Introduction
Materials and Methods
Plant material
Ripening treatments and storage
Respiration rate
Ethylene production
Fresh weight loss
Hunter ‘b’ and ‘L’ value
Firmness
Total soluble solids content
Statistical Analysis
Results and Discussion
Introduction
Banana belongs to the genus Musa, and is a member of the Musaceae family (Constantine and Rossel, 2001). It is one of the most important fruits in the world in terms of production and consumption (Aurore et al., 2009; INIBAP, 2000) and is an important fruit crop having a special place in human diets (Ahmad et al., 2006).
Dodo (2014) stated steady and rapid increase of the trade of bananas at the global stage over the lastcentury or so. Imports of banana of Republic of Korea has shown significant increase from 184, 200 tonnes in 2000 to 368,000 tonnes in 2012 (FAO, 2014). Korea Customs Service data showed that bananas have been the nation's leading imported commodity for ten consecutive years, and also have topped the list as the most-consumed fruit in the country (Korea Customs, 2013). The main exporters of bananas into the Republic of Korea are the Philippines (ranked 1st with over 97% share in total Korean import), Guatemala, and Peru (Kim and Ha, 2015).
Bananas are a typical climacteric fruit that exhibits a characteristic rise in ethylene production and respiration rate during ripening (Wyllie et al., 1998; Liu et al., 2004). Saltveit (1999) stated that the quality of some fruits such as avocados and bananas found to be increased when they are harvested at a mature but unripe stage that can withstand the rigors and duration of transport and then treated with ethylene to promote ripening before sale. Bananas are usually kept in airtight warehouses in which a system is controlling ethylene gas to achieve the quality of ripening (Liu et al. 1999; Kesari et al. 2010). Exogenous ethylene treatment of 100 ppm induces pre-climacteric bananas (Musa acuminata Collar. cv. Dwarf Cavendish) to ripen with increased respiration and endogenous ethylene production; 12 h treatment was slightly more effective than 6 h (Dominguez and Vendrell, 1994).
Bananas are harvested at a “mature-green” stage and exported to consumer countries and ripened in controlled conditions with ethylene gas (Ahmad et al., 2006; Giovanni et al., 2007; Kesari et al. 2010; Soltani et al., 2010), and banana importing (consumer) countries such as Republic of Korea, import bananas at “green-mature” stage and ripened artificially with commercial ethylene gas.
Since commercial ethylene is expensive and require careful management practices, ethylene producing tablet is considered as an alternative, because it is easy to use and readily available. This study was therefore initiated to assess the effect of ethylene producing tablet (Park and Jeong, 2014) with different concentrations and treatment periods on quality and storability of the imported banana fruit.
Materials and Methods
Plant material
Banana (Musa AAA Cavendish group) fruits of uniform size and free from physical defects, imported from the Philippines at mature green stage on July 10, 2014 were used for this research. The fruits were not treated with ethylene during shipping and storage.
Ripening treatments and storage
Mature green banana fruits were set in three air tight 62 L volume containers (ripening cells), and were treated with ethylene producing tablet at a concentration of 50 ppmv, 100 ppmv and control (standard commercial ethylene gas at 100 ppmv concentration), respectively. To uniformly spread the ethylene gas, the ripening cells were supplied with a ventilator fitted inside the ripening cells. The treatment lasted for 3 and 5 days at 15°C and 80+5% relative humidity. Evaluation was continued to assess their storability after releasing the treated bananas in storage room under the same temperature and relative humidity condition. The temperature was kept carefully at 15°C because banana fruits are highly susceptible to chilling injury below 14°C (Heliofabia et al., 2015).
Respiration rate
Respiration rates of banana were measured as a function of O2 and CO2 concentrations by using the closed system method. Banana fruit samples were placed in air tight 4 L volume container for 3 h and CO2 concentration was analyzed using PBI Dan-sensor (Check mate 9900, Denmark) gas analyzer. The result was expressed as mg·CO2·kg-1·hr-1.
Ethylene production
Banana fruit samples (3 fruits from each treatment) were sealed in an air-tight 4-L volume container and incubated for 3h (Neelam et al., 2003). A headspace 1 mL gas sample was taken from each container using a gastight syringe and injected into a gas chromatograph. The ethylene concentration was analyzed using a GC2010 Shimadzu gas chromatograph (Shimadzu Corporation, Japan) equipped with a BP 20 Wax column (30 m x 0.25 mm x 0.25 μm, SGE Analytical Science, Australia) and a flame ionization detector (FID). The detector and injector were operated at 127°C, the oven temperature was set at 50°C, and the carrier gas (N2) flow rate was 0.67 mL/s. The result was expressed as μL·C2H4·kg-1·hr-1.
Fresh weight loss
The fresh weight loss of banana during storage period was measured by subtracting sample weight from its previous recorded weight and the result was present as % of weight loss compared to its initial weight.
Hunter ‘b’ and ‘L’ value
The peel color of banana fruits was determined using Chroma meter, model CR- 400 (Minolta, Japan). Results were recorded in Hunter ‘b’ and ‘L’ values. Color variables were measured twice on both sides of each banana near the midsection and the average determined. A positive ‘b’ value represents the degree of yellowness and ‘L’ value represents degree of brightness (McGuire, 1992).
Firmness
Banana fruit firmness was measured using a Rheo meter (Sun Scientific Co. Ltd., Japan) with maximum force of 10 kg and a 3 mm diameter round stainless steel probe with a flat end. Measurements were taken at the middle part between stem end and distal end of the banana.
Total soluble solids content
The total soluble solids content (SSC) was measured using a refractometer (Model-Atago U.S.A. Inc., U.S.A.) and expressed as a percentage (%). Ten g of pulp was cut from a whole banana and homogenized in 50 mL of distilled water. The filtrate was used to measure the total soluble solids content.
Statistical Analysis
The experiment was conducted in a completely randomized design with nine replications per treatment. Measurements of parameters were analyzed by analysis of variance (ANOVA) at p ≤ 0.05 using SPSS statistics program (Version 21.0, SPSS Inc., Chicago, USA).
Results and Discussion
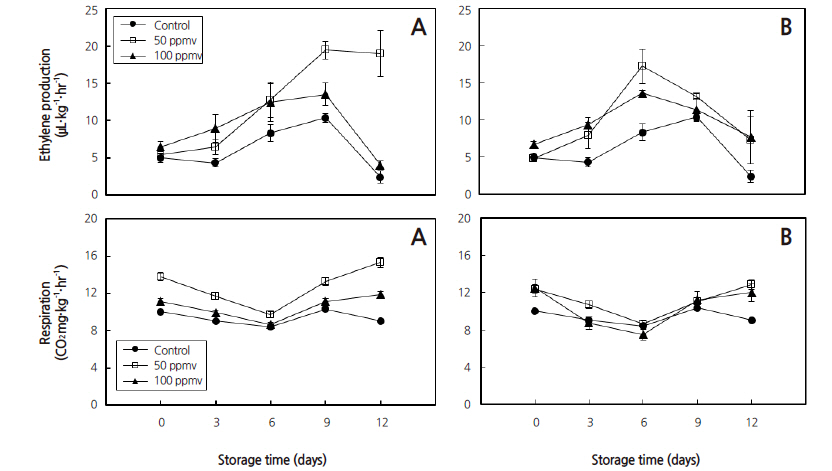
The ethylene-producing tablet in the present study has shown a higher endogenous ethylene production and cellular respiration rate at a relatively low concentration (50 ppmv) when compared to the control (Fig. 2). This result is in conformity with Saltveit (1999) who stated that ethylene concentrations from 10 to 1000 μL.L-1 are used commercially to promote the ripening of climacteric fruits such as bananas, avocados, mangos and tomatoes. In climacteric fruits, it has been accepted that ethylene plays an important role in ripening; in that a massive production of ethylene commences at the onset of the respiratory climacteric period, and exogenously applied ethylene induces ripening and endogenous ethylene production (Liu et al., 1999). The ethylene performs an important role during ripening by stimulating the development of color, texture, aroma and flavor and by reducing the ripening variability (Moya-Leon and Herrera, 2004; Palomer et al., 2005; Lee et al., 2015). Similarly, banana fruit is commercially ripened by treatment with exogenous ethylene, and the ripening process resulted in high respiration rate; change of color, texture, flavor and aroma (Li et al., 1997; Golding et al., 1998; Sogo Temi et al., 2014). The ethylene-producing tablet in the present study induced the same ripening process with an increase of the respiration rate resulting in a reduction of firmness, and similar Hunter ‘b’ and ‘L’ values after 9 days of storage at p ≤ 0.05 (Fig. 2, 4, and 5).
Slightly higher respiration rate was observed in banana fruits treated with 50 ppmv in both 3 and 5days storage as compared to the control at p ≤ 0.05 (Fig. 2). Longer storage time was recorded with banana fruits treated with 50 ppmv as compared to those treated with 100 ppmv; however, storability of the fruit was reduced after 9 days in all treatment groups as it was observed with high weight losses and respiration rate at p ≤ 0.05 (Fig. 3). It is evident from Fig. 2 that respiration rate increased significantly (p ≤ 0.05) as the ripening proceeded. This higher respiration rate also resulted in higher transpiration of water from the fruit surface which led to increase in percentage of weight loss and reduced firmness (Sabir et al., 2004).
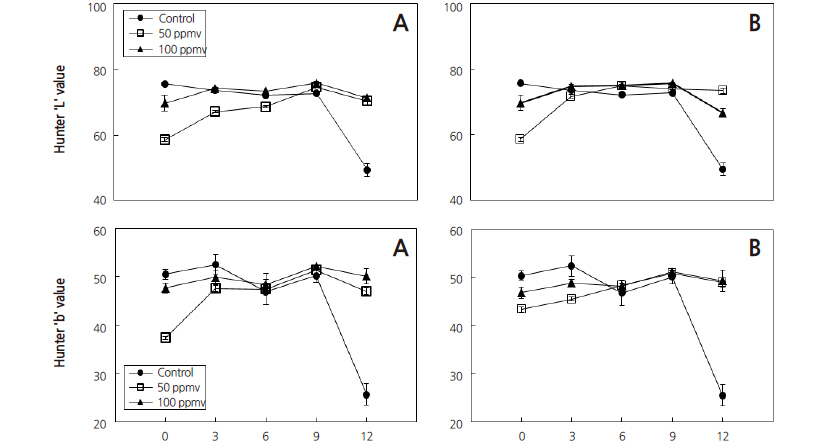
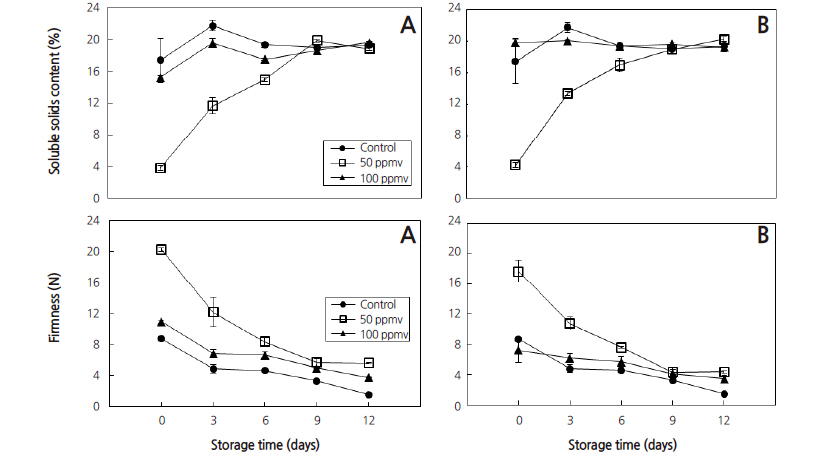
Firmness values were decreasing for all the treatments as the storage days proceeded. The highest firmness was recorded from 50 ppmv on the initial day of storage while the lowest was recorded from 100 ppmv on 12 day storage at p ≤ 0.05. This result is in conformity with the result reported by Saltveit (1999) and Ahmad et al. (2001) who stated that the reduced values of firmness of banana fruits with ethylene treatment are due to an increased softness of ripe bananas that resulted from hydrolysis of the starch and weight loss during ripening (Fig. 3 and 5). Moreover, with advances in banana fruit ripening, the mass ratio of pulp to peel increases due to an increase in water content, which in turn leads to decrease in peel firmness (Acedo and Bautista, 1989; Ahmad et al., 2001).
Fruits treated with 100 ppmv exogenous ethylene for five days showed a decrease in firmness when compared to those treated with 50 ppmv and the control stored for three days (p ≤ 0.05) (Fig. 5). Similar results were reported by Thompson (1996) who stated that the softening of banana fruit during ripening is associated with three processes: firstly, the conversion of starch to soluble carbohydrates, since starch granules have a structural function in the cell; secondly, the breakdown of pectin substances; and the third possibility is the movement of water from the peel of the banana to its pulp during ripening. Ahmad et al. (2001) explains that the movement of water from the peel of the banana to its pulp during ripening may have affected the turgidity of the skin, which would have been enhanced by transpirational losses. Saltveit (1999) and Ahmad et al. (2001) also reported reduced firmness of ripening banana fruits after ethylene treatment due to hydrolysis of the starch and weight loss. Similarly, Acedo and Bautista (1989), and Ahmad et al. (2001), stated that during fruit ripening, the pulpmass of a banana fruit increases due to mobilization of water from the banana peel to the pulp, resulting in decreased peel firmness.
The highest Hunter ‘b’ value (53) was recorded at the control stored for 3 days and decreased after that; while other treatments were similar up to the 9 day storage. Hunter ‘L’ and ‘b’ values for 50 ppmv and 100 ppmv showed an increasing trend for both 3 and 5 days treatments. The highest Hunter ‘L’ value (76) was recorded at 100 ppmv stored for 9 days. The results show that banana fruits could be stored for 12 days without loss of color in all treatments with the exception of the control (p ≤ 0.05). In the control treatment, the quality of fruits (in terms of color), rapidly decreased and lost marketability after 9 days of storage (Fig. 1 and 4). Similar results have been reported for bananas ripening at different temperatures for different storage times (Ward and Nussinovitch, 1996; Chen and Ramaswamy, 2002; Mendoza and Aguilera, 2004). These changes during the ripening period (loss of greenness, and increase in yellowness) took place as a result of the breakdown of the chlorophyll in the peel (Salvador et al., 2007).
Banana fruits treated with 100 ppmv for 5 days recorded higher SSC as compared to the 50 ppmv up to 9 day storage at p ≤ 0.05 (Fig. 5). Bananas treated with 50 ppmv for 5 days showed better storability with SSC content of 20% after 9 days storage and maintain the quality of banana fruits to be readily marketable. The increase in SSC content could be because during ripening starch is hydrolyzed into soluble carbohydrates, and that the SSC in the banana pulp comprised mainly soluble carbohydrates. Similarly Ahmad et al. (2001) reported that ethylene treated bananas possessed significantly greater SSC than untreated bananas.
The results revealed that ethylene tablets at 50 ppmv treated for 5 days or at 100 ppmv treated for 3 days have extended banana shelf life without affecting peel color, firmness and SSC content, which are very important quality attributes of banana. Therefore, the results indicated that ethylene tablets at 50 ppmv treated for 5 days or at 100 ppmv treated for 3 days could be applied for optimum ripening of banana to make ready for market and ultimate consumption.