Introduction
Materials and Methods
Plant Material and Growth Conditions
Fruit Quality Trait Determination
Protein Extraction and Quantification
2-D Electrophoresis
Image Acquisition and Analysis
MS (mass spectrometry) Analysis and Protein Identification
Statistical Analysis
Results and Discussion
Effect of Bagging on Fruit Quality
Proteomic Profiles in Natural and Bagged Peach Fruit during Development
Protein Identification and Functional Analysis
Proteins Associated with Stress Response and Defense
Proteins Associated with Photosynthesis
Proteins Associated with Carbohydrate Metabolism
Proteins Associated with Amino Acid Metabolism
Proteins Aassociated with Protein Synthesis and Degradation
Proteins Associated with Fatty Acid Metabolism, Aroma and Flavonoid Biosynthesis
Conclusion
Introduction
Peach (Prunus persica) is among the most economically important fruit producing rosaceous crops and a model species used in studies of stone fruit in the Rosaceae family (Shulaev et al., 2008). Fruit bagging while on the tree is extensively practiced in China to improve fruit quality via promotion of anthocyanin synthesis. Upon removal of the bag from the treated plants, the pigmentation of peach, apple, pear, and mango fruit is improved (Liu et al., 2013; Yu et al., 2012; Jia et al., 2005; Hofman et al., 1997). In addition to improving color, the practice of bagging prevents infestation by insect pests, reduces egg laying by female flies, inhibits disease, reduces physical damage (scars and scratches) (Amarante et al., 2002), and inhibits splitting and sunburn (Bentley and Viveros, 1992), while avoiding direct contact with pesticides and thus greatly reducing agrochemical residues. Although laborious, the practice of bagging is safer and easier than pesticide application, while providing farmers with more reliable estimates of projected harvests than are possible with non-bagging cultivation methods. Finally, bagging increases marketable fruit yield by reducing cosmetic defects.
Studies of the physiological mechanisms underlying the effects of bagging have primarily focused on anthocyanin synthesis following bagging of apple (Liu et al., 2013) and pear (Yu et al., 2012) trees. It is believed that bagging increases the sensitivity of fruit to light when the bag is removed, thus enhancing light-stimulated anthocyanin synthesis (although anthocyanin production in apple fruit also requires cool night temperatures). In addition, bagging may increase the abundance of volatile aromatic compounds in the skin of peach fruit, which may improve flavor (Jia et al., 2005). A recent study demonstrated that accumulation of n-hexanal and (E)-2-hexenal, which contribute to the characteristic aroma of peach fruit, is related to the particular sunlight transmission properties of the bags used to cover the fruit (Shen et al., 2014). In addition to color and aroma, fruit bagging also affects qualities of the flesh of the fruit, including sweetness and acidity. Most studies have reported that fruit bagging led to reduced contents of soluble carbohydrates, phenolic compounds, and organic acids (Lima et al., 2013) or had no changes in soluble carbohydrate content. Reports have mainly focused on the effects of fruit bagging on general fruit quality; however, fruit quality is the result of the combined action of numerous signaling and metabolic pathways during fruit development. Therefore, it is necessary to identify and quantify early responses to bagging at the protein level.
Recently, proteomics, the systematic study of global changes in protein expression, has been used in studies of peach fruit development (Hu et al., 2011; Prinsi et al., 2011; Nilo et al., 2010), post-harvest treatment (Zhang et al., 2011), and heat stress (Liu et al., 2012). Proteomics studies of peach fruit have provided insight into the physiological mechanisms underlying peach development. Feng et al. (2011) reported that fruit bagging altered the expression levels of numerous proteins in pear fruit.
The purpose of this study was to utilize proteomics to measure changes in protein expression following bagging of peach fruit, with the primary goals of identifying new biomarkers of fruit quality and evaluating alterations in adaptive responses in fruit, to provide a detailed characterization of the effect of fruit bagging on fruit quality at the proteomic level.
Materials and Methods
Plant Material and Growth Conditions
Peach (P. persica L. cv. ‘No. 24’) fruit were grown in an experimental peach orchard at the Beijing University of Agriculture (Pinggu District, Beijing, China) using conventional practices. Three neighboring peach trees with similar loads were the source of fruit. Bagging with two layers of pale-black fruit bags (Xintaiguodai factory, Shangdong, China) was carried out 50 days after full bloom (DAFB). Half of the fruit on each tree was bagged. Bagged and non-bagged fruit samples with no symptoms of pests and diseases were harvested at 125 DAFB from three different trees. On the same day, some of the bagged fruit was exposed to sunlight; this fruit was harvested at 133 DAFB. Non-bagged fruit was also harvested at 133 DAFB. For each treatment and control group, 21 fruits were harvested from three trees at random to avoid biasing the samples, after which the fruits were randomly divided into three groups, which served as three biological replicates (n = 7 in each replicate). The mesocarps from individual fruit in each treatment group in each replicate were pooled, immediately frozen in liquid nitrogen, and stored at -80°C. For determination of fruit quality, 10 fruits were harvested from each tree.
Fruit Quality Trait Determination
The fresh weight (FW), vertical diameter (VD), and transverse diameter (TD) were the averages of the measurements from 10 fruits. A TA-XT2i plus texture analyzer (Stable Micro System, Godalming, England) equipped with a 7.9-mm diameter head was applied for fruit firmness measurement as described by Zhang et al. (2010).
Skin color was visually assessed as the percentage of skin area showing red color and ranked on a scale of 0 (no red color) to 10 (intense red). Anthocyanin was extracted from mesocarp taken from the reddest part of each fruit with 25 mL of 1% HCl in methanol. The absorbance at 528 nm was measured by a spectrophotometer using the method reported by Chaovanalikit (2004). For the chlorophyll content assay, circular pieces were cut from the mesocarp and extracted with methanol using a mortar, after which the extracted pigments were centrifuged at 8000 × g (Sigma 3K-30, Germany) for 5 min to make the extract fully transparent. The resulting extracts were immediately assayed spectrophotometrically (SmartSpec Plus, Bio-Rad, USA). The specific absorption coefficients of Chl a and Chl b were taken from Lichtenthaler (1987).
Measurements of soluble solids content (SSC) and titratable acidity (TA) were conducted on juice samples collected from each treatment group. The SSC in opposite parts of each fruit was measured with a refractometer (Atago CM780N, Japan). TA was determined by titration to pH 8.1 with 0.05 N NaOH. The TA results were expressed in grams of malic acid per liter of juice.
Protein Extraction and Quantification
Total protein was extracted and purified from ground fruit mesocarp via phenol extraction followed by ammonium acetate methanol precipitation, as described by Faurobert et al. (2007) and Hu et al. (2011). The protein concentration in each extract was determined using the Non-Interfering Protein Assay TM Kit (EMD Biosciences, Germany) to remove interfering compounds, with bovine serum albumin (BSA) as the standard for the calibration curve. The re-solubilized protein samples were stored at -80°C prior to use.
2-D Electrophoresis
Three gels were run for each extract. Briefly, IPG strips (pH 4-7, 17 cm, ReadyStrip, Bio-Rad, Hercules, CA, USA) were actively rehydrated (50 V) overnight with 300 μL of IEF buffer containing 800 μg of total protein. Isoelectric focusing (IEF), equilibrating, and 2-D electrophoresis (2-DE) were performed according to the methods of Hu et al. (2011). 2-DE gels were stained with Coomassie Brilliant Blue-G250 (CBB) following a reported blue-silver protocol (Candiano et al., 2004).
Image Acquisition and Analysis
Gel images were acquired using a UMAX 2100 XL Scanner (UMAX Technologies, Dallas, TX, USA) and analyzed with PDQuest Advanced software version 8.0.1 (Bio-Rad, Hercules, CA, USA). Spot detection and matching were performed automatically, followed by manual inspection to correct any error prior to final data analysis. After normalization of the protein spot volume against the spot volume of the entire gel, the percentage volume of each spot was averaged for the three gels. Statistically significant changes in protein abundance were determined using three sequential data analysis criteria. First, a protein spot had to be present in all replicated gels. Next, a two-fold change in normalized spot volume was considered as indicative of a significant quantitative variation. Third, p-values ˂ 0.05 were considered to be statistically significant by Duncan’s multiple range test.
MS (mass spectrometry) Analysis and Protein Identification
Spots detectable in three replicates were excised and digested with trypsin according to the procedure of Hu et al. (2011). The extracted trypsin fragments were analyzed by 4800 MALDI TOF/TOFTM (AB Sciex, US) according to the manufacturer’s protocol. The raw spectra were manually filtered by PEAKERAZOR to detect potential contaminants and submitted to MASCOT software to search the NCBInr and SwissProt databases.
Statistical Analysis
All statistical analyses were performed using SPSS 14.0 (SPSS Inc., Chicago, IL, USA). Data were analyzed using analysis of variance (ANOVA), after which group means were compared by Duncan’s multiple range test (DMRT) with a significance threshold of p < 0.05.
Results and Discussion
Effect of Bagging on Fruit Quality
Differences in the external morphological characteristics of bagged and non-bagged peach fruit are shown in Fig. 1. At 125 DAFB, the ground color of bagged fruit was slightly yellow (Fig. 1B), while the skin of the non-bagged fruit was green (Fig. 1A). The bagged fruit rapidly turned red following bag removal (Fig. 1D), at which point the skin of fruit had better market acceptance than that of the non-bagged fruit (Fig. 1C). Bagging treatment increased the FW and size of peach fruit, but there was no difference in fruit firmness after bagging treatment (Table 1).
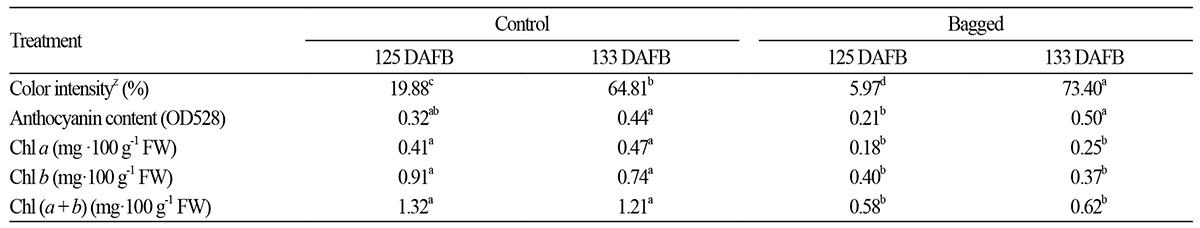
There was little difference in SSC content and no difference in TA content among the treatment groups. The solid/acid ratio of the bagged fruit at 125 DAFB and the debagged fruit at 133 DAFB were slightly lower than those of the non-bagged and bagged fruit at the same stages (Table 2). Furthermore, the solid/acid ratio increased with the developmental stage. Skin color was significantly affected by bagging (Table 3). The percentage of skin area with a blush of color was larger in the debagged fruit than in the non-bagged fruit at 133 DAFB. Anthocyanin content was not significantly changed by bagging treatment at 125 DAFB, whereas the fruit re-exposed to sunlight showed increased anthocyanin content at 133 DAFB (Table 3). In a previous study, fruit covered with bags appeared bright red and had high L values, which accounted for their good visual quality (Jia et al., 2005). The chlorophyll content of the non-bagged fruit was about twice that of the bagged fruit at 125 DAFB. In the fruit re-exposed to sunlight, the chlorophyll content in the debagged fruit was lower than that of the non-bagged fruit at 133 DAFB (Table 3).
Proteomic Profiles in Natural and Bagged Peach Fruit during Development
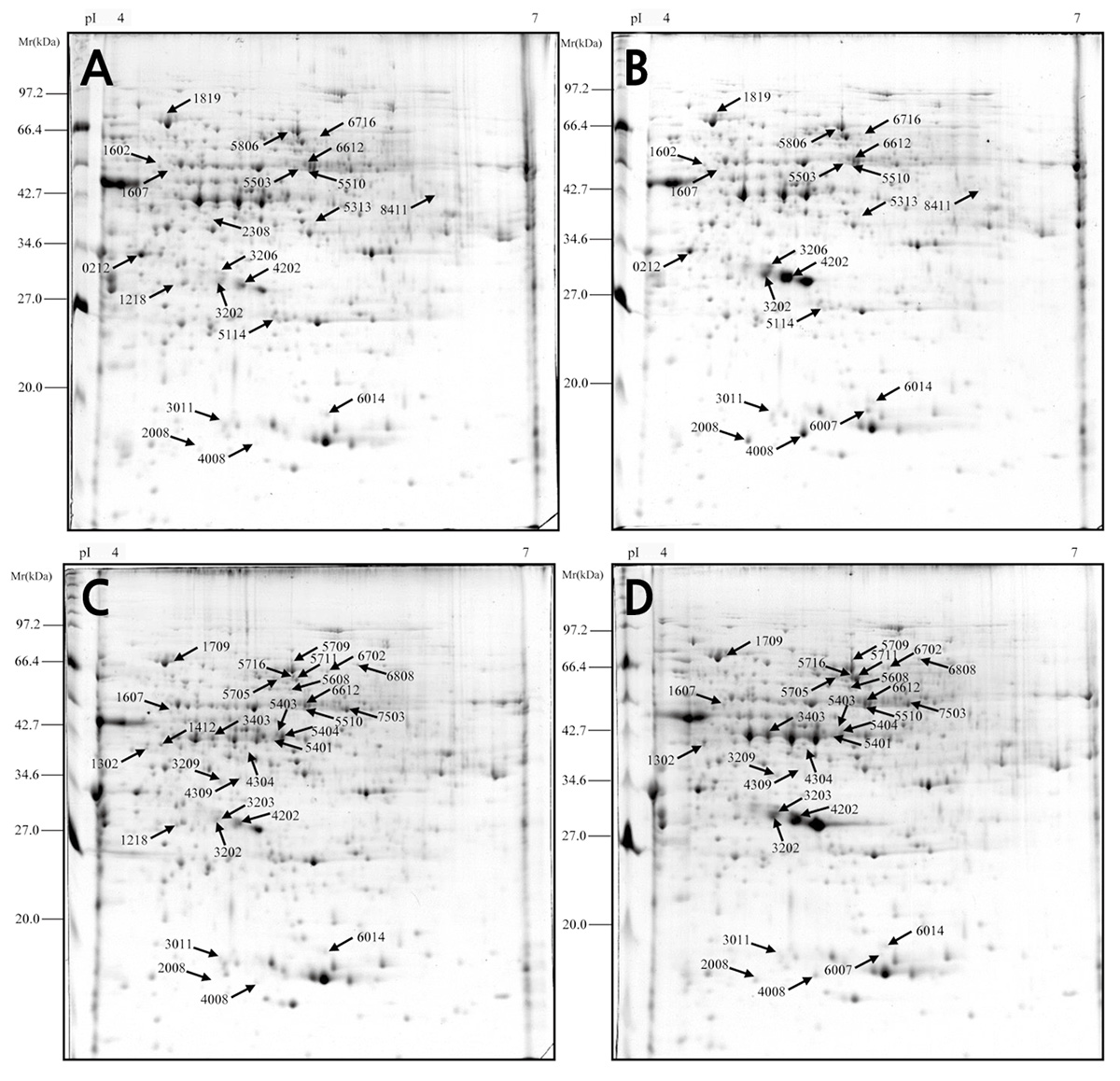
The proteomic profiles of the mesocarp from the bagged and non-bagged fruit were analyzed at 125 and 133 DAFB. Protein spots in all images were automatically detected and matched by PDQuest software, manually corrected spot-to-spot to identify various errors caused by noise and false spots, and subjected to analysis to calculate the effective number of spots (Fig. 2). Average proteomic maps showed approximately 500 spots for each stage. Based on the criteria described above, a total of 103 protein spots were subjected to mass spectrometry analysis, of which 68 spots showed a good quality MS signal and were subjected to a search in the NCBInr and SwissProt databases. Protein spots were analyzed by comparing their theoretical molecular weight (Mr.) and isoelectric point (pI) with experimental Mr. and pI as determined in 2-D gels. Twenty-one spots showed different expression levels in bagged and non-bagged fruit at 125 DAFB, whereas 30 spots showed different expression levels in bagged and non-bagged fruit at 133 DAFB, including up- and down-regulated proteins, as well as proteins present in some samples and not in others.
Protein Identification and Functional Analysis
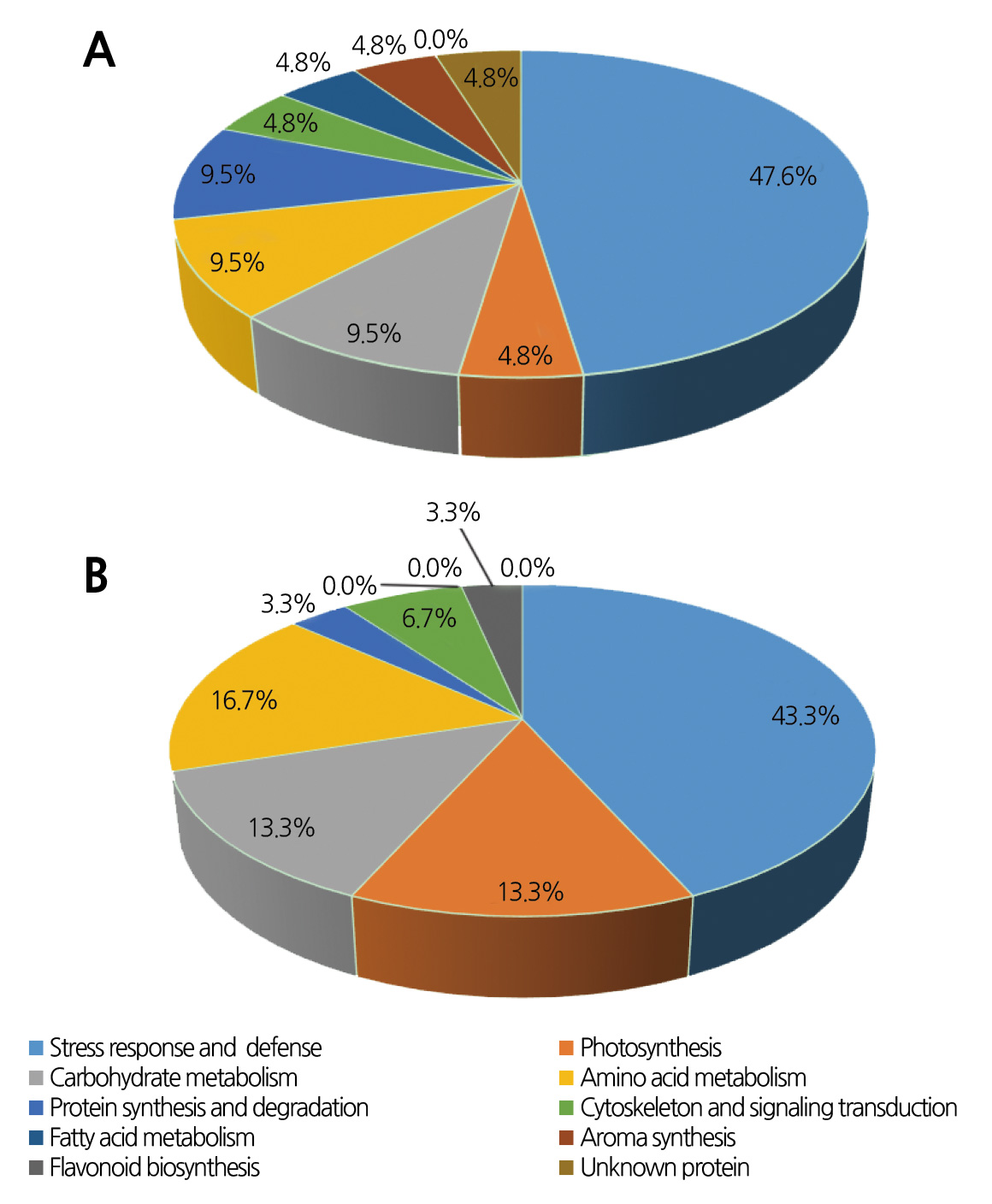
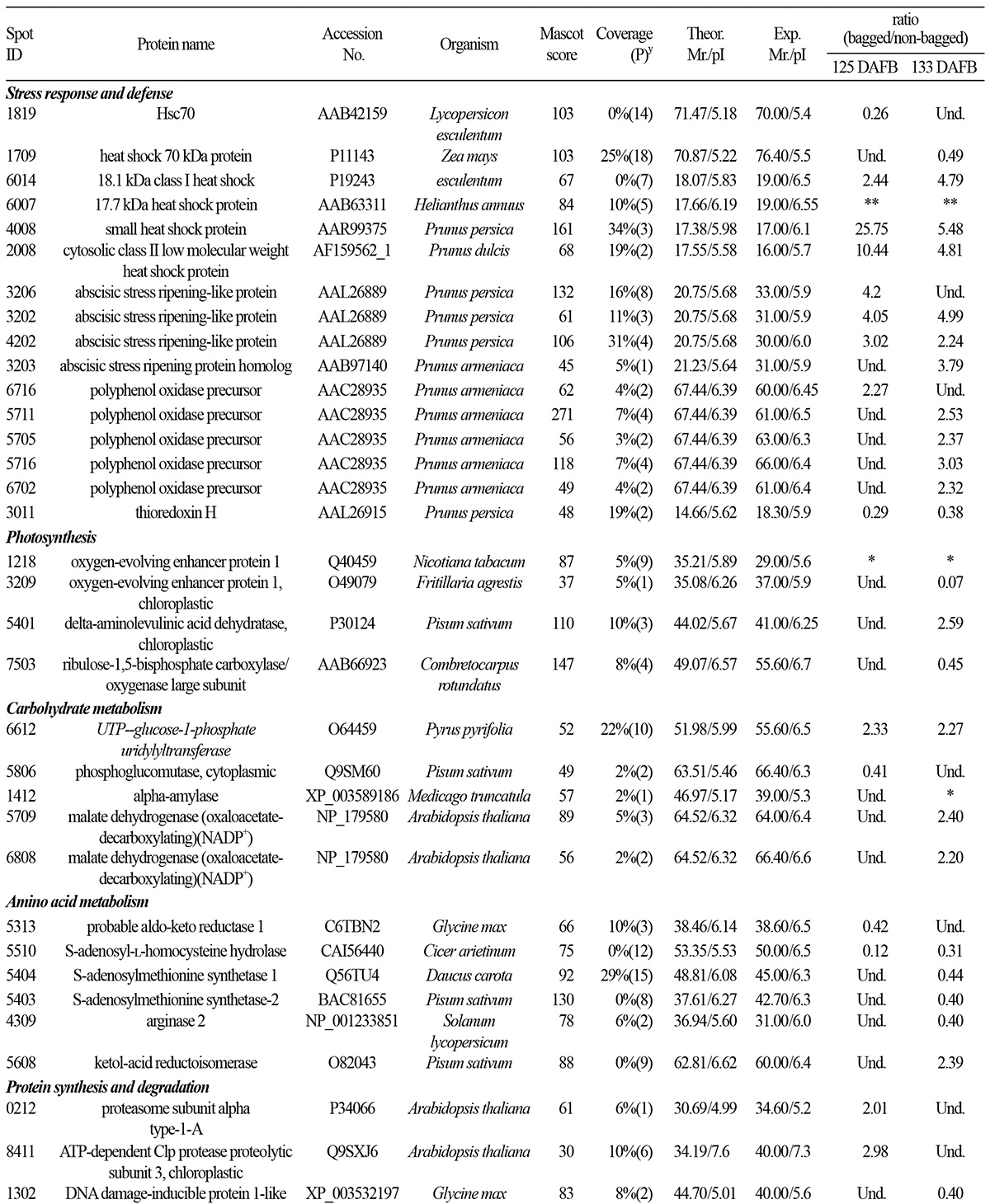
The identified proteins and their functions are listed in Table 4. The differentially expressed proteins in bagged and nonbagged fruit at 125 DAFB were classified into the following functional categories: stress response and defense (47.6%), photosynthesis (4.8%), carbohydrate metabolism (9.5%), amino acid metabolism (9.5%), protein synthesis and degradation (9.5%), cytoskeleton and signaling transduction (4.8%), fatty acid metabolism (4.8%), aroma synthesis (4.8%) and unknown protein (4.8%) (Fig. 3A). The differentially expressed proteins in bagged and non-bagged fruit at 133 DAFB were involved in stress response and defense (43.3%), photosynthesis (13.3%), carbohydrate metabolism (13.3%), amino acid metabolism (16.7%), protein synthesis and degradation (3.3%), cytoskeleton and signaling transduction (6.7%), and flavonoid biosynthesis (3.3%) (Fig. 3B). Stress/defense proteins were the most represented group, followed by components associated with photosynthesis.
Proteins Associated with Stress Response and Defense
Bagging subjects fruit to varying degrees of light deprivation and heat stress during the ripening process. Heat shock proteins (HSPs) can be induced by stimuli associated with bagging, including high temperature (Lara et al., 2009) and ripening (Nilo et al., 2010). Induction of small heat shock protein (sHSP) expression prevents protein misfolding under adverse conditions, including various stressors (Zou et al., 2009). In this study, four differentially expressed sHSPs (spots 6014, 6007, 4008, and 2008) were markedly induced following bagging and debagging treatment. The effects of stress and ripening caused by bagging were indistinguishable in our study.
The HSP70 family includes both heat-inducible and constitutively expressed members (D’Ambrosio, 2013). HSP70 protein levels increase upon exposure to various stressors (Ambrosia, 2013) and change in an isoform-specific fashion during fruit ripening (Huang et al., 2011). Giribaldi et al. (2007) reported that the expression of some HSPs decreased at the onset of ripening, consistent with a lower rate of protein synthesis and reduced protein yield. In our study, HSP70 (spot 1819 at 125 DAFB and spot 1709 at 133 DAFB) expression was down-regulated under bagging and debagging treatment. However, the stress caused by bagging generally increases HSP70 expression (Polenta et al, 2007); therefore, the decreased HSP70 expression observed in our study may be a result of changes in the ripening process caused by bagging treatment.
The abscisic stress ripening-like proteins belong to the abscisic acid/water deficit stress (ABA/WDS)-induced protein family, which consists of plant proteins induced by water deficit stress or ABA stress and ripening. Protein abscisic stress ripening-like proteins scavenge reactive oxygen species (ROS) in vitro (Kim et al., 2012). In this study, expression of abscisic stress ripening-like proteins (spots 3206, 3202, 4202, and 3203) was up-regulated in peach fruit by bagging treatment. Bagging generally caused earlier ripening, indicating that abscisic stress ripening-like proteins can serve as biomarkers of the peach ripening process. A previous study found that abscisic stress ripening-like proteins were differentially regulated in ripe fruit and fruit stored at a cold temperature (Nilo et al., 2010); further studies must be performed to determine the function of these proteins in peach fruit.
Polyphenol oxidase (PPO) catalyzes the hydroxylation of monophenols to o-dihydroxyphenols and the oxidation of o-dihydroxyphenols to o-quinones (Cabanes et al., 2007). In most cases, PPO activity progressively increases as fruit matures, with the greatest increase in the final days before harvest. PPO activity increases 1.5 times after ethylene and respiratory peaks in Calanda peach (Ferrer et al., 2005). Expression of 5 PPO precursors (spots 6716, 5711, 5705, 5716, and 6702) was up-regulated by bagging at 125 DAFB and 133 DAFB. Thus, bagging treatment caused earlier fruit ripening, which was associated with PPO expression. These results suggest that PPO can serve as a biomarker of the peach fruit ripening process.
Thioredoxin H (spot 3011) has been associated with fruit ripening in several species, including peaches (Callahan et al., 1993; Manrique-Trujillo et al., 2007; Levi et al., 2006). In addition, thioredoxin H has been found to be responsive to abiotic stressors, including chilling injury and drought (Yan et al., 2006; Hajheidari et al., 2007). In this study, thioredoxin H expression was down-regulated by bagging at 125 DAFB and debagging at 133 DAFB. We were unable to distinguish whether the change in thioredoxin H expression associated with bagging treatment was the result of changes in the ripening process or stress.
Proteins Associated with Photosynthesis
Oxygen-evolving enhancer protein 1 and chloroplast oxygen-evolving enhancer protein 1 are components of photosystem II (PSII), which generates ATP to allow plant growth and development. In addition, PSII provides a precise scaffold for pigments and cofactors that mediate vectorial primary charge separation and electron transfer (Feng et al., 2011). Oxygenevolving enhancer protein 1 (spot 1218) was only detected in non-bagged fruit, because such fruit have chlorophyll levels sufficient for photosynthetic activity. However, bagging treatment inhibited the accumulation and biosynthesis of chlorophyll. Even in fruit immediately exposed to sunlight after debagging, expression of chloroplast oxygen-evolving enhancer protein 1 (spot 3209) was markedly down-regulated. These results suggest that sunlight might be involved in the accumulation of phenolic compounds such as anthocyanin in peach peel.
Delta-aminolevulinic acid dehydratase catalyzes the synthesis of heme and Chl from the universal tetrapyrrole precursor (Shibata and Ochiai, 1977). Chl is the predominant tetrapyrrole in most plants. Delta-aminolevulinic acid dehydratase (spot 5401) expression was up-regulated in fruit that were re-exposed to sunlight after bagging. Chl is required for energy utilization when fruit are re-exposed to sunlight, leading to increased expression of delta-aminolevulinic acid dehydratase, which increases the photosynthetic capacity of the fruit.
Rubisco (spot 7503) is the key regulatory enzyme in the light-independent reactions of the Calvin cycle and catalyzes the first step in carbon fixation. The rate of photosynthesis was expected to decrease in bagged fruit subjected to low-light conditions. The bagged fruit at 125 DAFB and non-bagged fruit showed no difference in Rubisco expression. However, in comparison with non-bagged fruit, bagged fruit re-exposed to sunlight showed reduced Rubisco expression at 133 DAFB. The large change in photosynthesis occurring in fruit from 125 to 133 DAFB may have been a result of debagging.
These results show that expression levels of chloroplast oxygen-evolving enhancer protein 1, Rubisco, and deltaaminolevulinic acid dehydratase were changed in debagged fruit at 133 DAFB, indicating that fruit debagging affected biochemical pathways involved in photosynthetic carbon assimilation and protein turnover.
Proteins Associated with Carbohydrate Metabolism
Expression of UTP-glucose-1-phosphate uridylyltransferase (spot 6612), an enzyme involved in glycogenesis, was upregulated by bagging. UTP-glucose-1-phosphate uridylyltransferase catalyzes the formation of UDP-glucose from glucose- 1-phosphate and UTP. Bagging usually lowers the soluble carbohydrate content at harvest in apple (Watanabe et al., 2011) and pear fruit (Huang et al., 2009). Hiratsuka et al. (2012) reported that photosynthesis and phosphoenolpyruvate carboxylase (PEPC) activity were considerably inhibited after fruit bagging, while soluble carbohydrate content was reduced. In this study, the observed increase in UTP-glucose-1-phosphate uridylyltransferase expression was not associated with a change in soluble carbohydrate content. Other enzymes involved in soluble carbohydrate synthesis were not assessed in this study.
Malate dehydrogenase (MDH) reversibly catalyzes oxidation of malic acid to oxaloacetate via reduction of NAD+ to NADH and can thus reduce cellular malate acid concentrations. MDH expression was increased in bagged fruit at 125 DAFB and in debagged fruit at 133 DAFB (spots 5709 and 6808), but TA did not differ significantly among the treatment groups (Table 2). The contents of intermediate products may be affected by multiple factors. The predominant organic compounds in ripe peach fruit are malic and citric acids, while quinic acid is present in smaller amounts (Moing et al., 1998); however, no enzyme capable of citric acid metabolism was identified as being regulated by bagging.
Phosphoglucomutase is involved in carbohydrate metabolism in peach fruit. In tomato fruit, phosphoglucomutase activity declines during early fruit development, as does the expression level of its cytosolic isoform. In tomato fruit, the highest level of phosphoglucomutase expression was found in ripe fruit, which was not the case for fruit of wild accessions (Kortstee et al., 2007). Phosphoglucomutase (spot 5806) expression was down-regulated in bagged fruit. Alpha-amylase (spot 1412) catalyzes starch degradation in peach fruit. Alpha-amylase was expressed in non-bagged fruit, but not in bagged fruit. The decreased protein levels of phosphoglucomutase and alpha-amylase in bagged peach fruit suggest that bagging may inhibit carbohydrate metabolism. Future studies will be aimed at identifying other enzymes involved in carbohydrate metabolism that may be regulated by fruit bagging.
Proteins Associated with Amino Acid Metabolism
The aldo-keto reductase (AKR, spot 5313) superfamily is a large enzyme group of NADP-dependent oxidoreductases with numerous roles in metabolism (Kanayama et al., 2014). S-adenosyl-L-homocysteine hydrolase (SAHH, spot 5510) catalyzes the reversible hydrolysis of S-adenosyl-L-homocysteine into adenosine and L-homocysteine, which can be regenerated into S-adenosyl-L-methionine (SAM) through L-methionine and sustains 1-aminocyclopropane-1-carboxylic acid (ACC) synthesis and methylation. S-adenosylmethionine synthetase (spot 5404 and 5403) catalyzes the rate-limiting step of the methionine cycle. SAM is a methyl donor in transmethylation reactions and the propylamino donor in polyamine biosynthesis. In plants, arginase (spot 4309) is involved in nitrogen remobilization following protein degradation, especially during seed germination (King and Gifford, 1997; Goldraij and Polacco, 2000). AKR, SAAH, and arginase expression levels were down-regulated following bagging treatment, suggesting that bagging inhibited nitrogen metabolism during ripening.
Ketol-acid reductoisomerase (KARI) is an oxidoreductase that acts on the CH-OH group of a donor molecule and utilizes NAD+ or NADP+ as an acceptor. KARI (spot 5608) expression was up-regulated at 133 DAFB.
Proteins Aassociated with Protein Synthesis and Degradation
Proteasome subunit alpha, type-1-A (spot 0212) is part of a multicatalytic proteinase complex characterized by its ability to cleave arginine, phenylalanine, tyrosine, leucine, and glutamic acid at neutral or slightly basic pH. Chloroplast ATPdependent Clp protease proteolytic subunit 3 (spot 8411) is a Clp protease identified in Arabidopsis thaliana that is vital for chloroplast development and function (Sjögren et al., 2006). Removal of inactive polypeptides by proteases is particularly important during periods of stress, when the potential for protein damage is considerably increased. In bagged fruit, expression levels of proteasome subunit alpha, type-1-A and chloroplast ATP-dependent Clp protease proteolytic subunit 3 were up-regulated at 133 DAFB, suggesting that bagging altered protein metabolism.
DNA damage-inducible protein 1-like (spot 1302) acts as a linker between the 19S proteasome and polyubiquitinated proteins via UBA domains, facilitating degradation of ubiquitinated proteins. DNA damage-inducible protein 1-like expression was down-regulated at 133 DAFB, suggesting that proteasome-mediated degradation of ubiquitinated proteins may have been inhibited by bagging. Future studies will further evaluate changes in protein metabolism associated with fruit bagging.
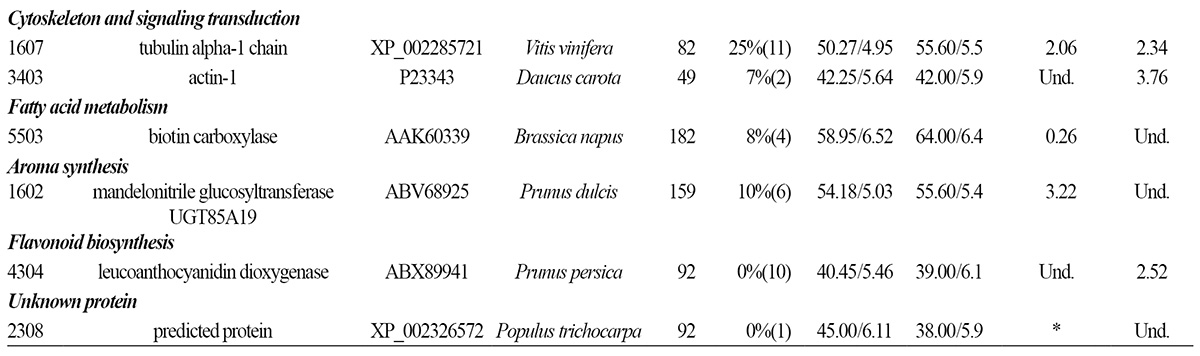
Proteins Associated with Fatty Acid Metabolism, Aroma and Flavonoid Biosynthesis
Biotin carboxylase (spot 5503) participates in fatty acid biosynthesis. In fruit, fatty acids serve as precursors of aromatic compounds (Krammer et al., 1991). In bagged fruit, biotin carboxylase expression was down-regulated at 125 DAFB, suggesting that the reduced aroma of bagged fruit may have been a result of reduced precursor abundance.
A recent study suggested that mandelonitrile glucosyltransferase (spot 1602) GT85A19 activity is associated with bitterness in almond kernels (Franks et al., 2008). In this study, mandelonitrile glucosyltransferase expression was upregulated in bagged fruit at 125 DAFB, which may have contributed to their reduced aroma. No further proteins with functions that could clearly be related to the aroma of peach fruit were identified as being differentially expressed in the bagged and non-bagged fruit.
Anthocyanidin synthase (spot 4304) participates in flavonoid biosynthesis. In this study, anthocyanidin synthase expression was up-regulated in bagged fruit at 133 DAFB, illustrating that bagging resulted in increased anthocyanin synthesis upon re-exposure of the bagged fruit to light.
Conclusion
Bagging has been used widely to improve fruit appearance, reduce pesticide residue levels, and increase commercial value. Fruit bagging is associated with changes in several characteristics of peach fruit, particularly fresh weight, skin color, and anthocyanin content. The present work reports a comprehensive proteomics analysis of peach fruit subjected to bagging in comparison with non-bagged fruit, with the goal of understanding the molecular mechanisms underlying the effects of fruit bagging. This investigation provides a preliminary overview of the important biological processes that contribute to the changes that occur in peach fruit following bagging. Functional classification of proteins with differential expression in bagged and non-bagged peach fruit revealed that the majority of regulated proteins were related to stress responses, photosynthesis, carbohydrate metabolism, amino acid metabolism, and synthesis of aromatic compounds. The functional analysis of the proteins regulated by bagging was consistent with some of the fruit phenotypes produced by bagging. Proteome analysis can be used to link genotype and phenotype during plant growth and development, but few proteins directly relatable to phenotype were identified in this study due to the limited sensitivity of 2-D methods. These results enrich the body of information concerning the effect of bagging on protein metabolism in peach fruit. Future studies should endeavor to further explore the molecular mechanisms underlying the effects of bagging on fruit.