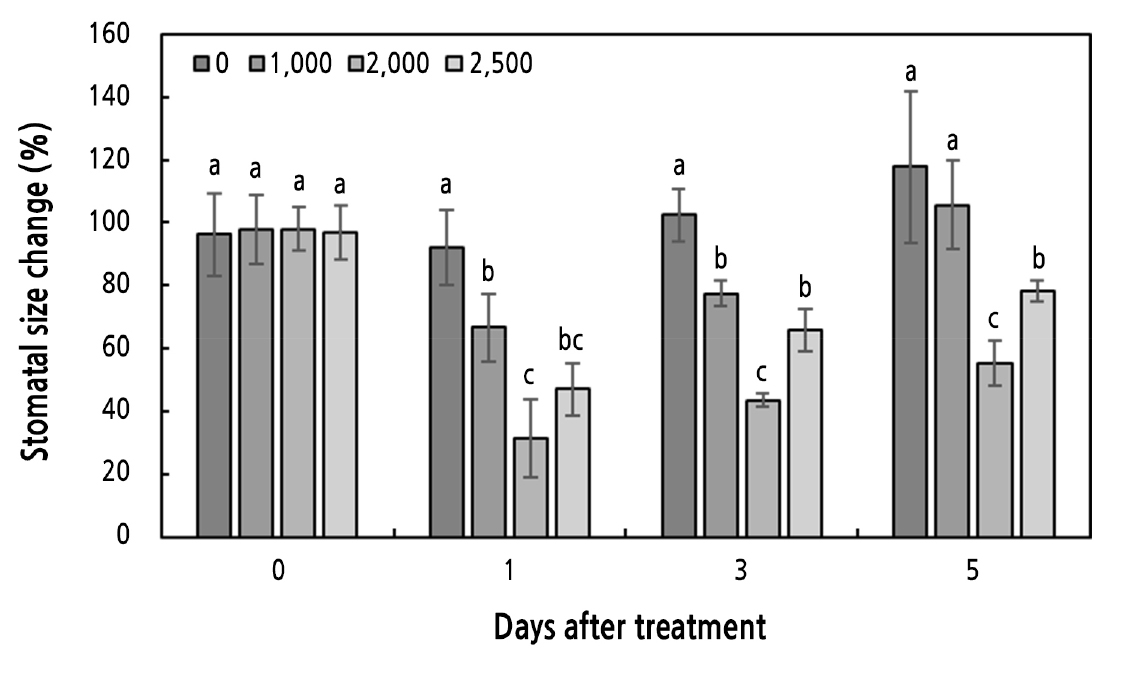
Fig. 2.
Change in stomatal size between stomatal closing (night) and opening (day) under different ABA concentrations (0, 1,000, 2,000, and 2,500 mg·L-1) in potted hydrangea. Bars indicate standard errors (n = 6). Different letters indicate significant differences between treatments according to Duncan’s multiple range test at p < 0.05.
Particularly, 2,000 mg·L-1 ABA treatment resulted in the smallest change in stomatal size between light and dark conditions up to 5 days post-treatment compared to the other treatments. This was likely because the stomata remained closed even under light conditions, resulting in a smaller change in stomatal size relative to that under dark conditions (Fig. 3G). On day 5 post-treatment, there was a considerable change in stoma size between day and night in plants subjected to the control and the 1,000 mg·L-1 ABA spray treatment, and stomata remained open even under light conditions (Fig. 3E and 3F). Thus, it is possible that potted hydrangea plants under the control and 1,000 mg·L-1 ABA spray treatments rapidly reached the end of their shelf lives due to transpiration caused by persistent stomatal opening. In turn, a lack of moisture likely caused the sepals and leaves to wilt.
Chlorophyll Contents, Maximum Quantum Yield of PSII, and Vegetation Indices (NDVI and SR)
The average leaf chlorophyll content on day 1 of the experiment was 70.9, with no significant differences between treatments (Fig. 4A). On day 5 of the experiment (the end of shelf life for potted hydrangea under the control treatment), chlorophyll content was lower than that observed on day 1 of the experiment. A higher SPAD value was obtained following the 2,000 mg·L-1 treatment compared to the control and the 1,000 mg·L-1 ABA treatment. Lee et al. (2019) reported that chlorophyll content decreased when cut hydrangea flowers senesced after harvesting. In this study, the chlorophyll content of potted hydrangeas also decreased with senescence, which negatively affected quality. When plants are subjected to stress, photosynthetic organs such as photosystem II can be directly or indirectly damaged. The response of photosystem II can be determined by measuring Fv/Fm in a non-destructive manner. The degree of damage can be digitized based on chlorophyll fluorescence before visual inspection of symptoms; this method is commonly utilized as an index to assess plant stress (Pagter et al., 2008). Although there were no differences in Fv/Fm among treatments on day 1 of this experiment, the Fv/Fm values obtained with ABA spray treatments were higher than those obtained with the control treatment on day 3. The 2,000 mg·L-1 ABA spray treatment resulted in the highest Fv/Fm value on day 5, at 0.79, whereas the control treatment resulted in the lowest value, at 0.63 (Fig. 4B). A normal Fv/Fm range in healthy plants is between 0.78 and 0.84 (Pagter et al., 2008); thus, only the 2,000 mg·L-1 ABA spray treatment resulted in a value within the normal range, indicating that plants subjected to the other treatments were stressed. These results are similar to those reported by Choi et al. (2001), who studied the Fv/Fm values of Aphelandra squarrosa, Ficus benjamina, and Pachira aquatica and found that Fv/Fm decreased below 0.6, in response to drought stress. This suggests that the 2,000 mg·L-1 ABA spray treatment effectively protected against water loss in potted hydrangea and that Fv/Fm could predict the degradation in quality caused by water loss in potted hydrangea. Other non-destructive evaluation methods (NDVI and SR) were examined in this study (Fig. 4C and 4D). Although there were no differences in the NDVI or SR between treatments on day 1 of the experiment, treatment-dependent differences were observed on days 3 and 5. On day 3, both NDVI and SR indices were higher with the ABA spray treatments than with the control. The NDVI index is obtained by calculating the difference in the reflectance of light energy between wavelengths of visible light and near-infrared light and is widely used to evaluate vegetative variables of plants, such as vegetation and biomass chlorophyll content (Payero et al., 2004). NDVI ranges between 0.2 and 0.9, with values between 0.5 and 0.9 indicating a healthy condition and those between 0.2 and 0.4 indicating that the plant is under stress (Payero et al., 2004). On day 5, plants subjected to the 2,000 mg·L-1 ABA spray treatment presented a higher NDVI than those under the control and 1,000 mg·L-1 ABA spray treatments. Nevertheless, all treatments resulted in an NDVI between 0.58 and 0.67, which was within the normal range. The SR index can also be used to determine the vegetation structure by dividing the reflectance of the near-infrared band by that of the red band. SR ranges from 1 to 10; healthy plants are scored between 6 and 10, and stressed plants are scored between 1 and 5 (Payero et al., 2004). On day 5, the SR value of plants under the 2,000 mg·L-1 ABA spray treatment was higher than that of plants under the other treatments, indicating that the 2,000 mg·L-1 ABA spray treatment effectively alleviated stress (Fig. 4D).

Fig. 4.
Chlorophyll content (A), maximum quantum yield of PSII (Fv/Fm) (B), normalized difference vegetation index (NDVI) (C), and simple ratio index (SR) (D) in leaves of potted hydrangea under different ABA spray concentrations (0, 1,000, 2,000, and 2,500 mg·L-1). Error bars indicate standard errors (n = 6). Different letters indicate significant differences between treatments according to Duncan’s multiple range test at p < 0.05.
The correlation between quality factors was examined on day 5 post-treatment, which represented the end of the shelf life of control plants (Table 2). There was a negative correlation between shelf life and change in stomatal size (r = -0.545, p < 0.01), indicating that the shelf life of potted hydrangea subjected to ABA spray treatments should be extended as they experience less change in stomatal size. Kitamura et al. (2017) reported that the quality of sepals in hydrangeas was affected by stomatal water loss and that drought caused color changes, such as browning, along with wilting in hydrangea sepals; however, the specific mechanisms underlying these phenomena remain unknown. Kitamura and Ueno (2015) also found that inhibiting transpiration in hydrangeas could positively impact quality, although ABA treatment could accelerate senescence. Therefore, the results of this study indicated that ABA treatment may be needed to reduce water losses during the distribution of potted hydrangea and that a 2,000 mg·L-1 ABA spray treatment effectively controls stomatal closure in potted hydrangea (‘Speedy Red’), thereby preserving quality.
Table 2.
Correlation between shelf life and measured physiological variables [change in stomatal size, maximum quantum yield of PSII (Fv/Fm), normalized difference vegetation index (NDVI), and simple ratio index (SR)] in potted hydrangea on day 5 post-treatment
| Variables | Shelf life | Stomatal size change | Fv/Fm | NDVI |
| Stomatal size change | -0.545** | 1 | ||
| Fv/Fm | 0.870* | -0.702* | 1 | |
| NDVI | 0.549** | -0.502** | 0.620* | 1 |
| SR | 0.832** | -0.829** | 0.540* | 0.719** |
There was a strong negative correlation between SR and change in stomatal size (r = -0.829, p < 0.01) (Table 2). This was consistent with the finding that potted hydrangea plants had a lower Fv/Fm value than the normal range under drought stress and that the 2,000 mg·L-1 ABA spray treatment prevented water loss by maintaining stomatal closure more efficiently than the other treatments, thereby effectively maintaining plant quality. The NDVI and SR indices were positively correlated with shelf life (r = 0.549, p < 0.01; r = 0.832, p < 0.01) (Table 2). It was speculated that the shelf life of potted hydrangea would be prolonged as the NDVI and SR indices fell within the normal range. Particularly, there was a strong correlation between SR index and shelf life, indicating that SR may have potential for use as a predictive indicator of shelf life and quality in potted hydrangeas. In addition to those examined in this study (NDVI and SR), there are various vegetation indices (Payero et al., 2004) that have not been studied in potted hydrangea. Therefore, further studies should focus on alternative indices that may be used to predict physiological activity.
These results suggest that ABA spray treatment at optimal concentrations before shipment from farms to wholesalers or retail stores significantly enhances the quality and shelf life of water-stress-sensitive potted hydrangeas. Based on our observations in potted hydrangea (‘Speedy Red’), a 2,000 mg·L-1 ABA spray treatment should extend shelf life through effective stomatal closing, thus inhibiting transpiration and preserving quality. Moreover, this study demonstrates that Fv/Fm and vegetation indices have potential for monitoring the quality of potted hydrangea under water stress in a non-destructive manner, which may facilitate the evaluation of potted flower quality on site.