Introduction
Materials and Methods
Growth Conditions of Asparagus Plants
Measurement of Plant Growth Parameters
Determination of Total Polyphenol and Flavonoid Content
Determination of Antioxidant Enzyme Activity
Determination of Antioxidant Activity
Statistical Analysis
Results and Discussion
Shoot Elongation Rate
Shoot Production
Plant Growth Characteristics
Total Polyphenol and Flavonoid Contents
Antioxidant Enzymes
ABTS Free-radical Scavenging Activity
Conclusion
Introduction
Asparagus (Asparagus officinalis L.) is considered a delicious vegetable and is also nutritious owing to the presence of bioactive compounds, such as vitamin C, α-tocopherol, carotenoids, and phenolic compounds, which are beneficial to human health (Li et al., 2012; Ku et al., 2018a; Siomos, 2018; Chitrakar et al., 2019; Guo et al., 2020; Zhang et al., 2020). Bioactive compounds extracted from asparagus have various pharmacological functions, including helping to prevent cancer, hyperglycemia, hyperlipidemia, and hypertension (Guo et al., 2020). In addition, the phenolic compounds extracted from asparagus are used in the functional food, cosmetic, pharmaceutical, and other industries (Rodríguez et al., 2005; Wang et al., 2011; Solana et al., 2015; Guo et al., 2020; Yu and Fan, 2020). Therefore, the cultivation and consumption of asparagus has increased worldwide.
Asparagus is a dioecious species that consists of distinct male and female plants (Kanno et al., 2017; Chitrakar et al., 2019). Male plants have higher spear yield, exhibit greater longevity in the field, and show higher resistance to plant diseases than female plants (Lazarte and Garrison, 1980; Kanno et al., 2017; Eum et al., 2020). Male cultivars of asparagus, including Atticus, Avalim, and Herkolim, are promising for spear production with high marketable and total yields in many countries (Mulder and Lavrijsen, 2008; Araki et al., 2009; Seong et al., 2013; Knaflewski et al., 2014; Haihong et al., 2017). Therefore, growers have become strongly interested in all-male cultivars in recent years.
In South Korea, asparagus plants encounter abnormal climate conditions, such as strong light intensity during summer cultivation. Excessive light intensity induces photoinhibition, negatively affecting plant growth and resulting in a decrease in crop yield (Szymańska et al., 2017; Toscano et al., 2019). Therefore, a cultivation system is required to protect plants from high light irradiance during the summer season in South Korea and to improve plant growth characteristics and yield performance. Mutiarawati Onggo (2012) found that 40–50% shade increased the number of asparagus shoots during the dry season in Indonesia. Saran et al. (2019) observed that the number of shoots of A. racemosus increased more when the plants were grown under 25% shade than under 75% shade when asparagus plants were grown in a semiarid and tropical climate. Male-female mixed asparagus cultivars, such as Atlas, Grande, and UC157, have been extensively cultivated in South Korea. We previously examined the effects of a 30% shade treatment on plant growth characteristics of these male-female mixed cultivars. Compared to high light intensity, growing the plants in 30% shade led to an increase in plant height in Atlas and Grande plants, spear number in Grande and UC157 plants, and shoot and bud numbers in UC157 plants. These results show that the effect of 30% shade treatment on plant growth characteristics of male-female mixed cultivars differs depending on the cultivar (Shawon et al., 2021). However, high light intensity elicits the synthesis and accumulation of beneficial phenolic compounds in asparagus spears (Maeda et al., 2005; Lee et al., 2010; Maeda et al., 2010; Toscano et al., 2019). Therefore, it is important to determine the optimal cultivars for high spear yields and high phenolic compound contents when cultivated in rain-shelter houses under shade conditions. In addition, to date, the effects of 70% shade treatment on plant growth characteristics and phenolic contents of all-male asparagus cultivars have not been extensively evaluated. The objective of this study was to investigate the effects of different levels of shade treatment on plant growth characteristics, phenolic compound contents, and antioxidant activities of three all-male asparagus cultivars (Atticus, Avalim, and Herkolim).
Materials and Methods
Growth Conditions of Asparagus Plants
The experiment was conducted in a rain-shelter house located at Wonkwang University, Iksan, South Korea (35º56' N; 126º57' E). Seeds of all-male asparagus cultivars (Atticus, Avalim, and Herkolim) were sown in plug trays filled with a commercial growing medium containing 68.51% coco-peat, 10% peat moss, 10% vermiculite, 5% zeolite, 6% pearlite, 0.18% fertilizer, 0.01% wetting agent, and 0.3% pH regulator (Alpha-Plus; Sang-Lim Company, Iksan, South Korea). Two-month-old seedlings were transplanted to pots (22 cm diameter × 25 cm height) and cultivated in three different areas in the rain-shelter house as follows: 1) 30% shade treatment (plants were grown in an area covered by an additional layer of roof polyethylene), 2) 70% shade treatment (plants were grown in an area covered by an additional layer of roof polyethylene and a black net), and 3) control treatment (plants were grown in an area without any additional layer of roof polyethylene or black net).
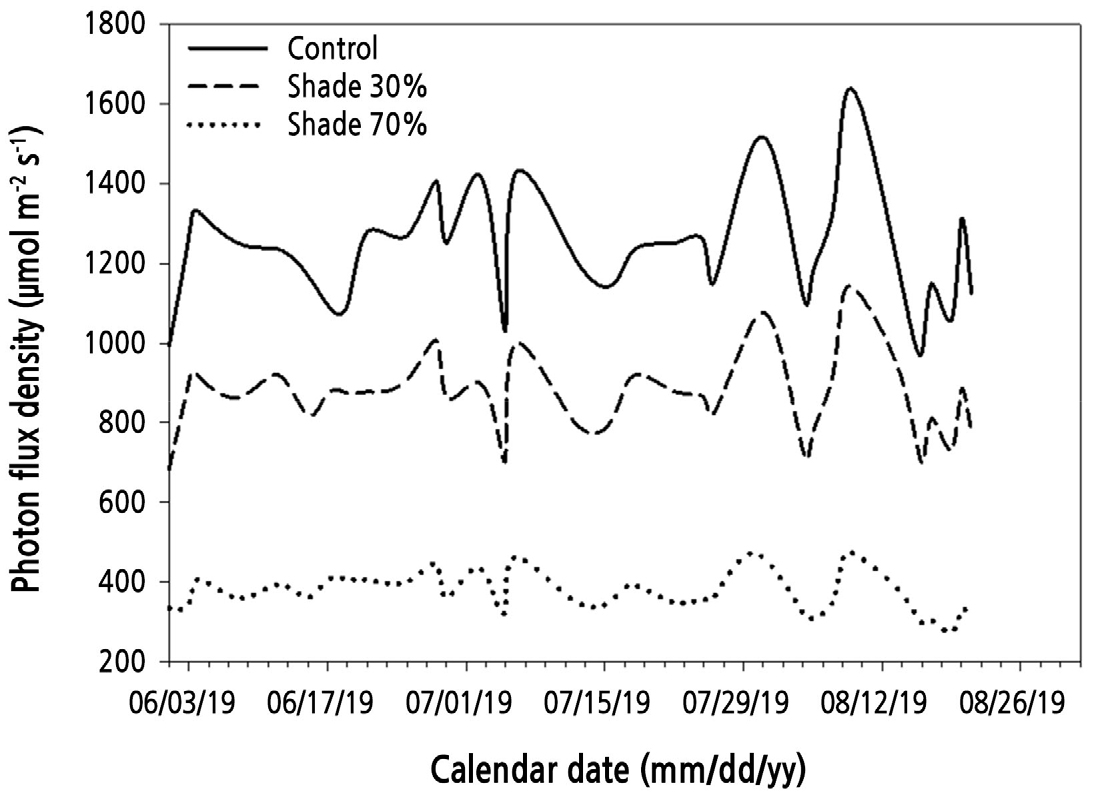
The light intensity in each treatment area (control, 30% shade, and 70% shade) of the rain-shelter house was monitored daily at 1 m above the ground using a SpectroPen-SP-110 spectrometer (Photon Systems Instruments, Brno, Czech Republic). The light intensity results are shown in Fig. 1. There were no differences in temperature among the three areas in the rain-shelter house (data not shown). The plants grown under the different shade conditions were watered at 2-day intervals. The amount of water used for watering each plant was controlled so that the moisture content in the soil was maintained at 30 ± 5%.
Asparagus spears (20 cm in length) of each plant were harvested from 11-month-old plants grown under the different shade conditions, frozen at –70°C in an ultra-low-temperature freezer (MDF-594, Panasonic; Panasonic Healthcare Co., Gunma, Japan), lyophilized for 4 d using a freeze-dryer (FD8512; Ilshin Biobase Co., Ltd., Seoul, South Korea), and ground into powder for further analyses.
Measurement of Plant Growth Parameters
One week after transplanting, the height of all shoots in each treatment were measured as the length from the upper surface of the growing soil to the tip of the shoot at one-week intervals using a tape ruler (CD-20APX, Mitutoyo Corporation, Kanagawa, Japan). The shoot elongation rate was defined as the increase in shoot height per week. The shoot number per plant was counted at one-week intervals beginning when the shoot length reached 1 cm. Shoot production number was determined as the increase in shoot number per plant per week. Ten weeks after the shading treatment, plants were uprooted from the pots. Plant roots were cleaned with tap water to remove soil, allowed to drain, and then the shoots and roots were carefully separated. Bud and root numbers were subsequently counted, and the root length was measured using a tape ruler. Bud and root diameters were measured using a digital caliper (CD-20APX; Mitutoyo Corp., Kanagawa, Japan). The roots and shoots of each sample were then weighed using an electronic balance to determine shoot, root, and total plant fresh weight. The roots and shoots were dried in an oven at 60ºC until reaching a constant weight prior to weighing for the determination of shoot, root, and total dry weight. The root-to-shoot mass ratio was calculated by dividing the root dry weight by the shoot dry weight.
Determination of Total Polyphenol and Flavonoid Content
The Folin-Ciocalteu method was used to determine the polyphenol content of the asparagus spears (Ku et al., 2018b). A spear extract was prepared by dispersing 20 mg of lyophilized asparagus powder in 1 mL of 95% ethanol. The extraction was carried out in an ultrasonic bath at 25°C for 60 min. The dispersion was filtered to remove insoluble materials. Folin-Ciocalteu reagent and sodium carbonate were added to the extract solution, and the absorbance was measured at 750 nm using a spectrophotometer (model 8452A; Hewlett-Packard, Rockville, MD, USA). The total polyphenol content is expressed as mg gallic acid equivalent (GAE) per g dry weight (DW) of spear.
The flavonoid content was determined by adding 0.3 mL of 5% (w/v) sodium nitrite, 0.5 mL of 2% (w/v) aluminum chloride, and 0.5 mL of 1 M sodium hydroxide to the extract solution (1 mL). The mixture was incubated for 10 min at 25°C, and then its absorbance was measured at 510 nm using a spectrophotometer. The flavonoid content is expressed as mg quercetin equivalent (QE) per g DW of spear.
Determination of Antioxidant Enzyme Activity
Superoxide dismutase (SOD) catalyzes the dismutation of superoxide anion into hydrogen peroxide (H2O2) and oxygen. Thus, SOD activity was determined based on the inhibition of the reduction of WST-1 (2-(4-iodophenyl)- 3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) by superoxide anion to formazan, whose absorbance was measured at 450 nm using an ultraviolet-visible (UV-VIS) spectrophotometer (Biochrom Ltd., Cambridge, UK). SOD activity is expressed as the percent inhibition per mg protein.
Catalase (CAT) decomposes H2O2 into water and oxygen. The rate of decomposition of H2O2 by CAT was determined by measuring the decrease in absorbance of the reaction solution at 240 nm (ε = 36 M-1 cm-1) using a UV-VIS spectrophotometer. The reaction was initiated by the addition of 11 mM H2O2 to the crude extract in 50 mM potassium phosphate buffer (pH 7.0). CAT activity is presented as µmol H2O2 decomposed per min per mg protein.
Ascorbate peroxidase (APX) catalyzes the reaction between ascorbic acid and H2O2. The rate of oxidation of ascorbic acid was measured by the change in absorbance at 290 nm (ε = 2.8 mM-1 cm-1) using a UV-VIS spectrophotometer. The reaction was initiated by the addition of crude extract to a mixture of 0.5 mM ascorbate and 0.2 mM H2O2 in 100 mM potassium phosphate buffer (pH 7.5). APX activity is expressed as µmol ascorbate oxidized per min per mg protein.
Peroxidase (POX) activity was measured spectrophotometrically based on its catalysis of the oxidation of guaiacol in the presence of H2O2 forming tetraguaiacol, which has a maximum absorbance at 470 nm (ε = 26.6 mM-1 cm-1). The reaction was initiated by the addition of crude enzyme extract to a mixture containing 1.5 mM guaiacol and 6.5 mM H2O2 in 40 mM potassium phosphate buffer (pH 6.9). POX activity is expressed as µmol tetraguaiacol formed per min per mg protein.
Determination of Antioxidant Activity
The 2,2'-azino-bis3-ethylbenzothiazoline-6-sulphonic acid (ABTS) free-radical scavenging assay was used to determine the antioxidant activity of asparagus spear extract. The ABTS reagent was prepared and stored in the dark for 16 h prior to being added to each solution of asparagus extract at different concentrations (500, 1000, 2500, 5000, 10,000, and 20,000 µg·mL-1). The reaction solution was kept in the dark for 10 min prior to the measurement of absorbance at 734 nm. The free-radical scavenging activity (%) was evaluated as the decrease in the absorbance of the reaction solutions in comparison with that of the control (no extract).
Statistical Analysis
Data analyses were conducted using IBM SPSS Statistics 24 for Windows (IBM Corp., Armonk, NY, USA). Duncan's multiple range tests were used to analyze significant differences at p < 0.05.
Results and Discussion
Shoot Elongation Rate
The 70% shade treatment significantly increased the shoot elongation rates of the three all-male cultivars compared with the control and 30% shade treatments (Fig. 2). The shoot elongation rates of the 30% shade-treated and control plants did not differ statistically. In addition, there were no significant differences in shoot elongation rate among the three male cultivars. In a previous study, a 30% shade treatment was investigated to reduce the high light intensity in summer in South Korea from the average value of 1,100 µmol·m-2·s-1 in the control to 800 µmol·m-2·s-1 in the 30% shade treatment. The 30% shade treatment stimulated shoot elongation of male-female mixed asparagus cultivars, including Atlas and Grande, when the plants were grown in the same rain-shelter house and the temperature conditions were not different between the control and 30%-shaded areas (Shawon et al., 2021). Under shade conditions, the stem elongation rate of shade-avoiding plants increases as a natural response to decreased light intensity (Hart, 2012; De Wit et al., 2016). Shade-avoiding plants tend to exhibit strong stem elongation growth away from surrounding shaded environments (Gommers et al., 2013). Similarly, the plant height of A. racemosus cultivated under 25% and 50% shade conditions was significantly higher than that of control plants (Saran et al., 2019).
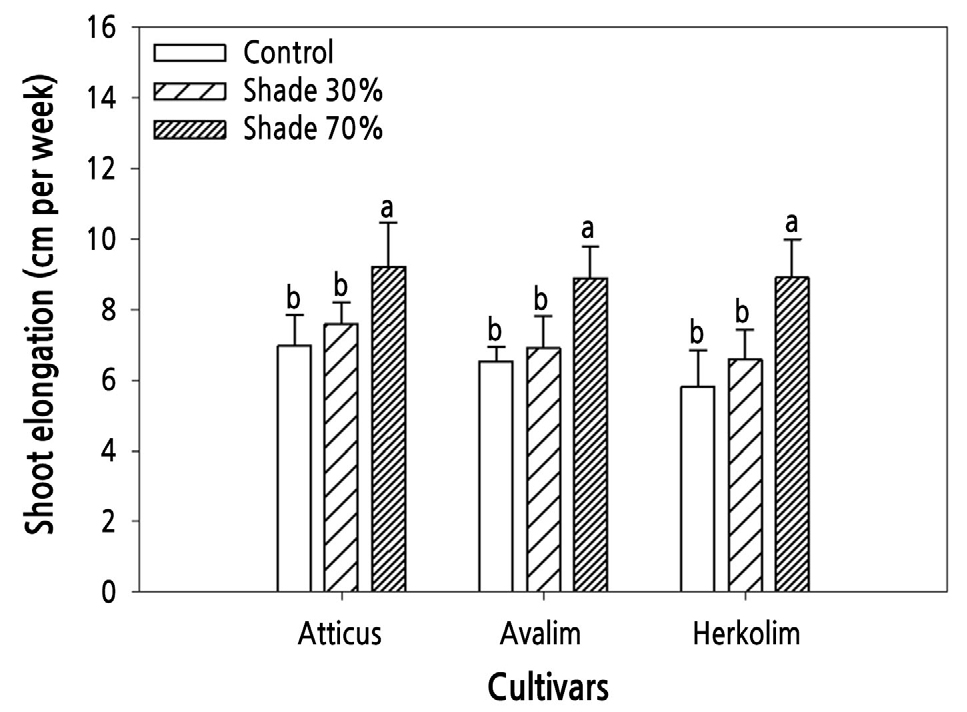
Fig. 2.
Shoot elongation of three all-male asparagus cultivars grown under control, 30% shade, and 70% shade conditions. Values represent the mean ± standard deviation (n = 5). Different lowercase letters above the bars indicate significant differences based on Duncan’s multiple-range test at p < 0.05.
Shoot Production
The number of shoots produced per week from Atticus and Avalim plants grown under 30% shade was significantly higher than that from the control and 70%-shaded plants (Fig. 3). Compared to that of the control plants, the shoot production number increased by 13.7% and 48.2% in Atticus and Avalim, respectively, when the plants were grown under 30% shade. Shade treatments did not affect the shoot production in the Herkolim cultivar. Shawon et al. (2021) similarly reported that a 30% shade treatment stimulated shoot production of asparagus cultivars, such as Apollo, UC157, and Walker Deluxe, compared to that in control plants. In the current study, the shoot production of Atticus and Avalim plants grown under the 70% shade treatment was significantly lower than that of the same cultivars grown under 30% shade. A decrease in shoot production under extreme shade conditions was also observed when A. racemosus was grown under 50%, 75%, and 90% shade conditions compared with under 25% shade (Saran et al., 2019).

Fig. 3.
The number of shoots produced per week for three all-male asparagus cultivars grown under control, 30% shade, and 70% shade conditions. Values represent the mean ± standard deviation (n = 5). Different lowercase letters above the bars indicate significant differences based on Duncan’s multiple-range test at p < 0.05.
The 30% shade treatment effectively stimulated the shoot production of Avalim compared to the control and 70% shade treatments. When grown under 30% shade, Avalim plants produced 1.2 shoots per week, whereas Atticus and Herkolim produced less than one shoot per week. Shawon et al. (2021) reported that male-female mixed cultivars, including UC157 and Walker Deluxe, grown under 30% shade produced approximately 1.5 shoots per week. These male-female mixed cultivars produced more shoots than the all-male Avalim cultivar in the current study.
Plant Growth Characteristics
Bud number is an important characteristic of asparagus plants because it potentially reflects spear production in the following harvest season (Drost and Wilcox-Lee, 1997; Gąsecka et al., 2009). The bud numbers of the Atticus and Avalim plants grown under 30% shade were significantly higher than those of plants of each cultivar grown under control and 70% shade conditions (Table 1). This result suggests that Atticus and Avalim plants need to be protected from natural sunlight at a level of approximately 30% shade to stimulate bud production and, presumably, to increase spear yield in the following season. Similar results were observed for UC157 and Walker Deluxe plants, in which the bud numbers in plants grown under 30% shade were significantly higher than those of the control plants (Shawon et al., 2021).
Table 1.
Plant growth characteristics of three all-male asparagus cultivars grown under control, 30% shade, and 70% shade conditions
| Cultivar | Shade treatment | Plant growth characteristic | ||||||||||||
|
Bud number (no.) |
Bud diameter (mm) |
Root number (no.) |
Root length (cm) |
Root diameter (mm) |
Fresh weight (g) |
Dry weight (g) |
Root: shoot | |||||||
| Shoot | Root | Total | Shoot | Root | Total | |||||||||
| Atticus | Control | 10.4zby | 4.37 a | 44.2 a | 31.4 a | 3.4 a | 46.1 a | 45.2 a | 91.3 a | 11.1 a | 10.7 a | 21.8 a | 0.98 a | |
| Shade 30% | 17.2 a | 4.15 a | 44.0 a | 34.2 a | 3.0 b | 50.9 a | 44.1 a | 95.1 a | 12.9 a | 11.7 a | 24.6 a | 0.93 a | ||
| Shade 70% | 8.8 b | 3.15 b | 20.4 b | 28.0 a | 2.1 c | 21.5 b | 7.1 b | 28.6 b | 5.3 b | 1.8 b | 7.0 b | 0.34 b | ||
| Avalim | Control | 10.2 b | 3.21 b | 43.8 a | 30.0 b | 3.0 a | 36.6 a | 35.4 b | 72.0 a | 9.8 a | 10.0 a | 19.8 a | 1.05 a | |
| Shade 30% | 16.6 a | 3.97 a | 46.0 a | 35.6 a | 3.1 a | 40.4 a | 42.9 a | 83.4 a | 10.8 a | 11.0 a | 21.8 a | 1.07 a | ||
| Shade 70% | 10.0 b | 3.90 a | 34.6 a | 32.8 ab | 2.7 a | 50.8 a | 21.9 c | 72.7 a | 12.7 a | 5.3 b | 18.0 a | 0.46 b | ||
| Herkolim | Control | 9.6 a | 4.67 a | 35.2 a | 29.2 b | 3.8 a | 33.4 b | 46.5 a | 79.9 a | 8.8 b | 10.8 a | 19.7 a | 1.27 a | |
| Shade 30% | 8.8 a | 4.64 a | 33.6 a | 35.8 ab | 3.3 b | 33.4 b | 36.3 ab | 69.7 a | 8.4 b | 8.9 a | 17.3 a | 1.09 a | ||
| Shade 70% | 9.2 a | 4.11 a | 28.6 a | 37.4 a | 2.7 c | 52.2 a | 23.2 b | 75.4 a | 12.4 a | 4.6 b | 16.9 a | 0.38 b | ||
| Significancex | ||||||||||||||
| Cultivar (A) | * | *** | ** | ns | *** | ns | ns | ns | ns | ns | ns | ns | ||
| Shade treatment (B) | *** | ** | *** | * | *** | ns | *** | *** | ns | *** | *** | *** | ||
| A × B | ** | *** | ** | ns | ** | *** | ** | *** | *** | * | *** | ns | ||
Bud diameter is another factor affecting the weight and yield of asparagus spears in the following harvest season (Drost and Wilcox-Lee, 1997). The bud diameter of the 70% shade-treated Atticus plants was lower than that of the control and 30% shade-treated plants, whereas the bud diameter of Avalim plants under 30% and 70% shade conditions was higher than that of the control Avalim plants (Table 1). Similarly, in another study, the bud diameter of Atlas asparagus plants grown in a rain-shelter house was significantly higher than that of Atlas plants grown in an open field and exposed to full natural sunlight (Ha et al., 2020). In the current study, shade treatments did not significantly affect the bud diameter of the Herkolim cultivar.
The number, length, and diameter of asparagus roots are important characteristics of asparagus plants that also affect spear productivity. A well-developed root system induces a high spear yield (Drost and Wilson, 2003). The root number of the shaded plants and the control plants of the three all-male cultivars did not differ statistically (Table 1). The only exception was the 70%-shaded Atticus plants, in which the root number decreased by 53.9% and 53.6% compared with that of the control and 30% shade-treated plants, respectively. Root length in Avalim and Herkolim plants was increased by the 30% and 70% shade treatments, respectively, compared to that of the control plants. The root lengths of Atticus and Herkolim treated with 30% shade did not significantly differ from those of the same cultivar grown under the control condition. In contrast, the root diameters of Atticus and Herkolim were significantly decreased under the shade treatments compared to those of the same cultivars under the control condition. Similarly, decreases in the root diameter of A. racemosus under different shade treatments were reported by Saran et al. (2019).
Shoot fresh and dry weights, root dry weight, total plant fresh and dry weights, and the root-to-shoot mass ratios of the three all-male cultivars grown under the 30% shade treatment did not significantly differ from those of plants grown under control condition. However, the root fresh weight of Avalim was significantly higher when grown under 30% shade than when grown under the control condition. The shoot fresh and dry weights, root fresh and dry weights, total fresh and dry weights, and the root-to-shoot mass ratio were lower in Atticus plants grown under 70% shade than in Atticus plants under control and 30% shade conditions. The decreases in root fresh and dry weights and total fresh and dry weights of Atticus under the 70% shade condition were ascribed to the decreases in root number and diameter of Atticus under the 70% shade condition (Table 1). The shoot fresh and dry weights of Herkolim were significantly higher under the 70% shade than under control and 30% shade conditions. This result was consistent with the higher shoot elongation rate of Herkolim under the 70% shade condition compared with that under the control and 30% shade treatments (Fig. 2). The root dry weight and the root-to-shoot mass ratio of Herkolim were significantly lower under 70% shade than under the control and 30% shade conditions. The decrease in the root dry weight of Herkolim under the 70% shade treatment might be associated with the decreased root diameter of this cultivar under the 70% shade condition compared with that of the cultivar under the control and 30% shade conditions (Table 1). There was a positive correlation between root dry weight and the root-to-shoot mass ratio of the three cultivars. Similarly, the ratio of root to shoot mass of male-female mixed cultivars was reported to have a positive correlation with the root dry weight of asparagus plants (Shawon et al., 2021).
Total Polyphenol and Flavonoid Contents
Total polyphenol content was significantly lower in the shade-treated plants than in the control plants of the three all-male cultivars, except for the 70% shade-treated Atticus plants (Table 2). The decreased polyphenol content in the spears under 30% and 70% shading may have been due to the decreased light intensity, which was more favorable for the growth of asparagus. In the present study, the average light intensity in the control area was 1,242.2 ± 149.3 µmol·m-2·s-1 (55,756.6 ± 6,793 lx) (Fig. 1), which appears to be higher than the suitable range for good growth of asparagus (Figs. 2, 3, and Table 1). When asparagus is grown under an excessive light intensity (e.g., the control condition), plants tend to synthesize phenolic compounds as a natural stress response to unfavorable environmental conditions (Bian et al., 2014; Laura et al., 2019). Similarly, Shawon et al. (2021) reported that asparagus plants grew well under 30% shading, with the light intensity varying from 660–890 µmol·m-2·s-1 (30,000–40,000 lx), and produced higher bud and spear numbers than those of plants grown under a control condition. In addition, asparagus cultivated in open fields, where the light intensity was high, had a higher polyphenol content than asparagus grown in rain-shelter houses or under lower light intensity (Maeda et al., 2005; Maeda et al., 2010; Kulczyński et al., 2016).
Table 2.
Total polyphenol and flavonoid content in asparagus spears of three all-male asparagus cultivars grown under control, 30% shade, and 70% shade conditions
| Cultivar | Shade treatment | Polyphenol content (mg GAE/g DW) | Flavonoid content (mg QE/g DW) |
| Atticus | Control | 31.2zay | 21.2 a |
| Shade 30% | 28.9 b | 18.1 b | |
| Shade 70% | 30.7 a | 19.2 b | |
| Avalim | Control | 30.8 a | 17.7 a |
| Shade 30% | 22.3 c | 14.5 b | |
| Shade 70% | 26.8 b | 18.3 a | |
| Herkolim | Control | 33.4 a | 19.4 a |
| Shade 30% | 25.3 c | 17.4 a | |
| Shade 70% | 28.2 b | 18.6 a | |
| Significancex | |||
| Cultivar (A) | *** | *** | |
| Shade treatment (B) | *** | *** | |
| A × B | *** | * |
The 30% shade treatment in this study caused a decrease in the flavonoid content of Atticus and Avalim plants compared to that of control plants (Table 2). The flavonoid content of Herkolim plants was unaffected by the shade treatments. The decrease in flavonoid content in the spears and the increases in the shoot production and bud number of Atticus and Avalim plants grown under the 30% shade indicate that the average light intensity of 872.65 ± 105.76 µmol·m-2·s-1 (38,927.9 ± 4,958.0 lx) under the 30% shade condition was most favorable to the growth of asparagus. The control plants and the 70% shade-treated plants received higher and lower levels of light, respectively, than the suitable light intensity for the growth of asparagus.
Among the three all-male cultivars, Atticus exhibited the highest polyphenol content under the 30% and 70% shade treatments, whereas Herkolim had the highest polyphenol content under the control condition. In addition, the flavonoid content of Atticus was higher than that of Avalim, but not significantly different from that of Herkolim under the control and 30% shade conditions.
Antioxidant Enzymes
The SOD activities of Atticus and Herkolim were significantly decreased by the shade treatments compared to those of the control plants, whereas the SOD activity of Avalim was unaffected by the shade treatments (Table 3). The CAT activities in the three male cultivars under the control condition were significantly higher than those of the respective plants under the shade treatments, except for the 70%-shaded Atticus plants. The CAT activities in Atticus, Avalim, and Herkolim grown under 30% shade decreased by 11.9%, 16.7%, and 20.1% compared to that in the control plants, respectively. The APX activity in Atticus plants treated with 30% shade was significantly lower than that in the control plants, whereas the APX activities in Avalim and Herkolim spears were unaffected by the shade treatments. Shading significantly deceased the POX activities of the three all-male cultivars compared to those of the control plants. The decreases in SOD, CAT, APX, and POX activities of the three all-male cultivars observed under shading suggest that the full natural light intensity of the control condition was stressful to the plants and not favorable for the growth of asparagus. Under high light intensities, such as in the control condition employed in this study, asparagus plants can be subjected to high light intensity stress, and the equilibrium between reactive oxygen species (ROS) generation and scavenging in plant cells can be perturbed. As a result, ROS accumulates in plant cells, ultimately activating the enzymatic antioxidant system (Gill and Tuteja, 2010; García-Caparrós et al., 2020).
Table 3.
Antioxidant enzyme activity of three all-male asparagus cultivars grown under control, 30% shade, and 70% shade conditions
| Cultivar |
Shade treatment |
Superoxide dismutase (SOD, % inhibition mg protein-1) |
Catalase (CAT, µmol H2O2 decomposed min-1 mg protein-1) |
Ascorbate peroxidase (APX, µmol ascorbate oxidized min-1 mg protein-1) |
Peroxidase (POX, µmol tetraguiaco l formed min-1 mg protein-1) |
| Atticus | Control | 80.4zay | 17.6 a | 675.9 a | 3.7 a |
| Shade 30% | 75.3 c | 15.4 b | 621.9 b | 2.9 b | |
| Shade 70% | 78.0 b | 16.2 ab | 645.9 ab | 3.1 b | |
| Avalim | Control | 73.5 a | 15.0 a | 607.8 a | 2.8 a |
| Shade 30% | 72.3 a | 12.5 b | 579.2 a | 2.2 b | |
| Shade 70% | 74.7 a | 13.1 b | 582.6 a | 2.1 b | |
| Herkolim | Control | 78.9 a | 16.9 a | 648.1 a | 3.8 a |
| Shade 30% | 73.9 b | 13.5 b | 638.2 a | 2.5 b | |
| Shade 70% | 74.5 b | 14.6 b | 598.0 a | 2.7 b | |
| Significancex | |||||
| Cultivar (A) | *** | *** | *** | *** | |
| Shade treatment (B) | ** | *** | * | *** | |
| A × B | ns | ns | ns | ns |
ABTS Free-radical Scavenging Activity
The shade treatments decreased the ABTS free-radical scavenging activities measured in spear extracts of the three cultivars compared to the control condition, except in Atticus and Avalim at a spear extract concentration of 5,000 µg·mL-1 (Table 4). The decrease in ABTS scavenging activity was most likely due to the decreased polyphenol content of the spears grown under 30% and 70% shading, as well as the decrease in flavonoid content under 30% shading compared with under the control condition.
Table 4.
ABTS free-radical scavenging activity of spear extracts of three all-male asparagus cultivars grown under control, 30% shade, and 70% shade conditions
| Cultivar | Shade treatment | Extract concentration (µg·mL-1) | |||||
| 500 | 1000 | 2500 | 5000 | 10000 | 20000 | ||
| Atticus | Control | 27.2zay | 38.5 a | 46.6 a | 60.7 a | 78.6 a | 85.4 a |
| Shade 30% | 22.2 b | 32.6 c | 43.3 b | 58.2 a | 72.7 b | 81.6 b | |
| Shade 70% | 23.8 b | 35.5 b | 43.1 b | 58.0 a | 76.3 a | 83.1 b | |
| Avalim | Control | 22.9 a | 33.0 a | 40.6 a | 54.6 a | 76.3 a | 83.1 a |
| Shade 30% | 20.7 a | 30.6 a | 34.4 c | 51.6 a | 70.7 b | 75.5 c | |
| Shade 70% | 20.6 a | 31.9 a | 36.9 b | 52.3 a | 70.4 b | 80.1 b | |
| Herkolim | Control | 24.4 a | 34.4 a | 42.2 a | 58.4 a | 78.1 a | 87.6 a |
| Shade 30% | 20.6 c | 33.4 ab | 37.4 c | 52.4 c | 71.5 b | 77.7 c | |
| Shade 70% | 22.4 b | 31.2 b | 39.5 b | 55.2 b | 74.6 b | 81.5 b | |
| Significancex | |||||||
| Cultivar (A) | *** | *** | *** | *** | *** | *** | |
| Shade treatment (B) | *** | *** | *** | *** | *** | *** | |
| A × B | ns | * | ns | ns | * | ** | |
The ABTS free-radical scavenging activity increased with the increase in spear extract concentration. In particular, the ABTS scavenging activity of the spear extract at a concentration of 500 µg·mL-1 was 22.8% on average and that at 20,000 µg·mL-1 was 81.7%. Similarly, a positive correlation between free-radical scavenging activity and spear extract concentration has been demonstrated in previous studies (Rodríguez et al., 2005; Kulczyński et al., 2016; Eum et al., 2020).
Conclusion
Shade treatments of 30% and 70% differentially affected the plant growth characteristics, phenolic content, antioxidant enzyme activities, and ABTS radical scavenging activity of the three all-male asparagus cultivars. The 30% shade treatment increased the shoot production rate and bud number of the Atticus and Avalim cultivars compared with that in control plants, which might result in an increase in spear production of these two cultivars in the following harvest season. Under the 30% shade treatment, the polyphenol content in the Atticus cultivar was significantly higher than that in the Avalim and Herkolim cultivars, and the flavonoid content in Atticus was higher than that of Avalim. The results suggest that a 30% shade treatment could be applied to the cultivation of Atticus to improve spear production in the following harvest season and to minimize the decreases in phenolic content.