Introduction
Materials and Methods
Preharvest Treatment and Harvest
1-MCP Treatment
Assessment of Fruit Quality Attributes
cDNA Synthesis and Quantitative Real-Time PCR (qRT-PCR)
Statistical Analyses
Results
Fruit Quality Attributes
Ethylene Synthesis and Gene Expression
Expression of Ethylene Receptor and Signaling Genes
Discussion
Introduction
The plum (Prunus subg. Prunus ‘Formosa’) is a climacteric fruit with a shortened shelf life after harvest due to the changes in physicochemical properties related to the ripening process, which are all stimulated by ethylene production (Singh and Khan, 2010). Furthermore, continuous respiration of plums after harvest also facilitates postharvest softening of plum fruit and decreases the commercial quality in a very short storage period (Lelièvre et al., 1997; Singh and Khan, 2010).
Senescence and ripening progression in plants accelerated by ethylene can be delayed by using inhibitors of ethylene biosynthesis and action. Aminoethoxyvinylglycine (AVG), an ethylene biosynthesis inhibitor, suppresses ethylene synthesis through inhibition of the activity of 1-aminocyclopropane- 1-carboxylic acid (ACC) synthase (ACS) enzyme, a key enzyme in the ethylene biosynthetic pathway (Capitani et al., 2002). AVG inactivates the structure of the ACS enzyme and blocks the conversion of S-adenosyl-L-methionine (SAM) to ACC (Capitani et al., 2002; Capitani et al., 2005). It has been shown that AVG treatment effectively decreases ethylene production and the postharvest disorders in many climacteric fruits, such as tomatoes (Saltveit, 2005), plums (Ozturk et al., 2015), apples (Yuan and Carbaugh, 2007), pears (Xie et al., 2015), and apricots (Muñoz-Robredo et al., 2012). The ethylene binding inhibitor 1-methylcyclopropene (1-MCP) has a strong chemical affinity to ethylene receptors, thereby preventing ethylene binding to the receptors and repressing the ethylene signaling pathway (Kamiyoshihara et al., 2012). The application of 1-MCP significantly prolongs the postharvest storage period and quality of many horticultural crops, such as broccoli, cucumbers, carnations, roses, petunias, delphiniums (Sisler et al., 1986; Serek et al., 1995; Cameron and Reid, 2001; Ha et al., 2020; Yoo et al., 2020), bananas, apples, avocados, pears, persimmons, and peaches (Watkins, 2006; Kim et al., 2018; Win et al., 2018).
Although 1-MCP treatment significantly retards fruit ripening and extends the postharvest life of plums and peaches (Khan and Singh, 2007; Hayama et al., 2008; Luo et al., 2009), it is not generally used in the postharvest industry of climacteric fruits due to its lower effectiveness during postharvest distribution of the fruit (Mathooko et al., 2001; Cin et al., 2006; Hayama et al., 2008). To maintain the high quality of plums during distribution, effective and practical techniques that can protect fruit against ethylene damage need to be developed. In this study, we investigated the effectiveness of suppressing ethylene action using preharvest AVG and postharvest 1-MCP treatments to maintain the fruit quality attributes of ‘Formosa’ plum fruit in a postharvest distribution system. Furthermore, we analyzed the expression patterns of ethylene biosynthesis and signaling genes in plums after AVG and 1-MCP treatments to elucidate the relationship between ethylene responses and postharvest quality of the fruit.
Materials and Methods
Preharvest Treatment and Harvest
Plum (Prunussubg. Prunus ‘Formosa’) fruit were harvested at a conventional plum orchard in Uiseong, Gyeongsangbuk-do, Republic of Korea. Ten days before harvest, the fruit were sprayed with 150 mg·L-1 AVG to inhibit ethylene biosynthesis. AVG powder (ReTainTM, AVG 15%, ValentBioSciences, Libertyville, IL, USA) was dissolved in distilled water to prepare a 150 mg·L-1 solution. Untreated (control) fruit were sprayed with distilled water. Plum fruit were harvested at commercial maturity stage (9 N firmness and 12% acidity ratio) and packed in cardboard boxes and transported within an hour to a postharvest physiology laboratory at the Division of Horticulture and Medicinal Plant, Andong National University in Andong, Republic of Korea. At the laboratory, disease-free fruit of a uniform size and quality were randomly selected for postharvest treatments.
1-MCP Treatment
Plum fruit were fumigated in the treatment chambers with 1 µL·L-1 1-MCP (Ecoplants Co., Ltd., Gyeonggi-do, Republic of Korea) for 24 h at 20 ± 1°C for 1-MCP treatment. Untreated control fruit were incubated in similar chambers with normal air. The 1-MCP (provided by Ecoplants Co., Ltd., Gyeonggi-do, Republic of Korea) was applied via FreshLongTM sticks in the treatment chambers at 20 ± 1°C. To prepare 1 µL·L-1 1-MCP gas, the stick was broken twice and directly put into the chambers. After the treatment, the fruit were packed in cardboard boxes and stored in the chambers in the dark at 20 ± 1°C and 60 ± 2% relative humidity for postharvest quality evaluation.
Assessment of Fruit Quality Attributes
Flesh firmness, fresh weight loss, soluble solid content (SSC), titratable acidity ratio, skin color, decay, and ethylene production of fruit were measured during the postharvest period. After removing the skin of the fruit, the flesh firmness of each fruit was measured using a fruit texture analyzer fitted with ø 5-mm cylindrical probe (TA-XT2 Texture Analyzer, SMS, UK). For SSC and acidity ratio measurements, the fruit tissues were ground into a juice using a mortar and pestle. Subsequently, 1 g of fruit juice was poured into a beaker containing 50 mg distilled water and diluted by gentle stirring. The SSC and acidity ratio of the fruit were measured using a Pocket Brix-Acidity Meter (Plum) (Atago Co., LTD, Tokyo, Japan). The quality of plum fruit was also assessed by determining the extent of decay with three replicates per treatment. Plums were assessed for decay symptoms at 6 and 10 d after storage when the fruits showed a visible decay symptom on the peel. The fruit decay was assessed on a relative scale of 0 to 3 according to the percentage of rotten area on the fruit skin, as follows: 0: 0%, 1: 1-30%, 2: 31-50%, and 3: 51-100%. The peel color of fruit was measured over three replicates using a chromameter (CR-10, Minolta Corp., Osaka, Japan). Ethylene production was measured using gas chromatography (GC, Hewlett-Packard 5890, Palo Alto, CA, USA) equipped with a flame ionization detector (FID) and alumina column. The temperatures for the injector, column, and detector were 110°C, 50°C, and 250°C, respectively. Plum fruit were incubated in a 1 L jar at 20 ± 1°C for 1 h, and 1 mL of air was taken from the headspace of the jar and injected into the GC for ethylene analysis.
cDNA Synthesis and Quantitative Real-Time PCR (qRT-PCR)
Six flesh portions were detached from each plum fruit on day 6 of the storage period. The fruit flesh (200 mg) was mashed with liquid nitrogen using a pestle and mortar for total RNA extraction using the Ribospin Plant kit (Gene All, Gen All Biotechnology Co., LTD, Seoul, Republic of Korea). The first strand of cDNA was synthesized from sterile water, total RNA, and oligo (dT)15 primers by heating to 70°C for 5 min. After placing on ice for 1 min, the reagents were supplemented and mixed gently as the follows: 5× RT buffer (4 µL), dNTP (2 µL), DTT (2 µL), and AMV RT enzyme (0.5 µL) (INTRON Biotechnology, Inc., Gyeonggi-do, Republic of Korea). The reverse transcription was conducted in a Bio-Rad PTC-100 Programmable Thermal Controller (MJ Research, Inc., Hercules, CA, USA) at 42°C (1 h) and heated to 70°C (5 min) to complete the reaction.
The expression levels of five ethylene biosynthesis (PsACS1, PsACS3, PsACS4, PsACS5, and PsACO1) and signaling genes (PsETR1, PsERS1, and PsCTR1) in plums were detected using the Bio-Rad CFX Connect Real-Time System (Life Science, Hercules, CA, USA). Primer sequences used for qRT-PCR are shown in Table 1. The reaction mixtures contained 5 µL cDNA as a template, 10 µL iQ™ SYBR® Green Supermix (Bio-Rad, Life Science, Hercules, CA, USA), 1 µL forward and reverse primers (10 µM), and 4 µL water. The qRT-PCR reaction was performed using the following thermocycling pattern: 95°C for 60 s for pre-denaturation (one cycle), followed by 40 cycles of 95°C for 10 s (denaturation), 65°C for 10 s (annealing), and 72°C for 40 s (extension).
Table 1.
The primer sequences used for gene expression analysis in plum fruit
Statistical Analyses
All measurements were conducted on three biological replicates for each treatment and three fruits per replicate. Data are shown as mean ± standard error (SE). Data were subjected to two-way analysis of variance at p = 0.05 using SPSS 20.0 (IBM, Somers, NY, USA), and Duncan's multiple range test was used to compare the differences among the treatments.
Results
Fruit Quality Attributes
Changes in postharvest quality attributes of fruit during storage period were investigated to assess the effectiveness of the AVG and 1-MCP applications. After 6 d of postharvest storage, untreated control fruit exhibited a dark red color of the skin through an increase in the a* value. Color changes were effectively delayed by the combination treatment of AVG and 1-MCP at 6 d after storage, compared with the result from other treatments (Figs. 1 and 2A).
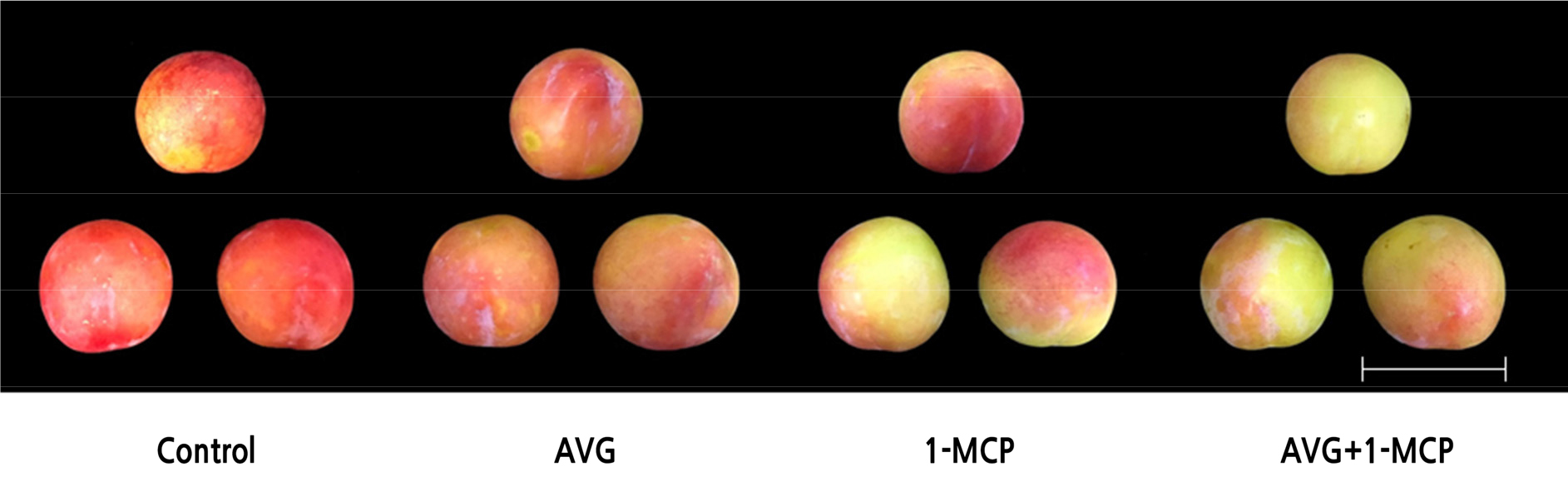
Fig. 1.
Effect of AVG and 1-MCP treatments on the appearance of plum fruit after 6 d of storage in the dark at 20 ± 1°C and 60 ± 2% relative humidity. Plum fruit were sprayed preharvest with 150 mg·L-1 AVG and postharvest with 1 µL·L-1 1-MCP for 24 h. Control, untreated fruit; AVG, 1-MCP, and AVG+1-MCP, fruit were treated with AVG (aminoethoxyvinylglycine), 1-MCP (1-methylcyclopropene), and combination of AVG and 1-MCP. Scale bar = 6 cm.
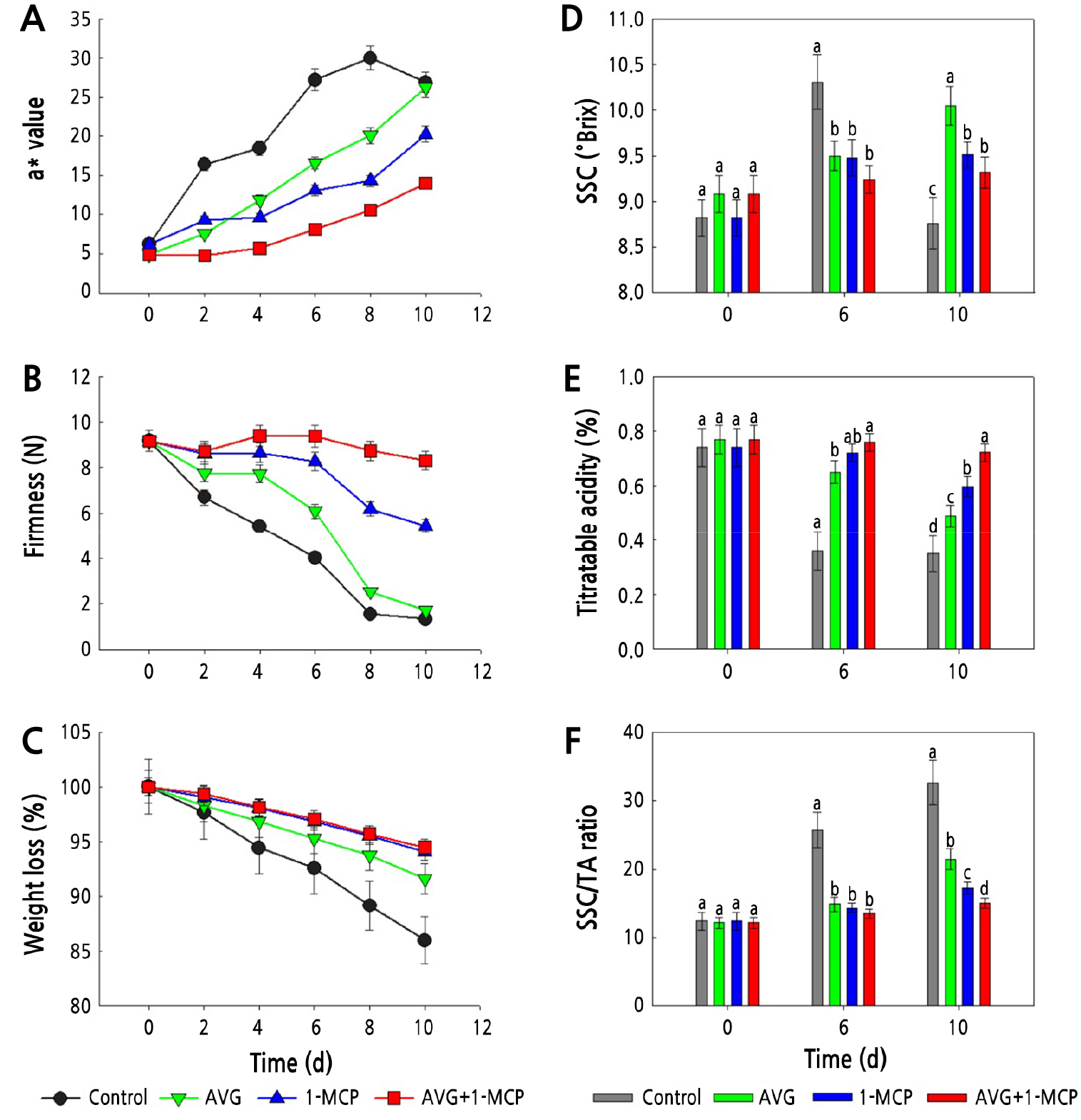
Fig. 2.
Effect of AVG and 1-MCP treatments on skin color change (A), flesh firmness (B), weight loss (C), SSC (D), titratable acidity ratio (E), and SSC/TA ratio (F) of plum fruits during storage in the dark at 20 ± 1°C and 60 ± 2% relative humidity. Plum fruit were sprayed preharvest with 150 mg·L-1 AVG and postharvest with 1 µL·L-1 1-MCP for 24 h. Control, untreated fruit; AVG, 1-MCP, and AVG+1-MCP, fruit were treated with AVG (aminoethoxyvinylglycine), 1-MCP (1-methylcyclopropene), and combination of AVG and 1-MCP. Error bars are SE of 3 replications. The values followed by different letters in D, E, and F are significantly different (p < 0.05) based on Duncan's multiple range test (n = 3).
Flesh firmness of untreated control plums rapidly decreased with progression in storage duration (Fig. 2B). The flesh firmness values were higher in AVG and single 1-MCP treated plums, compared with untreated control plums (Fig. 2B). However, the combination treatment of AVG and 1-MCP was the most effective treatment in delaying fruit softening and maintaining flesh firmness during postharvest storage period (Fig. 2B). Weight loss increased in untreated control plums as the postharvest storage period increased (Fig. 2C). In contrast, weight loss was lowest in the combination treatment of AVG and 1-MCP, 1-MCP, and AVG, compared with untreated control fruit (Fig. 2C).
The SSC of plum fruit juice was significantly decreased by the combination treatment of AVG and 1-MCP (Fig. 2D). The highest change in acidity ratios was observed in control fruit during postharvest storage (Fig. 2E). Both preharvest AVG treatment and postharvest 1-MCP treatment also reduced SSC acidity ratios in the fruit juice of plums (Fig. 2F).
Fruit decay was also determined during the storage period to assess the effectiveness of AVG and 1-MCP treatments during the storage period. All treatments significantly reduced fruit decay, compared with the untreated control plums on days 6 and 10 of the storage period (Fig. 3). Notably, the combination treatment of AVG and 1-MCP did not result in any fruit decay on day 6 and furthermore showed the lowest fruit decay (< 5%) on day 10 (Fig. 3). In contrast, untreated control plums exhibited the highest decay (9% and 27%) on days 6 and 10 of storage, respectively (Fig. 3). Overall, these results revealed that the combination treatment of AVG and 1-MCP effectively decreased the deterioration of plum fruit, thereby prolonging fruit storability of plums.
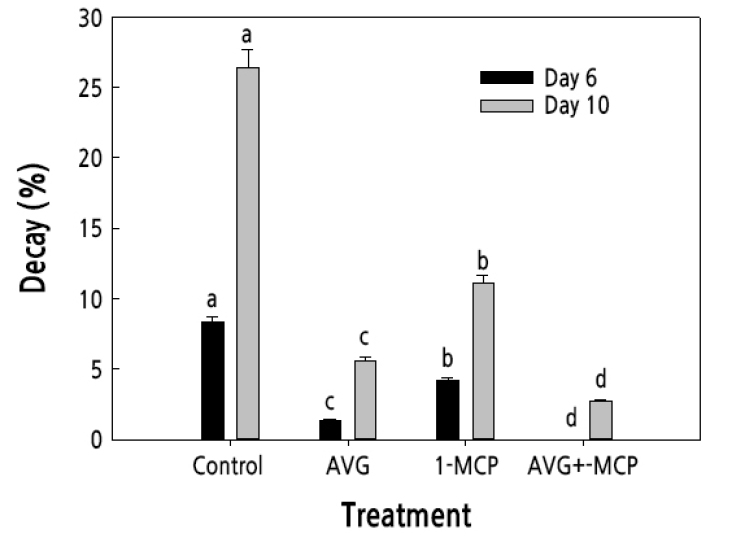
Fig. 3.
Effect of AVG and 1-MCP treatments on fruit decay on days 6 and 10 of storage in the dark at 20 ± 1°C and 60 ± 2% relative humidity. Plum fruit were sprayed preharvest with 150 mg·L-1 AVG and postharvest with 1 µL·L-1 1-MCP for 24 h. The fruit decay was assessed on a relative scale of 0 to 3 according to the percentage of rotten area on the fruit skin, as follows: 0: 0%, 1: 1–30%, 2: 31–50%, and 3: 51–100%. Control, untreated fruit; AVG, 1-MCP, and AVG+1-MCP, fruit were treated with AVG (aminoethoxyvinylglycine), 1-MCP (1-methylcyclopropene), and combination of AVG and 1-MCP. Values followed by different letters are significantly different (p < 0.05) based on Duncan's multiple range test (n = 3).
Ethylene Synthesis and Gene Expression
The expression level of the ethylene biosynthesis genes (PsACS1, PsACS3, PsACS4, PsACS5, and PsACO1) and ethylene production were characterized in plum fruit during the storage period (Fig. 4A-F). The ethylene production level of ‘Formosa’ plums was very low in the early stage (2-4 d after storage) and was similar among the treatments (Fig. 4F). However, untreated control plums showed an increase in ethylene production after 6 d of storage and exhibited a climacteric ethylene production peak with ripening at day 10 (Fig. 4F). Both postharvest 1-MCP treatment and the combination treatment of AVG and 1-MCP completely suppressed ethylene production for 10 d of the storage period (Fig. 4F). The single AVG treatment also decreased the ethylene production of plums, compared with untreated control fruit. However, its effectiveness was relatively lower than that of the postharvest 1-MCP treatment and the combination treatment of AVG and 1-MCP (Fig. 4F).
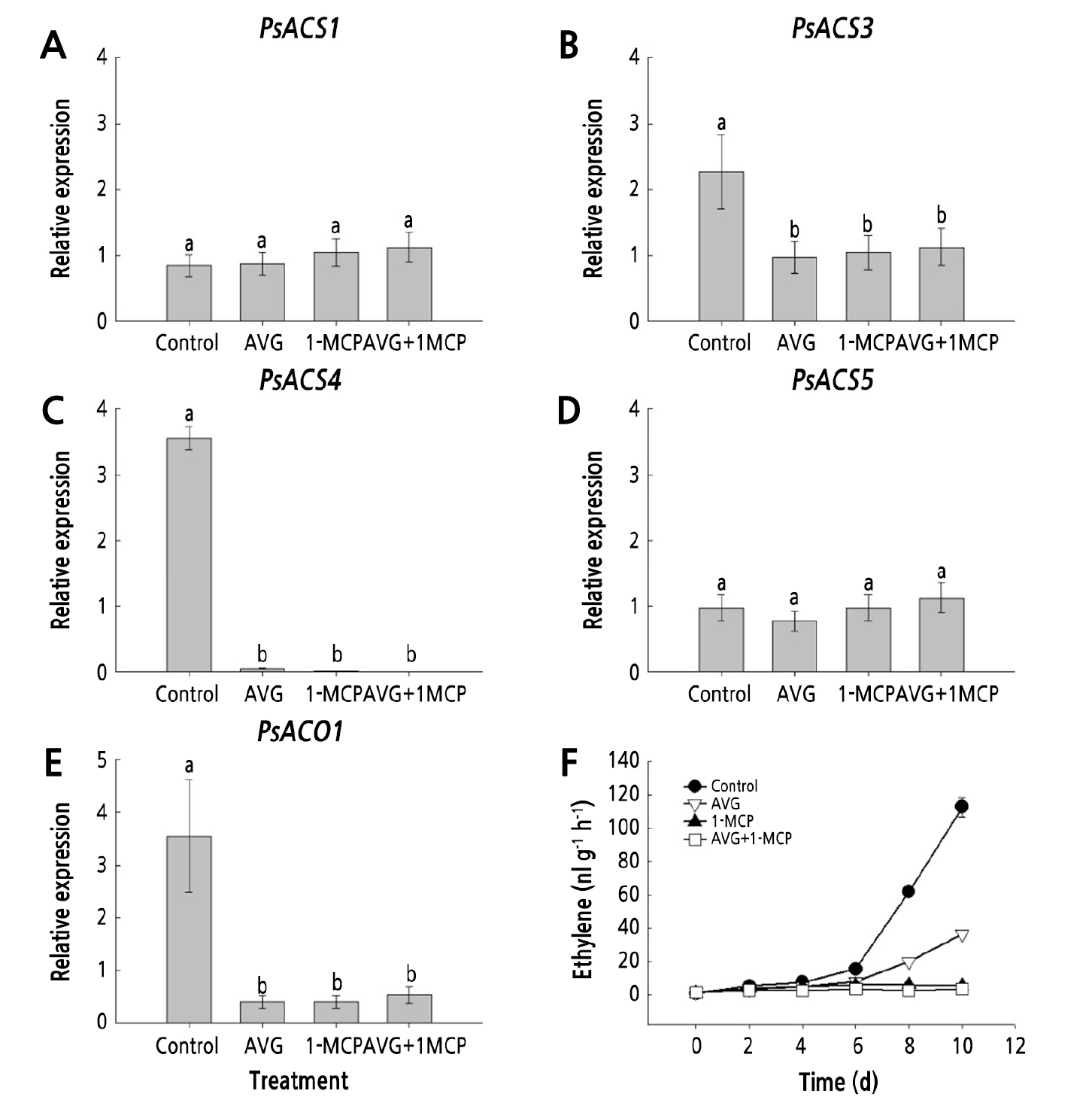
Fig. 4.
Effect of AVG and 1-MCP treatments on the transcript levels of PsACS1 (A), PsACS3 (B), PsACS4 (C), PsACS5 (D), PsACO1 (E), and ethylene production (F) in plum fruit on day 6 of the storage in the dark at 20 ± 1°C and 60 ± 2% relative humidity. Plum fruit were sprayed preharvest with 150 mg·L-1 AVG and postharvest with 1 µL·L-1 1-MCP for 24 h. Control, untreated fruit; AVG, 1-MCP, and AVG+1-MCP, fruit were treated with AVG (aminoethoxyvinylglycine), 1-MCP (1-methylcyclopropene), and combination of AVG and 1-MCP. Values followed by different letters are significantly different (p < 0.05) based on Duncan's multiple range test (n = 3).
The transcript levels of the ethylene biosynthesis genes (PsACS3, PsACS4, and PsACO1) in untreated control plums also markedly increased on day 6 (Fig. 4B, 4C, and 4E). Treatments with AVG, 1-MCP, and AVG+1-MCP suppressed the transcript levels of PsACS3, PsACS4, and PsACO1 in plum fruit (Fig. 4B, 4C, and 4E). The transcript levels of PsACS1 and PsACS5 were unaffected by the treatments (Fig. 4A and 4D), indicating that PsACS1 and PsACS5 may be related to fruit growth and maturation rather than fruit ripening and softening.
Expression of Ethylene Receptor and Signaling Genes
The expression patterns of PsETR1, PsERS1, and PsCTR1 were also analyzed in plum fruit after treatment with AVG and 1-MCP. The transcript levels of PsETR1, PsERS1, and PsCTR1 were lowest in untreated control fruit on day 6 (Fig. 5). The PsETR1 and PsERS1 reduction was suppressed by AVG and 1-MCP treatment (Fig. 5A and 5B). The AVG+1-MCP treatment significantly increased the expression of PsCTR1 in plum fruit on day 6, but the single AVG or 1-MCP treatment did not clearly affect the expression of PsCTR1, compared with the untreated control plums (Fig. 5C). Overall, the combination treatment of AVG and 1-MCP was the most effective in inhibiting the ripening-related degradation of the ethylene receptors and the signaling genes in plums.
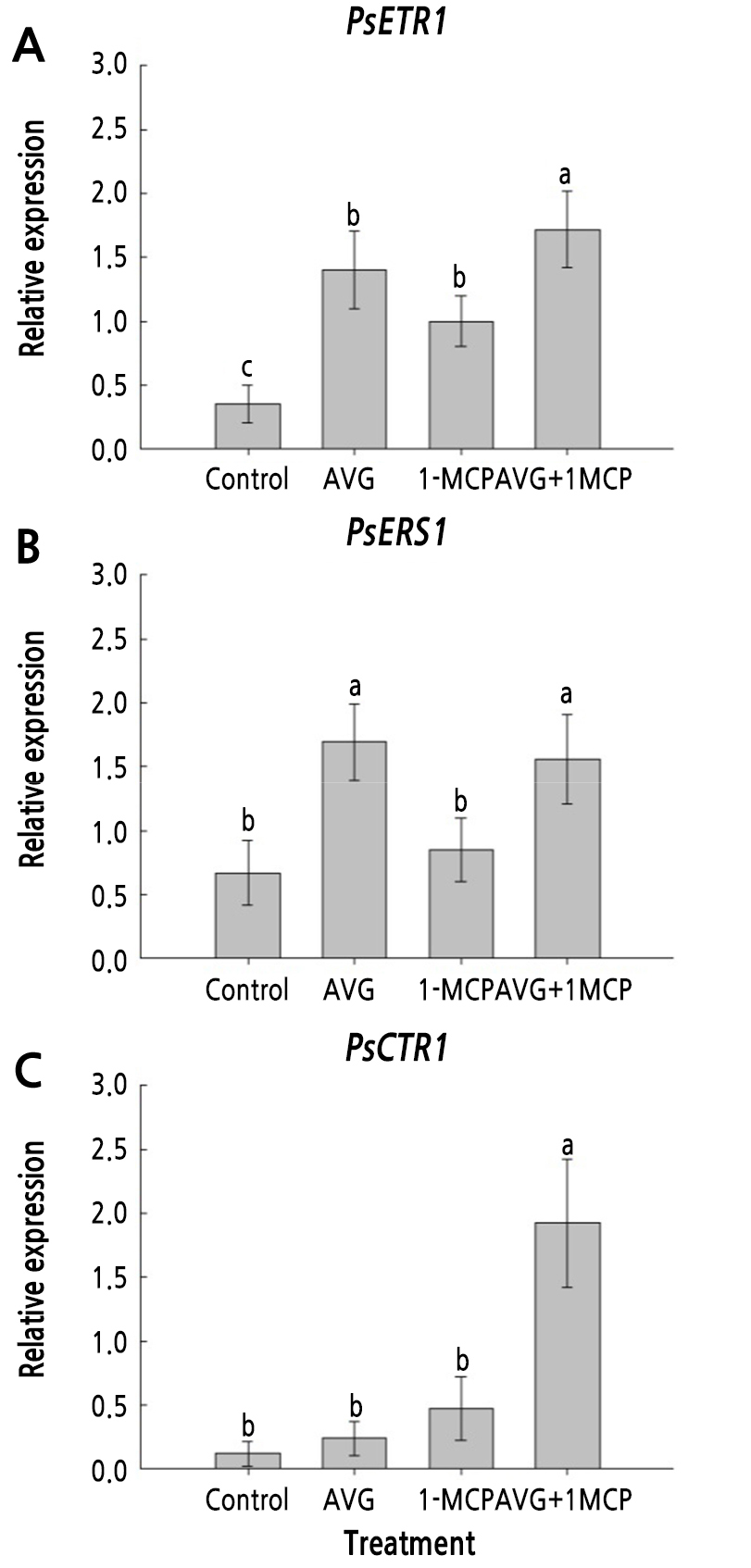
Fig. 5.
Effect of AVG and 1-MCP treatments on the transcript levels of ethylene signaling genes PsETR1 (A), PsERS1 (B), and PsCTR1 (C) in plum fruits on day 6 of storage in the dark at 20 ± 1°C and 60 ± 2% relative humidity. Plum fruit were sprayed preharvest with 150 mg·L-1 AVG and postharvest with 1 µL·L-1 1-MCP for 24 h. Control, untreated fruit; AVG, 1-MCP, and AVG+1-MCP, fruit were treated with AVG (aminoethoxyvinylglycine), 1-MCP (1-methylcyclopropene), and combination of AVG and 1-MCP. Values followed by different letters are significantly different (p < 0.05) based on Duncan's multiple range test (n = 3).
Discussion
The ethylene production during ripening of climacteric plum fruit is altered based on harvest maturity, cultivar, and pre/postharvest treatments (Singh and Khan, 2010). During storage, the ethylene production in ‘Formosa’ plum fruit was different in each treatment. High ethylene production leads to fruit ripening and consequently shortens the shelf life of plums. Ethylene antagonists (AVG and 1-MCP) are used to retard the ripening process in climacteric fruits (including plums) due to their strong effectiveness in inhibiting ethylene production and respiration rates (Sisler and Serek, 1997). Although the ripening accelerated by ethylene in climacteric fruits can be suppressed by AVG or 1-MCP application, the effectiveness is stable only in the short term because of the differences in ethylene sensitivity of the genotypes and the recovery of hormone sensitivity after the treatments (Sisler and Serek, 1997). Our results indicated that the combination treatment of AVG and 1-MCP effectively reduced ethylene production in fruit during the storage period, decreased postharvest losses, and extended the shelf life of ‘Formosa’ plums, compared with the single treatment of AVG or 1-MCP. These results indicate that the primary cause of early ripening in plum fruit would be ethylene and the ethylene-inducible process would be effectively delayed by the simultaneous suppression of ethylene binding and biosynthesis with 1-MCP and AVG. Previous studies have also indicated that the combined application of 1-MCP and AVG would be more effective in delaying fruit ripening and softening than a single treatment of the ethylene antagonists (Hayama et al., 2008).
In this study, the expression of three ethylene biosynthesis genes (PsACO1, PsACS3, and PsACS4) exhibited a rapid increase with the climacteric rise of ethylene synthesis in plum fruit at a later stage (day 6). This result indicates that PsACO1, PsACS3, and PsACS4 are presumably involved in autocatalytic ethylene synthesis (system 2 ethylene), which is responsible for senescence and ripening in plums. Our results imply that the autocatalytic ethylene synthesized by untreated control fruit was bound to ethylene receptors and thereby decreased the expression of the receptor genes, PsETR1 and PsERS1. This leads to a decrease in the downstream protein kinase, PsCTR1, resulting in an ethylene response in the fruit. It is well known that the accumulation of the ethylene biosynthesis genes (ACSs) during plant senescence may be regulated by the degradation of protein kinase, CTR1 (Jones, 2003; In et al., 2013; Xu and Zhang, 2014). In this study, the combination treatment of AVG and 1-MCP significantly inhibited an increase in the expression of PsACO1, PsACS3, and PsACS4 and the degradation of PsETR1, PsERS1, and PsCTR1, and thereby strongly suppressed the progression of fruit ripening. These results are supported by previous work showing that the simultaneous application of 1-MCP and AVG in carnations suppressed the ethylene-inducible increase of DcACSs and degradation of DcCTRs, which consequently progressed senescence in the petals (In et al., 2013). In this study, although the single application of 1-MCP decreased ethylene production in plum fruit, the transcript levels of PsETR1, PsERS1, and PsCTR1 were lower than the AVG+1-MCP treatment. It has been shown that fruit treated with 1-MCP alone can overcome ethylene inhibition caused by generating new ethylene receptors that have the ability to bind with the hormone (Sisler and Serek, 1997; Kevany et al., 2007; In et al., 2013). Thus, in plums treated with 1-MCP alone in this study, ethylene receptors synthesized anew or dissociated from ethylene presumably regained ethylene responsiveness in the short period after the treatments. This reversible binding of ethylene to the receptors had been observed in AtETR1 and AtETR2 and in carnation flowers (Schaller and Bleecker, 1995; O'Malley et al., 2005; In et al., 2013).
Acidity ratio, SSC, color, and flesh firmness are the most important postharvest quality attributes in plum fruit (Crisosto et al., 2007; Singh and Khan, 2010). Ethylene production and respiration during ripening results in various biochemical and physical changes in plums, such as the degradation of pigment, softening of fruit tissues, and variations in organic acids and soluble carbohydrates (Valero et al., 2003; Singh and Khan, 2010). Flesh firmness in plums is negatively correlated with ethylene production during ripening (Ozturk et al., 2015). Anthocyanins are responsible for the red coloration in plum fruit during maturity and ripening, and they are regulated by ethylene production (Singh and Khan, 2010; Fang et al., 2016). Since the ethylene responses in plum fruit are strongly blocked by the combination treatment of AVG and 1-MCP, the transcript levels of softening-related genes (endo-PG, exo-PG, pectin esterase, and endo-1,4-β-glucanase), polyphenol oxidase, and anthocyanin-related genes (MYB and bHLH) (Khan and Singh, 2007; Fang et al., 2016; Lee et al., 2020) can be inhibited after harvest. Therefore, AVG+1-MCP strongly inhibited the color change, maintained the firmness, and significantly reduced the decay of plum fruits during the storage period. Our results are consistent with a previous study indicating that a combined application of AVG and 1-MCP was effective in delaying softening and stabilizing the acidity in peach fruits (Hayama et al., 2008). Similar to our results, inhibition of ethylene synthesis and binding by AVG and 1-MCP decreased ethylene production and the activity of softened enzymes, maintained the hardness, and decreased red peel color development in ‘Qingnai’ and ‘Tegan Blue’ plum fruit (Khan et al., 2009; Luo et al., 2009). In this study, AVG was not effective in retarding the early change in color and the loss of flesh firmness in fruit. The limitation can be overcome by the simultaneous application of AVG and 1-MCP pre- and postharvest in plum fruit.
Overall, our results indicated that AVG+1-MCP treatment effectively suppressed ethylene production and ripening, and consequently resulted in longer shelf life of plum fruit. The results also revealed that the ethylene-inducible reduction of ethylene receptor transcripts (PsETR1 and PsERS1) was blocked by the AVG+1-MCP treatment, thereby the active PsCTR1 and suppressed ethylene signaling, resulting in delayed ethylene responses in plum fruit. Our results facilitate the improvement of postharvest treatment techniques for plums that show a climacteric rise of respiration and ethylene synthesis. Based on the current results, further research on the effectiveness of AVG and 1-MCP on postharvest quality of fruit at different storage temperatures is needed to develop a practical postharvest treatment technique for plums.


