Introduction
Materials and Methods
Plant Materials
Precooling by Hydrocooling with Micro-bubbled Water and Pressurized Air
1-MCP Application
Ethylene Production
Firmness of Fruit Pulp
Soluble Solids Content and Titratable Acidity
Trichome Distribution on the Fruit Surface
Statistical Analysis
Results
Ethylene Production and Tissue Softening of ‘Janghowon Hwangdo’ Peach Fruit
Changes in Fruit Firmness by the Hydrocooling Method
Changes in Ethylene Production in the Fruits Treated with Hydrocooling and 1-MCP
Changes in Firmness of the Fruit Treated with Hydrocooling and 1-MCP
Changes in Sweetness and Acidity of the Fruit
Trichome Distribution on the Surface of the Fruit by Hydrocooling
Discussion
Ethylene Production and Pulp Softening in Peach Fruit During Ripening
Effect of Hydrocooling and 1-MCP Treatment
Trichome Removal by Hydrocooling
Introduction
Peach (Prunus persica (L.) Batsch) fruit is one of the most popular summer fruits in Korea and possesses a unique taste, juiciness and aroma. Peach fruit can be divided into melting and non-melting types based on the rate of softening of flesh tissue during fruit ripening (Haji et al., 2001). Melting peaches have become more favored for fresh consumption due to their palatability. However, handling and marketing the melting-type fruit is difficult due to a short shelf-life and a narrow harvesting period. Studies have shown that the melting-type peach fruit undergoes rapid tissue softening after harvest due to senescence-related changes as well as physical damages (Lester et al., 1994; Abbott, 1999; Barry and Giovannoni, 2007; Seymour et al., 2013; Yong et al., 2016). Thus, it is important to develop appropriate methods to reduce postharvest loss by maintaining the freshness and palatability of peach fruit.
Peach fruit belongs to a typical climacteric fruit. As fruit ripening progresses, an increase in ethylene and carbon dioxide production, disappearance of greenish background of the fruit and concurrent increase in skin color development, and loss in firmness are manifested within a couple of weeks (Fan et al., 2002; Barry and Giovannoni, 2007; Schotsmans et al., 2009; Chun et al., 2010). Among these changes, ethylene evolution plays a critical role in the ripening process of peach fruit. For example, loss of fruit firmness is likely due to a breakdown of cell wall structure in flesh tissue by the activity of pectin degrading enzymes including polygalacturonases (PG) (Choi and Lee, 2003; Goulao and Oliveira, 2008). It has been reported that ethylene stimulates an increase in the expression of PG during fruit ripening (Pech et al., 2008; Hocking et al., 2016).
1-Methylcyclopropene (1-MCP) has been widely used for prolonging the shelf-life of fresh produce, mainly because besides its role in suppressing ethylene action, the gas is considered safe for the environment and humans (Watkins, 2006; Park, 2012; Zhang et al., 2020; Lwin and Lee, 2021; Win et al., 2021). 1-MCP binds to the ethylene receptors localized in subcellular membranes such as endoplasmic reticulum (ER) and Golgi body, thereby inhibiting ethylene action and biosynthesis (Chang et al., 1993; Bleecker and Schaller, 1996; Sisler and Serek, 1997; Blankenship and Dole, 2003).
Studies on delaying peach fruit ripening have been carried out using 1-MCP alone and combined with other treatments such as low temperature (Fan et al., 2002), aminoethoxyvinylglycine (AVG) (Hayama et al., 2008; Win et al., 2021), edible coatings using aloe extracts (Sortino et al., 2020), and sterilizers such as ozone and TiO2 (Du et al., 2020). The outcome of these studies is varied and depends on cultivars and maturity of the fruit being tested. Cooling of fresh fruit products is often considered as an efficient way of maintaining freshness. Precooling, which quickly lowers the pulp temperature of fresh products by means of cold water and/or cold air, diminishes microbiological activity, respiration, and ethylene production of the fruit (Fan et al., 2002; Kader et al., 2002; Majeed and Jawandha, 2016). These conditions enhance freshness and prolong the subsequent shelf-life of the produce. The objective of the present study was to determine the effect of hydrocooling and 1-MCP application by single or combined treatments on the quality of peach fruit (cvs. ‘Mibaekdo’ and ‘Janghowon Hwangdo’) during storage.
Materials and Methods
Plant Materials
Two cultivars of flesh-melting peach (Prunus persica (L.) Batsch), ‘Mibaekdo’, representing a mid-season harvesting type, and ‘Janghowon Hwangdo’, a late season harvesting type, were harvested from an orchard at Eumseong, Chungcheongbuk-do in Republic of Korea and used in this experiment. Maturity of peach fruit of ‘Janghowon Hwangdo’ was classified into 5 stages based on the extent of the green background on the surface of the fruit, from 100 (M1), 80 ± 10 (M2), 60 ± 10 (M3), 40 ± 10 (M4), and 20 ± 10 % (M5). After monitoring the changes in ethylene production and loss in tissue firmness from the fruit at various stages of maturity during subsequent storage at 25°C, M3-stage fruit were routinely harvested for the subsequent experiments. Those fruits displaying uniform coloration and similar size with no defect on the surface were sampled. ‘Mibaekdo’ fruit were also harvested at the M3 stage based on surface color development as described for ‘Janghowon Hwangdo’ fruit.
Precooling by Hydrocooling with Micro-bubbled Water and Pressurized Air
A water tank equipped with a micro-bubble generator at National Institute of Horticultural and Herbal Science was filled with 0°C ice water. Peach fruit were placed within the tank while retaining the temperature of the water at 0.0±1.0°C. A preliminary test on hydrocooling with 0°C water showed that it took approximately 20 min for the temperature of peach fruit (300 ± 50 g) to reach 10°C, which represents ¾th cooling. The precooling of peach fruit was carried out in a circulating water tank by submerging fruit into the water for 40 min at 0.0±1.0°C. After cooling, the fruit were returned into the packaging box and the surface water of the fruit was fully dried in ambient air for 1-2 hrs. The fruit was kept at 10°C for 15 days, when decay symptoms became visible. For the comparison of cooling methods, pressurized air-cooling was applied with a 1/2 hp (horse power) Sirocco fan at 0°C for 4 hrs.
1-MCP Application
In the preliminary test with 1.0, 2.0, and 3.0 µl·L-1 of 1-MCP, 3.0 µl·L-1 of 1-MCP at 20°C for 12 hrs was effective to maintain the green color on the fruit surface longer than control fruit. The fruit in a cardboard box were put in a chamber (378 L) and 1-MCP was added into the chamber according to the manufacturer’s manual (e-fresh, Republic of Korea). After treatment, fruit were kept at 10°C for 15 days and ethylene evolution as well as quality of the fruit were examined.
Ethylene Production
Ethylene production from the fruit was monitored every 24 hours. A peach fruit was placed in an airtight plastic bottle (1.24 L) and sealed for an hour. Then a 3 mL gas-tight syringe with a #22 needle was used to collect the gas sample inside the container. The sample was injected into a gas chromatography machine (M600D model, Younglin, Korea). These steps were executed 3 times for each treatment. The ethylene analysis was carried out using an Al2O3Na/column (Chromopack, 0.53 mm x 50 m) and a FID (flame ionization detector) with an oven temperature of 70°C. Both injector and detector temperature were maintained at 220°C. Helium gas was used as a carrier gas.
Firmness of Fruit Pulp
During the storage period of 15 days at 10°C, the firmness of fruits were measured after 0, 1, 2, 4, 8, 12, and 15 days in storage. Tissue samples were made by punching out the tissue from the equatorial region of the fruit using a cork-borer (diameter, 2.0 cm). Firmness was measured with a 5.0 mm probe of the Texture Analyzer (TA-XT Express, England). Finally, the highest peak value on the texture profile chromatogram was expressed in Newtons (N).
Soluble Solids Content and Titratable Acidity
While storing the peaches at 10°C, soluble solids content (SSC) and titratable acidity (TA) of the fruits were examined. The SSC was measured using a hand-refractometer (CR-10, Atago, Tokyo, Japan). The TA was determined by taking 10 ml of extracted juice and titrating with 0.1N NaOH until it reached pH 8.2. Malic acid (Sigma-Aldrich) was used to calculate TA.
Trichome Distribution on the Fruit Surface
The spatial distribution of trichomes on the surface of the fruit before and after hydrocooling with micro-bubbled water was monitored using a digital camera (Coolpix 5700, NIKON, Tokyo, Japan) at 8.0-fold magnification.
Statistical Analysis
All statistical analyses were performed using SAS statistical software version 9.3 (SAS Institute Inc., Carry, NC, USA). Data were presented as mean ± standard error (SE). Analysis of variance was applied to evaluate the significance of main factors and the interaction effects during storage. This was followed by the least significance difference (LSD) test to determine the mean difference at p < 0.05.
Results
Ethylene Production and Tissue Softening of ‘Janghowon Hwangdo’ Peach Fruit
The ripening behavior of peach (cv. Janghowon Hwangdo) fruit harvested at different maturities was monitored by investigating ethylene production and tissue softening during storing at 25°C. Development of the fruit was divided into five stages (M1-M5) based on the surface color development (Fig. 1A). M1 fruit consistently maintained lower amounts of ethylene up to 4 days in storage and then ethylene production began to increase slowly, but steadily, during the subsequent storage period (4-12 days). M2 fruit began to increase sharply in ethylene production from 4 days after harvest, which consistently increased until 12 days in storage (Fig. 1B). Fruit harvested at M3 stage immediately began producing ethylene after harvest and peaked at 6 days followed by a gradual decline until 12 days in storage, showing a typical climacteric pattern. However, fruit of M4 stage, the conventional harvest stage of ripeness, showed significantly higher levels of ethylene even at harvest (0 days), suggesting that M4 stage fruit peak in ethylene production at 3 days in storage (Fig. 1B). The fruit harvested at M5 stage, of which is the customary practice, showed consistently reduced levels of ethylene, indicating that these fruit are in a post-climacteric ethylene production stage.
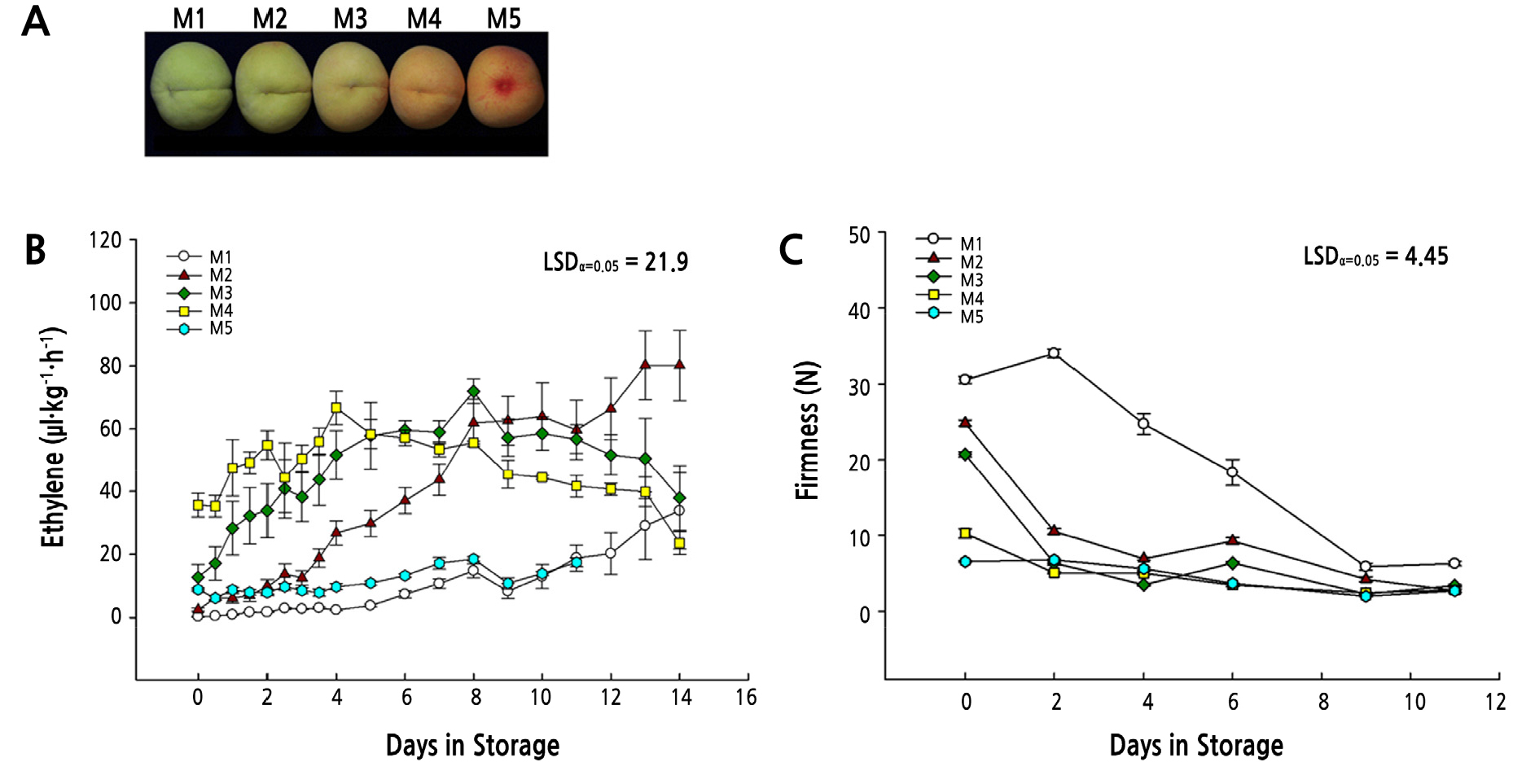
Fig. 1.
Ethylene production (B) and loss in firmness (C) of peach ‘Janghowon Hwangdo’ fruit harvested at different maturities (A: M1-M5) during ripening at 25°C. Maturity of the fruit was classified based on surface color development as shown in the picture on the top. Details for defining the maturity of the fruit were described in the materials and methods.
The firmness of peach fruits harvested at M1 stage exhibited more than 30 N during the initial 2-3 days of storage, and thereafter sharply decreased to less than 10 N in 7 days, at which it reached complete softness (Fig. 1C). Immediately after storage, fruit at M2 and M3 stages exhibited a rapid decline in pulp firmness, whereas fruits of the M4 and M5 stage were already soft and remained constant through storage (Fig. 1C). The results shown in Fig. 1 indicated that loss of pulp firmness of the peach fruits began earlier, 3 to 4 days earlier in fruit harvested at M1 stage, than the increase in ripening-associated ethylene production.
Changes in Fruit Firmness by the Hydrocooling Method
Cooling treatment on harvested fruit can improve postharvest fruit quality. We investigated the effect of two different cooling methods (pressurized-air cooling and hydrocooling) on reduction of fruit temperature (Fig. 2). The temperature of the air and water during cooling treatment was maintained at 0°C. To reach a fruit temperature of 10°C, which represents ¾ cooling, the pressurized-air cooling method took approx. 180 minutes, whereas it took about 20 minutes using the hydrocooling method (Fig. 2). Fruit firmness was determined before and after 1 hr of the two different cooling treatments. Before cooling, the firmness of the fruit (cv. ‘Janghowon Hwangdo’) was 39.5 ± 5.0 N, and then decreased sharply to 29.5 ± 5.0 N after 1 hour at ambient temperature (Table 1). Firmness of the fruit treated with the pressurized-air cooling method declined to 29.0 ± 1.5 N, showing a similar level of firmness to control fruit. Meanwhile, the fruit treated with hydrocooling increased to 44.0 ± 7.5 N (Table 1), indicating that precooling with 0°C cold water significantly increased the firmness of the peach fruit when compared to untreated fruit or pressurized-air cooling.
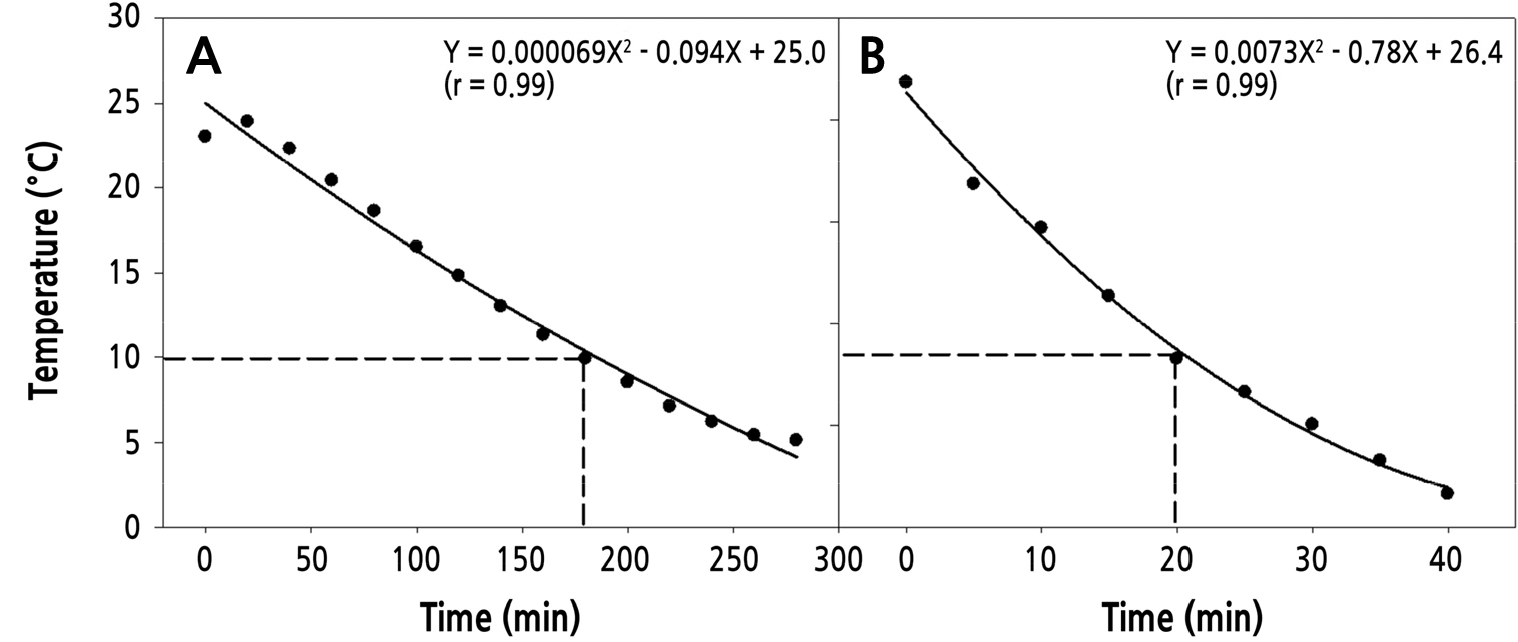
Fig. 2.
Temperature changes of peach ‘Janghowon Hwangdo’ fruit during pressurized-air cooling (A) and hydrocooling (B). Air temperature for pressurized-air cooling and water temperature for hydro-cooling was 0°C during cooling treatments. Details of cooling were described in the Materials and Methods.
Table 1.
Comparison of the cooling method with regard to changes in firmness of peach fruit (‘Janghowon Hwangdo’)
| Treatment | Firmness (N) | |
| Before | After | |
| No cooling | 29.5 ± 5.0 | |
| Pressurized-air cooling | 39.5 ± 5.0 | 29.0 ± 1.5z |
| Hydro-cooling | 44.0 ± 7.5z | |
Changes in Ethylene Production in the Fruits Treated with Hydrocooling and 1-MCP
The ethylene production in ‘Mibaekdo’, a mid-season harvesting type, was monitored according to the different treatments. In the control, ripening of the ‘Mibaekdo’ peach fruit progressed rapidly and the ethylene level continuously increased until 11 days after storage at 10°C (Fig. 3A). In the hydrocooling treatment, peach fruit did not show a significant difference in ethylene production compared to the control. However, there was a reduced ethylene level in the fruit treated with 1-MCP alone or hydrocooling plus 1-MCP (Fig. 3A). At the climacteric peak of ethylene production, the control fruit produced 55.0 µL·kg-1·h-1 of ethylene while the fruit treated with hydrocooling plus 1-MCP produced 38.0 µL·kg-1·h-1 of ethylene, approximately 70% of the control (Fig. 3A).
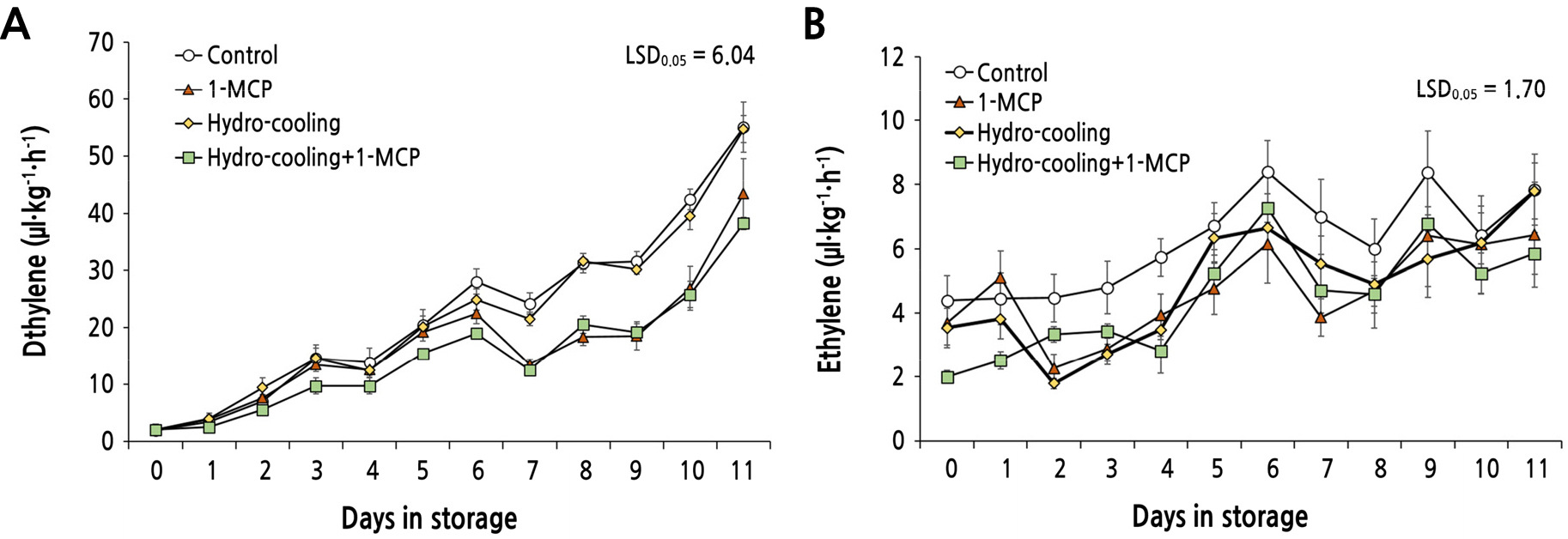
Fig. 3.
Changes in ethylene production from peach ‘Mibaekdo’ (A) and ‘Janghowon Hwangdo’ (B) fruit treated with 1-MCP, hydro-cooling, and their combination during storage at 10°C. The maturity of the fruit used in the experiment was when the green background of the fruit surface was 60 ± 10%. Data were expressed as mean ± SE of five fruits.
Late season harvesting type, ‘Janghowon Hwangdo’, fruit also exhibited characteristic climacteric ethylene production with a slightly slower rate and much lower amount of ethylene (Fig. 3B) compared to ‘Mibaekdo’ peach fruit. ‘Janghowon Hwangdo’ fruit treated with both hydrocooling and 1-MCP showed little significant impact on suppression of ethylene production (Fig. 3B). Rather, control fruits were similar or even lower in ethylene content throughout storage. This result indicates that the effectiveness of ‘hydrocooling’ and/or ‘1-MCP’ treatment on ethylene production of peach fruit might differ depending on the cultivars.
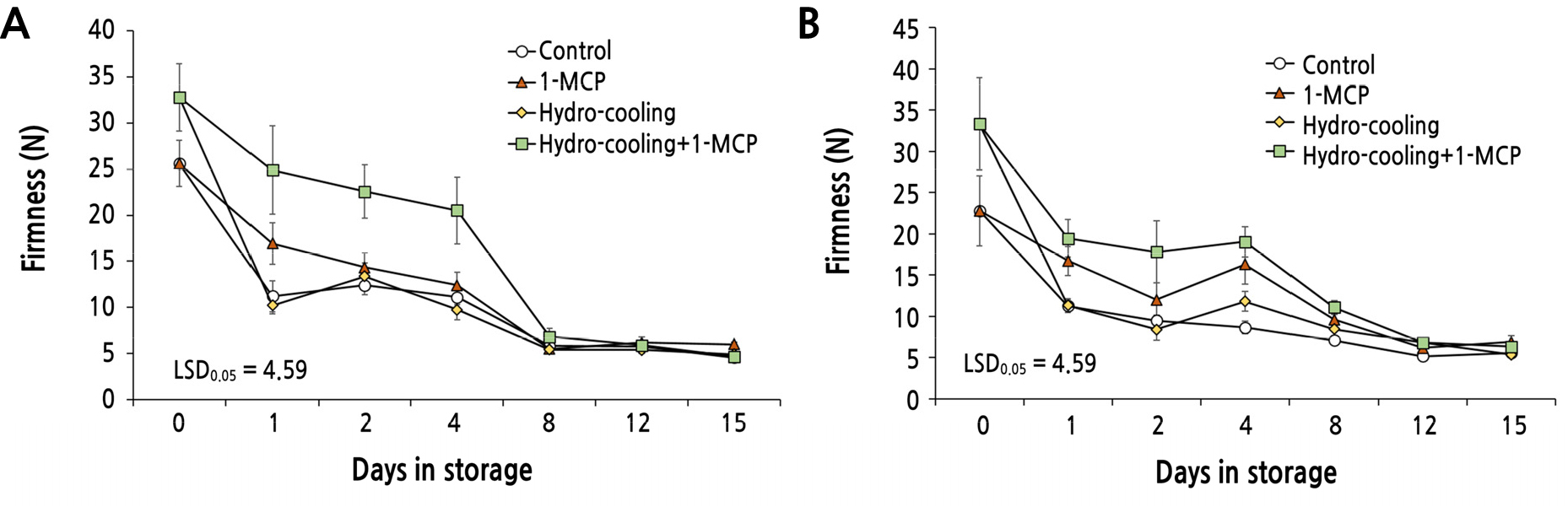
Changes in Firmness of the Fruit Treated with Hydrocooling and 1-MCP
Hydrocooling or 1-MCP, and the combination of both treatments affected pulp softening of peach fruit during subsequent storage at 10°C. There was a substantial delay in the loss of firmness of the fruit applied with the combined treatment compared to the control in both ‘Mibaekdo’ and ‘Janghowon Hwangdo’ fruit (Fig. 4). First, the combined treatment of hydrocooling and 1-MCP on ‘Mibaekdo’ peach fruit clearly suppressed pulp softening for approximately 1 week after treatment (Fig. 4A). However, after 1 week, the firmness of all fruit reached below 7 N, which is the lower limit of firmness for marketability of peach fruit. In addition, no significant difference was observed in pulp softening from the fruits treated with hydrocooling or 1-MCP compared to the control in ‘Mibaekdo’ fruit (Fig. 4A). In ‘Janghowon Hwangdo’ fruit, 1-MCP resulted in a small but significant delay in pulp softening (Fig. 4B). However, a single treatment of hydrocooling did not produce a significant effect on retaining pulp firmness in both cultivars (Fig. 4A and 4B).
Changes in Sweetness and Acidity of the Fruit
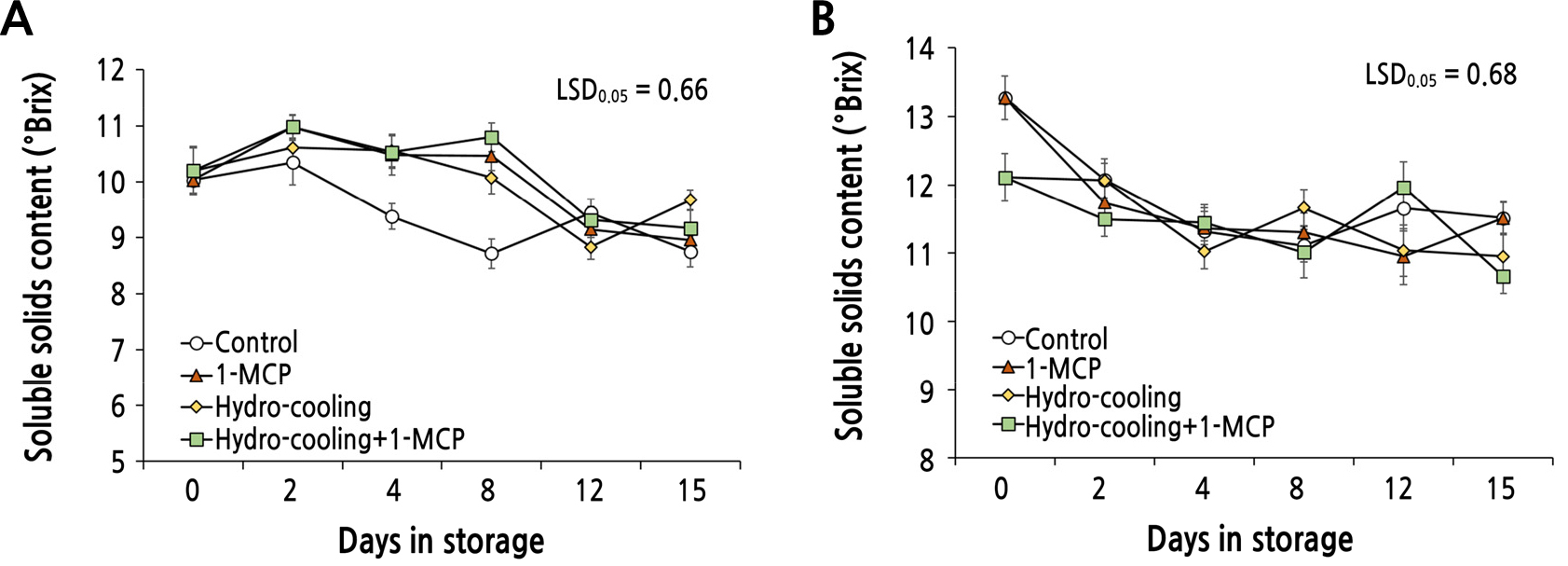
The soluble solids content (SSC; green rectangle in Fig. 5A) of the ‘Mibaekdo’ fruit treated with both hydrocooling and 1-MCP was maintained at 10-11% during the 1st week of storage, and thereafter declined to 9% in the 2nd week of storage. However, the SSC of the control fruit dropped noticeably to 8.7% during the 1st week of storage (Fig. 5A). This indicates that both hydrocooling and the combined treatment on ‘Mibaekdo’ peach fruit positively affect the SSC for at least 1 week compared to the untreated control (Fig. 5A). In the case of ‘Janghowon Hwangdo’ fruit, the control and 1-MCP treatment exhibited a significant drop of SSC during the initial 4 days in storage, and thereafter was maintained at a similar level (Fig. 5B). Meanwhile, the SCC in the combined treatment and the hydrocooling only treatment remained roughly unchanged throughout storage (Fig. 5B).
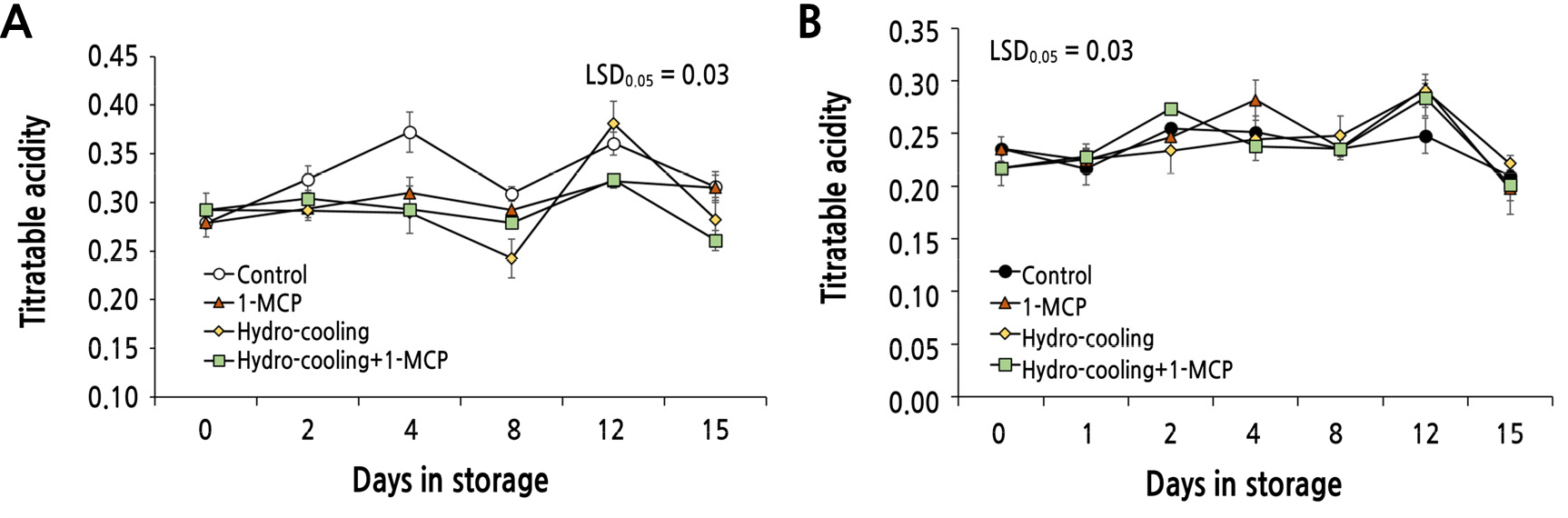
Next, we investigated the effect of hydrocooling and/or 1-MCP treatment on the acidity of both ‘Mibaekdo’ and ‘Janghowon Hwangdo’ fruit (Fig. 6). The TA of ‘Mibaekdo’ fruit applied with the combined treatment was significantly lower after the 2nd week of storage (Fig. 6A), while the other treatments had no effect on the acidity of ‘Mibaekdo’ peaches. The initial acidity of the ‘Janghowon Hwangdo’ peach fruit was lower than that of ‘Mibaekdo’ fruit, approximately 0.23. The TA of ‘Janghowon Hwangdo’ fruit remained mostly unchanged among treatments (Fig. 6B).
The results shown in Figs. 5 and 6 regarding to SSC and TA of the fruit indicate that the quality of the peach fruit treated with hydrocooling and/or 1-MCP is temporarily improved compared to the control during early storage.
Trichome Distribution on the Surface of the Fruit by Hydrocooling
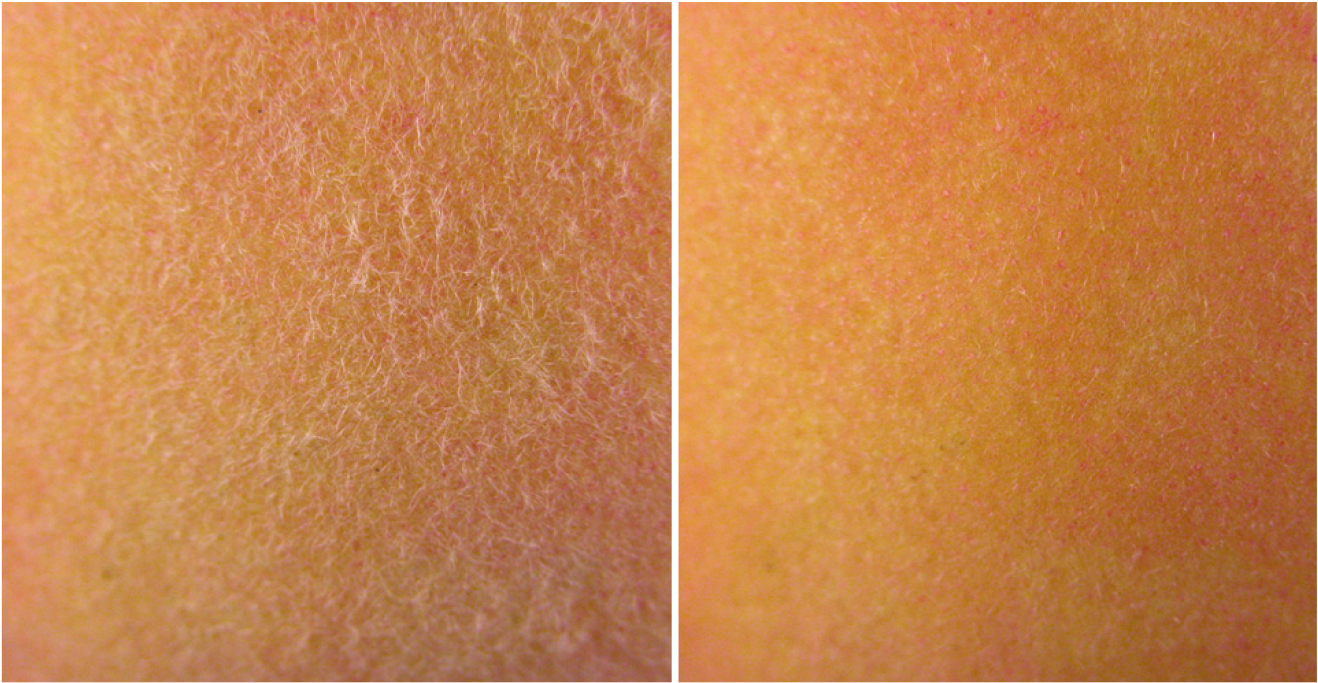
The surface of both ‘Mibaekdo’ and ‘Janghowon Hwangdo’ fruit is covered with dense hairs. Previous studies on peach fruit pubescence have elucidated that the surface of peach is covered with trichomes and cuticles, which may serve various protective purposes (Fernández et al., 2011). Studies on the application of 1-MCP to peach fruit have been controversial, mainly due to the presence of trichomes on the surface of the fruit, which may serve as a barrier to gas diffusion. 1-MCP may not be as effective as expected. As shown in Fig. 5, hydrocooling removed most of the hair on the peach fruit which may allow 1-MCP to diffuse through the skin tissue. As depicted in Fig. 3, 1-MCP application to ‘Mibaekdo’ fruit had a positive effect by suppressing ethylene production, lending to firmer tissue during storage for 2 weeks. An increase in the firmness of peach fruit using hydrocooling with 0°C cold water could extend the market life as well as storability of the fruit. In addition, removal of trichomes from the surface of the fruit by the treatment may enhance the marketability since consumers have frequently experienced an allergy-like response to peach fruit. Further studies should be conducted regarding the removal of trichomes of peach fruit on storability and water loss through the surface.
Discussion
Ethylene Production and Pulp Softening in Peach Fruit During Ripening
When ‘Janghowon Hwangdo’ peach fruit was harvested based on development of the green background color of the surface of the fruit, ranging from 20 to 100%, the ethylene production pattern differed during the subsequent storage at 10°C as shown in Fig. 1. Among them, M3-stage fruit exhibited typical climacteric ethylene production, which comprises the pre-climacteric, peak in climacteric, and post-climacteric stage of development. Numerous reports showed that the pre-climacteric stage of fruit development is the optimum stage for investigating the effect of postharvest treatment including ethylene, ethylene inhibitors, and other biotic and abiotic stimuli on shelf-life (Kader, 2002; Schotsmans et al., 2009). Based on the result shown in Fig. 1, we determined that M3 stage fruit is the optimum maturity for investigating the effect of hydrocooling and/or 1-MCP treatment on peach fruit ripening and pulp softening, as was also done in ‘Mibaekdo’ peach fruit.
It is noteworthy that a loss in flesh firmness of ‘Janghowon Hwangdo’ peach fruit begun during the pre-climacteric stage, that is, before an increase in ethylene production as shown in M1 and M2 stage fruit. Immediately after harvest, the firmness of M4 and M5 stage fruit was already at the lowest threshold for marketing (Fig. 1). ‘HetsalHaunkeybee’ peach fruit also showed a decrease in pulp firmness with little increase in ethylene production during storage at 10°C (Yoo et al., 2019). In addition, Trainotti et al. (2003) provided the gene expression profiles involved in fruit softening and ethylene biosynthesis in ripening peaches, explaining that fruit softening begins well before the climacteric increase in ethylene production. Similar changes have been reported in fruits such as banana, kiwi, melon, and persimmon during maturation and ripening (Kader, 2002; Pech et al., 2008). These fruits are known to be highly sensitive to ethylene, indicating that tissue softening is initiated in the presence of very low concentrations of the gas, often well before the onset of a climacteric rise (Choi and Lee, 2003; Liguori et al., 2004; Barry and Giovannoni, 2007).
It has been widely accepted that climacteric fruits have system 1 and system 2 ethylene production, whereas non-climacteric fruits such as citrus, grape, and strawberry possess only system 1 ethylene production (McMurchie et al., 1972; Barry and Giovannoni, 2007). System 1 is ethylene auto-inhibitory and responsible for producing basal levels of ethylene in all tissues including the pre-climacteric stage of fruits. System 2 is functional during the ripening of climacteric fruit when ethylene operates auto-stimulatory, called ‘autocatalytic’. Further studies should elucidate the mechanism by which loss in pulp firmness of peach fruit before an increase in ethylene production is initiated by system 1 and/or system 2 ethylene production or other mechanisms that underlie fleshy softening.
Effect of Hydrocooling and 1-MCP Treatment
The increase in pulp firmness by hydrocooling, but not in pressurized-air cooling, may come from the inhibition of water loss and/or the increase in cell turgor pressure by the cold water. In ‘Hass’ avocado fruit, immersion in ice water for 30 min delayed ripening associated changes including pulp softening and ethylene production. Inhibition of fruit softening was associated with a decrease in polygalacturonase (PG) and endo-𝛽-1,4-glucanase activities; both enzymes are involved in pectin degradation in cell wall carbohydrates (Chen et al., 2017). Precooling at 3°C for 30 min also increased the mechanical strength of potato tissues (Alvarez and Canet, 1997).
The rate of decrease in pulp firmness was not significantly different between the fruit treated with 1-MCP or hydrocooling. However, fruit applied with the combined treatment had significantly delayed pulp softening at least 4 days into storage, indicating that the combined treatment brought a synergistic effect. This effect was more pronounced in ‘Mibaekdo’ peach, a mid-season harvesting and faster-melting type than ‘Janghowon Hwangdo’, a late-season harvesting and slower-melting type. The fact that during ripening, the ‘Mibaekdo’ peach produced much more ethylene than the ‘Janghowon Hwangdo’ fruit (Fig. 3) implies the difference of the effect of 1-MCP and hydrocooling on after-ripening of theses fruits.
Though peach fruit are climacteric and undergo pulp softening as reported in previous studies, the relationship between ethylene production and fruit softening is not fully understood (Fan et al., 2002; Barry and Giovannoni, 2007; Schotsmans et al., 2009; Chun et al., 2010). Since peach fruit softening begins significantly earlier than the increase in ethylene evolution as shown in Fig. 3 as well as previous studies (Tonutti et al., 1991), 1-MCP could be effective in slowing softening. However, this inhibitory effect occurs only while the inhibitor is present and for 1-2 day after the end of treatment (Rasori et al., 2002; Liguori et al., 2004). Delaying ripening of peach fruit using 1-MCP combined with other treatments such as low temperature treatment (Fan et al., 2002) has been reported with a pronounced effect over 1-MCP alone. Thus, treatment of 1-MCP alone could inhibit climacteric ethylene production but may not be enough for suppressing pulp softening. It is noteworthy that in apple fruit, for example ‘Fuji’, 1-MCP blocks ethylene production to a basal level and retains fruit firmness for several month.
The results shown in Figs. 3 and 4 regarding SSC and TA of the fruit indicate that quality of peach fruit treated with both hydrocooling and 1-MCP is improved for several days compared to the control during early storage.
Trichome Removal by Hydrocooling
The surface of both ‘Mibaekdo’ and ‘Janghowon Hwangdo’ fruits exocarp is covered with a dense indumentum constituted by trichomes from 100 to 1,000 µm in length (Fig. 1 in Fernández et al., 2011). Previous studies on the pubescence of peach fruit skin have reported that the surface of the peach is covered with trichomes and cuticles, which may have various protective functions (Fernández et al., 2011). Studies on the application of 1-MCP to peach fruits have proven difficult, mainly because of the presence of trichomes that cover the surface of the fruit, which may serve as a barrier to gas penetration into pericarp tissues. 1-MCP may not be as effective as expected in terms of penetration of the gas into the interior of the fruit, which may be hindered by the hairs that develop densely on the surface of the fruit. However, in the present experiment, 1-MCP application to ‘Mibaekdo’ fruits did have a positive effect, suppressing ethylene production and allowing the fruit to remain firmer during storage for two weeks. In addition, hydrocooling removed most of the hair on the peach fruits (Fig. 7), which may facilitate the diffusion of 1-MCP into flesh tissues through the epidermal layers.
Increased firmness of peach fruits using hydrocooling with cold water at 0°C could extend the market life and improve the storability of the fruit. In addition, removal of trichomes from the surface of the fruit may enhance the marketability of the fruit, since some consumers have frequently experienced allergy-like responses to peach fruit upon touching. Further studies should investigate the effects of trichome removal from peach fruits on storability and water loss through the surface layers during postharvest handling to promote marketability of the fruit.