Introduction
Materials and Methods
Plant material and experimental conditions
Preparation of the treatment solutions
Plant sampling and data collection
Statistical analysis
Results and Discussion
Conclusions
Introduction
Nutrient management is the fundamental aspect of strawberry farming because they are very susceptible to disorders influenced by fertilizers (Ariza et al. 2021). Therefore, the seedlings need an adequate amount of nutrients for identical vegetal augmentation and maximum output with better quality fruit (Sharma 2002). Studies have shown that an appropriate amount of nutrient application to seedlings immensely influences the vegetative growth of strawberries (Hargreaves et al. 2009).
The growth and development of strawberries highly depend on the macronutrients in the root media, particularly nitrogen (N), phosphorus (P) and potassium (K). Leaf number, leaf area, chlorophyll content, diameter of crowns and most importantly plant yield depend on the nutrients listed previously. A proper amount of macronutrients from organic and inorganic fertilizers can give the maximum vegetative growth which is indispensable for a higher yield and better-quality fruit (Khalil and Agah 2017). Though N, P, and K have the most important roles in strawberry cultivation, a good proportion of all macro and micronutrients give uniform vegetative growth and high-quality fruit. Among all the essential nutrients, N is the most growth-limiting factor for strawberries. An increase in the rate of N fertilizer resulted in a higher vegetative growth, where the absence of N showed a poor and weak above-grown biomass for all cultivars (Biscaro et al. 2022). On the other hand, a P deficiency interrupts the growth and devolvement of the branches, hampers stem elongation and the weather susceptibility of plants, for example, temperature and pathogen stress (Choi et al. 2013). Choi and Lee (2012) indicated that shoot size is reduced followed by leaf yellowing with an excessive presence of P in strawberry plant tissues. They also showed that the iron (Fe) and zinc (Zn) is mitigated because of the excessive P in the tissue content. K and Ca are also required to cultivate strawberries to get the maximum vegetative growth which is vital for a high yield with quality fruit (Pettigrew 2008; Easterwood 2012). A well grown and developed plant is very important to achieve a high yield with quality fruit. Combined application of N and K in a solution formed with water influence plant growth and enhance the fruit quality and plant development (Reinaldo et al. 2015). In contrast, excessive use of K and Ca beyond the recommended amount can damage the plants, can damage the plants, both vegetatively and reproductively. The growth and production of strawberries decrease with an increase in the K and Ca concentrations in the nutrient solution (Andriolo et al. 2010; Sousa et al. 2014). On the other hand, Choi and Latigui (2008) stated that Magnesium (Mg) is very important in maintaining ionic balance (K+, Ca++ and Mg++) in the nutrient solution and has the most important role in the quantity of chlorophyll in the leaves. The amount of chlorophyll decreases with a reduction of Mg in the nutrient solution and increases the ionic imbalance which leads to lower growth and development of seedlings. Santiago et al. (2018) declared that Sulfur (S) is a key element in strawberry cultivation and is responsible for shoot and fruit development. S increases the accumulation of selenium (Se) in strawberry plants and enhances the growth and yield of strawberries, but the positive relation is disturbed with the increase of the S concentration in the nutrient solution.
The excessive application of fertilizers can enhance growth and increase the yield, but it can cause environmental pollution and waste fertilizer which will increase costs (Chandini et al. 2019; Kang et al. 2018). On the other hand, the application of the optimum amount of fertilizer is the best solution for attaining the necessary vegetative growth which is a precondition for quality fruit with maximum yield (Vance et al. 2017).
However, nowadays, growers are also showing eagerness in sustainable production schemes by limiting fertilizer use to maintain soil fertility and reduce environmental degradation (Habibzadeh et al. 2019). Farmers are facing different difficulties in raising strawberry seedlings for producing quality fruit and further propagation, as a consequence of superfluous information with respect to nutrient uptake and tissue mineral content. Considering that, further studies are required to investigate the optimal fertilizer use. Therefore, this study examined the effects of specific macronutrient deletion in mother plants (N, P, K, Ca, Mg, and S) before low temperature treatment on runner plant occurrence and growth of three different strawberry cultivars in the Spring season. The main goal of this study was to explore deficiencies that could provide insight into the relationship between plant development and tissue mineral concentrations in the content of overhead dried substances and the quantity of the tissue mineral contents associated with reduced vegetative growth.
Materials and Methods
Plant material and experimental conditions
The experiments with strawberry seedlings were carried out inside a glass covered greenhouse at Chungnam National University, South Korea (36° 20' N, 127° 26' E) to examine the effects of specific macronutrient deletion (N, P, K, Ca, Mg, and S) on growth and development of mother plants before low temperature treatment on runner plant occurrence and growth of three different strawberry cultivars in the Spring season. Five strawberry seedlings with three true leaves were transplanted in a zigzag row in plastic containers (length 64.3 cm × width 23.5 cm × height 17 cm). The beds were prepared using a commercially prepared root medium (coco peat + perlite + peat moss + vermiculite; 52.5, 30, 15, and 2.5%, respectively), followed by irrigation. The physical properties of the root medium were measured as previously described (Choi et al. 1999) and were as follows: total porosity, container capacity and air-filled porosity were 83.9, 74.2, and 9.7%, respectively. After transplanting the seedlings, they were irrigated twice a day, and the application of a nutrient solution was started seven days after transplantation and applied three times a day (9 am, 1 pm and 4 pm) using a timer based automatic irrigation channel with a duration of 3 minutes. During the entire experiment, the reproductive growth of the strawberries was restricted manually. The inside average temperature was 24°C during the day and 14°C at night.
Preparation of the treatment solutions
Nutrient solutions for six different fertilizer stresses were prepared (Table 1). The ingredients of the other essential nutrients were equal in every treatment, with the absence of N, P, K, Ca, M, and S. All the fertilizers were formulated and applied until the cold treatment of the mother plants. The pH of the solutions was calibrated to 5.8–6.0 using hydrochloric acid (HCl), whereas the electrical conductivity (EC) was 0.6. Fertilizers were applied three times a day at a rate of 2 L per pot before cold treatment. After low temperature treatment N-P2O5-K2O (18-18-18, Haifa Chemicals Ltd. Matam-Haifa, Israel) was applied using a drip irrigation system (3 times a day with a 3-minute duration), and Proplex-Ca 20 (Plant Health LTD, United Kingdom) was drenched once a week. The experiment was replicated three times for each treatment.
Table 1.
Composition of the fertilizer solutions used to investigate the effects of specific macronutrient deletion in the Fall season on vegetative growth and runner plant production of strawberry in spring season
Plant sampling and data collection
Mother plants were uprooted to collect the vegetative component for growth investigation 90 days after transplantation. Eleven plants from each treatment were selected randomly, and the average values of all were presented as one nutrient treatment. Growth was measured as numerical values as follows: plant height (cm) and number, length (cm), and width (cm) of leaves, length of petiole (cm), crown diameter (cm), fresh weight and dry weight (g) of the mother plants, number of runners with their length (cm) and fresh weight (g) and dry weight (g), and the number of runner plants along with the fresh weight (g).
Tissue contents were analyzed using oven dried (75°C for 48 hours) leaves, sampled from each treatment and rinsed in detergent followed by distilled water. After proper drying, the leaves were ground by pestle and mortar. Then, they were sieved in a 0.5 mm sieve. Thereafter, 0.5 g of the leaf sample from every treatment were dry-ashed at 500°C for 6 hours and burnt in a hooded chamber using 2 mL of 95% H2SO4. Then, 50 mL of 0.5 N HCl were added to the dark burnt leaf sample, and a clear liquid sample was gained after filtering with Advantec No. 2 filter paper (Toyo Roshi Kaisha, Ltd., Tokyo, Japan). Then K, Mg, Ca, Fe, Mn, Cu, and Zn were analyzed using an Atomic Absorption Spectrophotometer (AA-7000, Shimadzu Co., Kyoto, Japan).
The total nitrogen concentration present in the plant tissue was measured by using ground up leaves (0.1 g) and a mixture of sulfuric acid and salicylic acid (5 mL) at a ratio of 20:1 (v/w) in glass tubes. The tubes were kept at room temperature and allowed to settle down for about one hour, and then, the mixture was digested for 5 minutes in pre-heated (270°C) digestion blocks. The hot tubes were carefully taken out from the blocks and cooled down after which the Kjeldahl digestion mixture (K2SO4:CuSeO3·2H2O: pumice in 970: 19: 11 w/w/w) was added (2 g / tube). By that time, the temperature of the digestion blocks reached 400°C, and the sample tubes were placed again in the block to digest for about 30 min. Properly digested light green colored samples were taken out and kept aside to be chilled and come to room temperature which then were diluted using distilled water (20 mL). Total nitrogen was analyzed using the method of Eastin (1978), and the appliance was the Kjeldahl Digestion and Distillation Unit (manufactured in Korea).
Root media were collected and kept for air drying and then used to prepare a saturated paste with the help of distilled water at a ratio of 1:10 (root media : distilled water, g / g). The paste was then kept at room temperature for two hours followed by the addition of 1–2 drops of a soil wetting agent to reach equilibrium. A clear solution of root media and distilled water was achieved after filtering with the help of a three-layered white gauze, which was then used to evaluate the pH and EC utilizing a pH/EC meter (Multi meter CP-500L, Istek Co., Seoul, Korea). After that, one drop of preservative was added, and the solution was preserved in a refrigerator to analyze the ion concentrations present in the root media with the help of ion chromatography (883 Basic IC plus, Metrohm, Switzerland). All the processes were done with three replications.
Statistical analysis
Data on seedling growth, tissue contents, pH, electrical conductivity, and nutrients present in root media were analyzed by Duncan’s multiple range test (p < 0.05) using CoStat Version 6.311 (CoHort Software, Monterey, California, USA).
Results and Discussion
The composition of the nutrient solution strongly influences the vegetative growth of strawberry plants. The relative ratio among the nutrients controls the vegetative equilibrium in seedlings and mother plants. Excessive or very low nutrient availability showed drastic effects in both shoot formation and growth (Savini et al. 2005). All essential nutrients, especially macro-nutrients, are limited in strawberry cultivation. Optimum application and uptake of these nutrients must occur to get better growth and yield of strawberries. The absence of any of these nutrients leads to deficiency symptoms and retreaded growth (Domoto 2011). A reduction in any of the essential nutrients depresses the vegetative growth of the plants (Guttridge 1985). The vegetative growth of strawberry varieties particularly depends on the fertility of root media and the availability of water during the growing season, which is a prerequisite for maximum fruit yield. Therefore, to gain steady growth and high yield with quality fruits, proper plant nourishment is necessary, which is only possible by providing adequate nutrients (Sharma 2002). According to Eshghi et al. (2008), plants treated with proper nutrients had the maximum vegetative growth, which led to the highest numbers of floral buds, fruit yield and shoot and root fresh and dry weights.
In the present study, different macronutrient deletion showed a substantial impact on the average values of growth for all strawberry cultivars (Table 2). Amidst the six nutrient stress treatments, seedlings treated with controlled N had a lower growth, i.e., the height of the plant, leaf length, width and number, length of petiole, SPAD, crown diameter, fresh weight, and dry weight of the mother plants compared to the other stress treatments.
Table 2.
Influence of the different macronutrient stresses (N-, P-, K-, Ca-, Mg- and S-) in the Fall season on growth and development of ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ mother plants 90 days after transplantation
| Treatment |
Plant height (cm) |
Leaf length (cm) |
Leaf width (cm) |
No. of petiole (cm) |
Petiole length (cm) | SPAD |
Crown dia. (cm) |
Fresh weight (g) |
Dry weight (g) |
| Altaking | |||||||||
| -N | 45.8 bz | 10.8 b | 7.3 c | 9.1 a | 35.9 b | 34.3 c | 0.9 a | 49.5 b | 7.8 b |
| -P | 48.2 a | 12.3 a | 8.6 b | 9.3 a | 37.3 a | 39.7 a | 1.1 a | 58.1 a | 12.0 a |
| -K | 49.1 a | 11.0 ab | 7.6 c | 8.6 a | 35.1 b | 40.2 a | 1.1 a | 49.6 b | 9.4 b |
| -Ca | 49.7 a | 11.6 ab | 14.3 a | 8.7 a | 36.8 ab | 39.9 a | 1.2 a | 55.0 a | 9.9 a |
| -Mg | 48.0 a | 11.3 ab | 8.0 c | 8.6 a | 37.7 a | 40.0 a | 1.1 a | 45.0 b | 9.5 b |
| -S | 49.2 a | 11.2 ab | 7.5 c | 9.0 a | 37.8 a | 38.9 b | 1.1 a | 55.2 a | 10.2 a |
| F-sig. | * | NS | ** | NS | NS | ** | NS | ** | ** |
| Kuemsil | |||||||||
| -N | 43.6 b | 11.3 c | 7.5 c | 9.5 a | 33.3 a | 40.8 a | 0.9 a | 43.5 a | 8.2 a |
| -P | 48.6 a | 11.6 bc | 8.5 b | 9.6 a | 34.5 a | 42.9 a | 1.0 a | 49.0 a | 9.2 a |
| -K | 49.9 a | 12.8 ab | 8.8 b | 9.7 a | 34.4 a | 43.4 a | 1.0 a | 55.3 a | 10.1 a |
| -Ca | 48.9 a | 12.4 ab | 8.7 b | 9.3 a | 34.8 a | 43.6 a | 0.9 a | 49.3 a | 9.5 a |
| -Mg | 48.8 a | 11.6 bc | 8.6 b | 9.4 a | 33.6 a | 42.5 a | 0.9 a | 57.6 a | 11.1 a |
| -S | 49.7 a | 13.1 a | 9.3 a | 9.6 a | 33.5 a | 44.0 a | 0.9 a | 55.1 a | 10.7 a |
| F-sig. | * | NS | ** | NS | NS | *** | NS | ** | ** |
| Vitaberry | |||||||||
| -N | 43.7 b | 12.7 a | 8.4 b | 10.3 a | 33.9 a | 40.6 c | 1.1 a | 53.9 b | 10.0 b |
| -P | 44.8 ab | 13.4 a | 9.7 a | 11.0 a | 32.0 a | 42.1 a | 1.1 a | 59.8 a | 11.4 a |
| -K | 45.8 ab | 13.9 a | 9.6 a | 10.2 a | 31.2 a | 43.5 a | 1.1 a | 61.0 a | 12.3 a |
| -Ca | 47.4 ab | 13.3 a | 9.2 a | 10.2 a | 32.9 a | 41.5 b | 1.0 a | 60.8 a | 11.8 a |
| -Mg | 45.1 ab | 12.4 a | 8.6 b | 10.3 a | 32.1 a | 41.4 b | 1.1 a | 54.1 b | 10.7 b |
| -S | 48.9 a | 11.8 a | 9.3 a | 10.6 a | 29.7 a | 40.8 c | 1.0 a | 51.1 b | 10.3 b |
| F-sig. | * | NS | ** | NS | NS | ** | NS | ** | ** |
Nutrient deletion resulted in varying plant heights across all strawberry varieties, with noticeable disparities primarily seen in controlled nitrogen (N) conditions (Table 2). Notably, ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ reached their tallest average heights of 49.7 cm, 49.9 cm, and 48.9 cm, respectively, under calcium (Ca), potassium (K), and sulfur (S) controlled treatments. Despite no significant overall differences in plant height among treatments, the N- controlled treatment yielded the lowest heights. When considering maximum leaf dimensions, ‘Altaking’ and ‘Kuemsil’ exhibited optimal length and width under Ca and S control, while ‘Vitaberry’ displayed differing trends with maximum length under K control and maximum width under P control. Additionally, the highest number of petioles for each cultivar was observed under S, K, and P control for ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’, respectively. Petiole length varied with ‘Altaking’ showing the maximum under S control, ‘Kuemsil’ under Ca control, and ‘Vitaberry’ under N control. Although leaf length, petiole number, and petiole length showed no significant differences across cultivars, leaf width exhibited partial significance. Notably, dry weight data (Table 1) indicated the most robust growth in ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ under P, Mg, and K control, respectively, consistent with findings by Eshghi et al. (2008).
The highest occurrence of runner plants (Table 3) in ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ was noted under P, S, and K- controlled conditions, respectively. Conversely, the N- controlled treatment resulted in the lowest growth across all tested strawberry cultivars. Runner growth displayed partial significance and was notably suppressed under N- controlled conditions for all cultivars. Crown diameter remained unaffected by nutrient deletion treatments and was statistically non-significant, yet consistently lowest under N-control for ‘Altaking’ and ‘Vitaberry’. At harvest, maximum SPAD values for fully expanded new leaves were observed under K-control for ‘Altaking’ and ‘Vitaberry’, and under S-control for ‘Kuemsil’. Numerical data suggests that while various nutrient controlled treatments support strawberry mother plant growth, they may not be optimal for commercial cultivation. This observation aligns with findings by Savini et al. (2005) and Yoon et al. (2018), who emphasized the importance of adequate nutrient application in improving strawberry yield through enhanced crown diameter, leaf area, and both fresh and dry weights, ultimately promoting vegetative growth and successive flowering. The experiment highlights the adverse effects of N- controlled treatment on both mother and runner plant growth. This result is also supported by Sharma et al. (2006), who stated that the abundant vegetative growth of strawberry seedlings is consistent with the excessive use of nitrogenous fertilizers, and low or no nitrogen resulted in stunted growth with a smaller number of petioles and a lower plant height.
It is noted that there was no significant difference in the treatments with respect to the number of runners in the cultivated strawberry varieties, but the growth of runners in terms of the length and fresh and dry weights of the runners were significantly different and were the lowest in the N- controlled treatment, which is very important for the production of runner plants. The highest fresh weight of runner plants in ‘Altaking’ and ‘Kuemsil’ was observed in the S- controlled treatments while in ‘Vitaberry’, it was observed in the Mg- controlled treatment. However, in all cultivars, the lowest occurrence and growth of runner plants and the number, fresh weight and length of runners were found in the N- controlled treatment compared to the other treatments (Table 3). The above result was confirmed by Biscaro et al. (2022), who mentioned that among all the essential macro and micronutrients, N is the most growth-limiting factor in strawberries, and it agrees with the outputs of Agehara (2021). The applications of nitrogen in the early season results in higher vegetative growth of the mother plants, which is responsible for further reproductions and yield of fruit. An increase in the rate of N fertilizer resulted in higher vegetative growth, where the absence of N showed poor and weak above-grown biomass for all cultivars. On the other hand, Lundblad (2019) mentioned that an immoderate application of fertilizers, especially N, may increase vegetative growth, but it can inhibit the occurrence of runner plants. Additionally, to get the maximum vegetative growth with higher emergence of runner plants, the key factor is to use of an adequate amount of all essential nutrients.
Table 3.
Influence of the different macronutrient stresses (N-, P-, K-, Ca-, Mg- and S-) in the Fall season on the growth and development of strawberry seedlings (runners and runner plants) 90 days after transplantation
| Treatment | Runner plants | Runner | |||||
| No. | Fresh weight (g) | No. | Fresh weight (g) | Dry weight (g) | Length (cm) | ||
| Altaking | |||||||
| -N | 7.6 bz | 25.3 b | 5.9 a | 18.8 b | 2.7 b | 506.3 c | |
| -P | 9.2 a | 32.9 a | 6.8 a | 22.7 a | 3.5 a | 606.1 a | |
| -K | 8.2 a | 32.2 a | 6.3 a | 19.4 b | 3.0 a | 526.9 c | |
| -Ca | 8.7 a | 33.3 a | 6.3 a | 22.2 a | 3.3 a | 545.6 b | |
| -Mg | 8.6 a | 29.1 b | 6.3 a | 19.8 b | 2.9 b | 569.8 b | |
| -S | 8.8 a | 33.7 a | 6.4 a | 21.6 a | 3.2 a | 598.6 a | |
| F-sig. | * | * | NS | * | * | ** | |
| Kuemsil | |||||||
| -N | 5.8 b | 25.5 c | 5.0 a | 14.8 c | 2.2 c | 512.0 c | |
| -P | 7.8 a | 35.1 b | 5.2 a | 20.4 ab | 2.9 b | 562.1 b | |
| -K | 7.1 a | 31.6 b | 5.4 a | 16.8 bc | 2.5 b | 539.4 a | |
| -Ca | 7.6 a | 31.6 b | 5.0 a | 18.9 ab | 2.8 b | 619.8 a | |
| -Mg | 7.6 a | 37.3 a | 5.7 a | 19.9 ab | 2.9 b | 539.6 b | |
| -S | 7.8 a | 40.0 a | 5.6 a | 22.7 a | 3.3 a | 627.1 a | |
| F-sig. | * | * | NS | * | * | ** | |
| Vitaberry | |||||||
| -N | 10.6 b | 37.1 b | 6.0 a | 28.7 b | 4.3 b | 609.6 c | |
| -P | 11.8 a | 44.6 a | 6.6 a | 34.2 a | 5.2 a | 667.2 b | |
| -K | 13.1 a | 39.7 a | 6.6 a | 35.3 a | 5.6 a | 765.0 a | |
| -Ca | 12.6 a | 43.2 a | 6.4 a | 33.5 a | 5.1 a | 697.0 b | |
| -Mg | 12.1 a | 45.7 a | 6.4 a | 33.6 a | 5.4 a | 691.5 b | |
| -S | 11.6 a | 39.1 b | 5.9 a | 29.2 b | 4.5 b | 661.2 b | |
| F-sig. | * | * | NS | * | * | ** | |
Different nutrient deletion treatments impacted the tissue contents of all strawberry cultivars (Table 4). Specifically, the N- controlled treatment exhibited the lowest total nitrogen levels alongside the highest manganese (Mn) content. Conversely, the K- controlled treatment showed the highest levels of phosphorus (P), calcium (Ca), and magnesium (Mg) across all cultivars, with partial significance observed. Additionally, the highest potassium (K) content was observed in the N- controlled treatment for ‘Kuemsil’ and ‘Vitaberry’. However, in ‘Altaking’, both P- and Mg- controlled treatments showed the highest percentage of K content. A significant difference was absent in the amount of K present in the tissue content. In all the cultivars, the amount of Cu and Fe was the highest in the P- and Ca- controlled treatments, respectively. On the other hand, the amount of Zn was highest in the Ca- controlled treatment in ‘Altaking’ and ‘Kuemsil’, but in ‘Vitaberry’, it was in the K- controlled treatment. The amount of tissue contents in plant sap depends on the proportion of fertilizers applied to the root media, which also has been reported by Agehara (2021). They further said that the vegetative growth of the strawberry mother plants is influenced by the properties of the plant sap in the strawberry seedlings. Nevertheless, based on the findings of Yamasaki and Yano (2009), the amount of NPK in the shoot apex and leaves depends on the fertilizers applied in the root media during the vegetative growth; this finding is also supported by Eshghi and Tafazoli (2007). However, the soil solution pH regulates the amount of K, Ca, Mg, and S contents. The concentration of the mentioned macronutrients is reduced in soil solution if the root media become acidic, which results in decreased plant sap. Acidic root media interrupt the root apex in the uptake of alkali ions; on the other hand, rhizosphere acidification takes place due to excess application of nitrogenous fertilizers (Sas et al. 2003). Nonetheless, Khalil and Agah (2017) confirmed that the addition of fertilizers in root media increases the assimilation and accumulation of macro and micronutrients which lead to enhanced tissue nutrient contents in strawberry plants. However, the tissue content in ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ at their highest growth of mother plants regarding their dry weight was as follows: 3.59, 3.81, and 4.00% total nitrogen, 1.11, 1.51, and 1.51% P, 3.75, 3.72, and 3.48% K, 2.17, 1.72, and 3.09% Ca, 1.02, 1.07, and 1.74% Mg, 5.63, 3.68, and 4.38 mg kg-1 Cu, 115.06, 162.64, and 143.16 mg kg-1 Fe, 114.72, 119.23, and 119.16 mg kg-1 Mn, and 19.63, 25.25, and 26.48 mg kg-1 Zn, respectively.
Table 4.
Influence of the different macronutrient stresses (N-, P-, K-, Ca-, Mg- and S-) in the Fall season on the tissue nutrient content of strawberry mother plants 90 days after transplantation
| Cultivar | Treatment | T-N | P | K | Ca | Mg | Cu | Fe | Mn | Zn |
| --------------------- % --------------------- | ---------------- mg kg-1 ------------------ | |||||||||
| Altaking | -N | 2.48 cz | 1.41 a | 3.69 ab | 1.43 b | 1.13 b | 4.12 b | 128.36 c | 136.44 a | 17.44 b |
| -P | 3.59 b | 1.11 c | 3.75 a | 2.17 a | 1.02 b | 5.63 a | 115.06 d | 114.72 c | 19.63 ab | |
| -K | 3.30 b | 1.43 a | 3.53 b | 2.92 a | 1.87 a | 3.73 c | 138.11 b | 113.54 c | 21.21 a | |
| -Ca | 3.83 a | 1.39 a | 3.68 ab | 0.91 c | 1.31 b | 3.96 b | 168.34 a | 122.61 b | 23.39 a | |
| -Mg | 3.90 a | 1.38 a | 3.75 a | 1.82 b | 0.92 c | 3.66 c | 165.76 a | 122.77 b | 25.27 a | |
| -S | 3.79 a | 1.32 b | 3.71 a | 1.93 b | 1.73 a | 4.18 b | 126.45 c | 114.11 c | 14.55 b | |
| F-sig. | ** | ** | NS | *** | *** | *** | *** | ** | NS | |
| Kuemsil | -N | 2.51 b | 1.36 b | 3.77 a | 1.16 b | 1.09 c | 4.13 c | 119.47 c | 123.96 a | 15.36 b |
| -P | 3.71 a | 1.13 c | 3.68 ab | 2.33 a | 1.01 c | 6.61 a | 112.68 c | 111.83 c | 18.58 ab | |
| -K | 3.65 a | 1.52 a | 3.47 b | 2.95 a | 1.76 a | 5.32 b | 142.19 b | 117.54 b | 21.56 a | |
| -Ca | 3.72 a | 1.47 a | 3.72 a | 0.84 c | 1.38 b | 4.19 c | 163.97 a | 112.48 c | 25.44 a | |
| -Mg | 3.81 a | 1.51 a | 3.72 a | 1.72 b | 1.07 c | 3.68 d | 162.64 a | 119.23 b | 25.25 a | |
| -S | 3.76 a | 1.31 b | 3.75 a | 2.11 a | 1.68 a | 4.42 c | 131.61 b | 112.85 c | 15.33 b | |
| F-sig. | * | ** | NS | *** | ** | *** | *** | ** | NS | |
| Vitaberry | -N | 2.72 b | 1.41 a | 3.75 a | 1.47 c | 1.45 b | 4.17 e | 117.91 c | 126.22 a | 19.11 b |
| -P | 4.10 a | 1.26 c | 3.73 a | 2.59 b | 1.21 c | 6.37 a | 111.73 c | 121.56 a | 19.63 b | |
| -K | 4.00 a | 1.51 a | 3.48 b | 3.09 a | 1.74 a | 4.38 d | 143.16 b | 119.16 b | 26.48 a | |
| -Ca | 4.02 a | 1.42 a | 3.64 ab | 1.16 c | 1.51 a | 4.64 c | 162.84 a | 124.66 a | 20.19 b | |
| -Mg | 4.10 a | 1.49 a | 3.74 a | 2.32 b | 1.12 c | 4.13 e | 159.87 a | 123.49 a | 20.24 b | |
| -S | 3.93 a | 1.33 b | 3.70 a | 2.46 b | 1.66 a | 5.39 b | 135.22 b | 116.28 b | 15.13 c | |
| F-sig. | * | ** | NS | ** | ** | *** | *** | * | ** | |
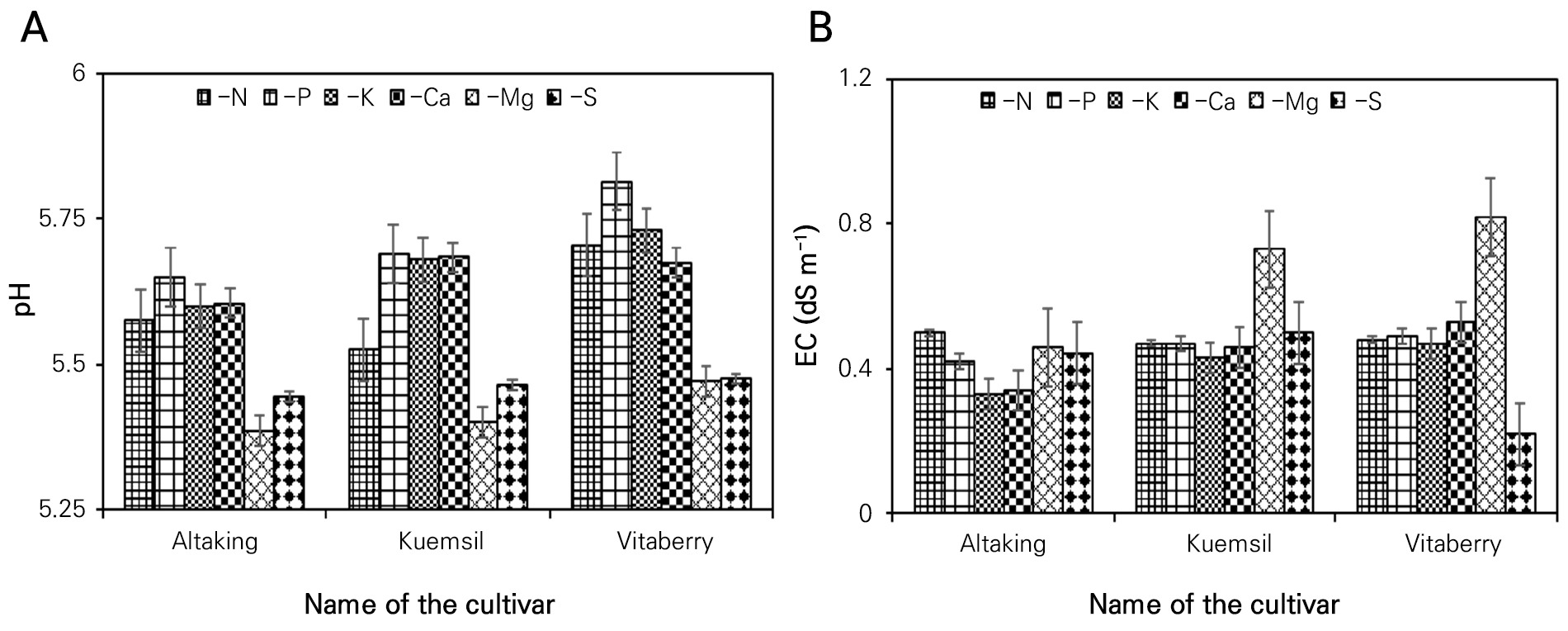
Fig. 1 shows the results of the numerical values of the pH and EC analysis in the root media solution at 90 days after seedling transplantation. The applied fertilizer treatments are dominant for nutrient availability in the root zone, plant uptake and pH regulation in the root substrates. Excessive application of fertilizers is corelated with an increase in the root zone pH. pH values can increase with an increase in the quantity of applied fertilizers as a result of residual effects. Additionally, the residual effects of nutrients along with liming may affect the soil acidity which has been found to last up to 15 years (Hargreaves et al. 2009). However, in the present study, a significant statistical difference was absent in the root medium pH (Fig. 1A) among the treatments (5.23–5.82) but was lowest in the Mg- controlled treatment in all cultivars. Treatments with a higher pH showed a lower presence of NO3 and PO4 ions in the root media. This result was also demonstrated Kang et al. (2011); according to that study, not only NO3 and PO4 but the uptake and presence of K, Ca, Mg, Fe, Mn, and Zn in the shoots were also significantly affected by the pH in the root zone. They stated that a high pH had inhibitory effects on the nutrient contents of the shoot tissues. According to Sas et al. (2003), the pH and the amount and form of fertilizer applied have a reciprocal relationship and influence the availability of other necessary nutrients.
In contrast, the electrical conductivity (EC) showed a significant difference with respect to the different nutrient-controlled treatments and was highest in the Mg- controlled treatment in all three cultivated strawberry varieties (Fig. 1B). It had a comparatively lower value in other nutrient deletion treatments, especially in the N-, P- and K- controlled treatments. This result indicates that the EC is highly influenced by different nutrient stresses in fertilizer solutions, and the absence of macronutrients has an impact on the soil solution EC. This result was also supported by Kang et al. (2011), showing that an increase in the concentration of nutrients in the fertilizer solution is directly responsible for raising the nutrient concentration of the root zone and the shoot and highly influences the electrical conductivity of the root media solution. This result was demonstrated by Choi et al. (2016), who reported that the root zone EC concentration depends on the concentration of the applied nutrient solution.
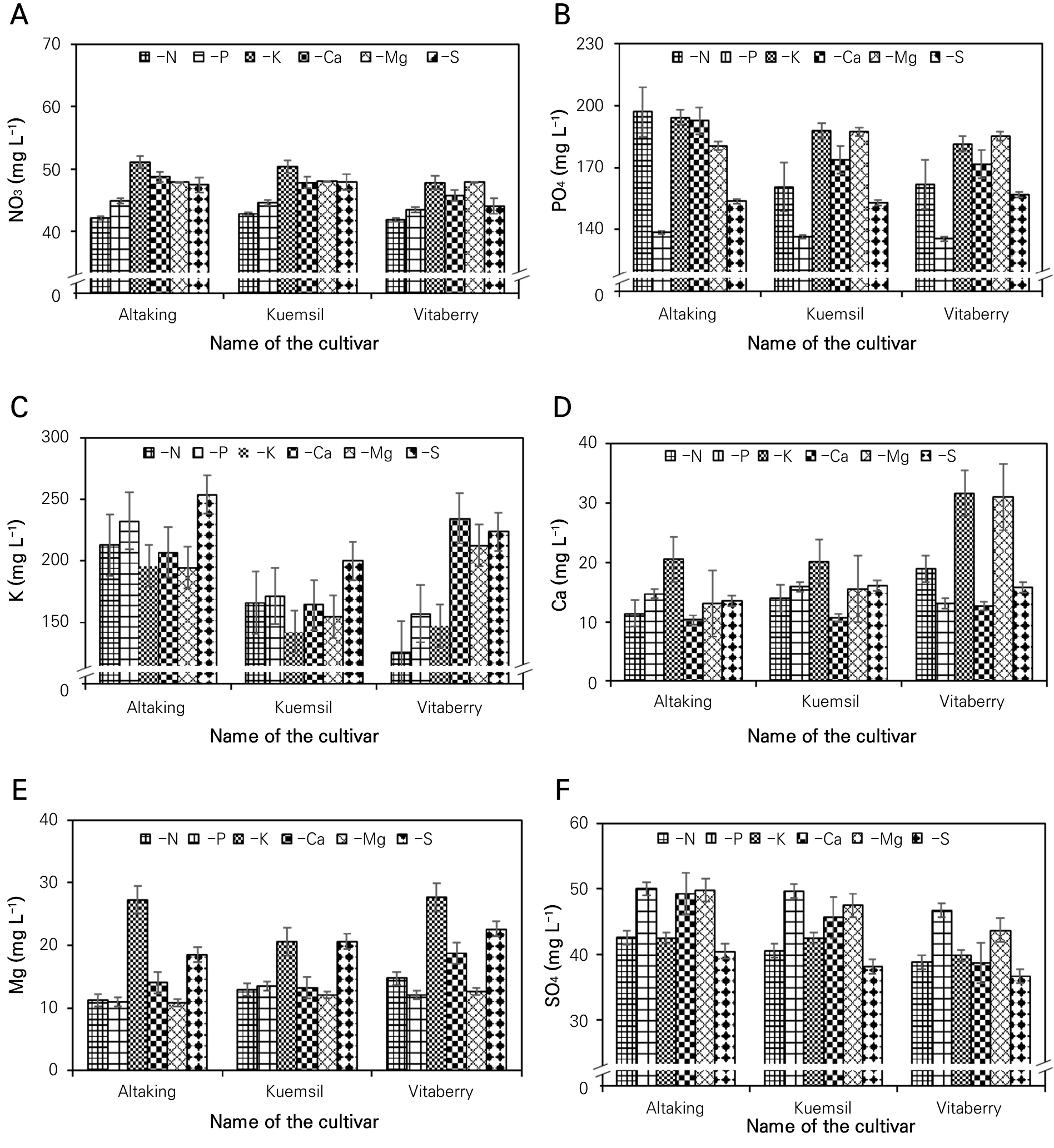
Fig. 2 shows the available macronutrients concentration in the root substrate solution at 90 days after the strawberry seedlings were transplanted. The root medium nutrient concentrations in ‘Altaking’ and ‘Vitaberry’ at their highest mother plant growth and incidence of runner plants were as follows: 44.9 and 47.8 mg L-1 NO3-; 138.5, and 181.4 mg L-1 PO43-; 232.4, and 147.1 mg L-1 K+; 14.7, and 31.6 mg L-1 Ca2+; 11.0, and 27.7 mg L-1 Mg2+, and 50.0, and 39.9 mg L-1 SO42-, respectively. On the other hand, the root media nutrients of ‘Kuemsil’ at the highest growth of the mother plants were 48.0, 187.3, 174.6, 15.5, 12.1, and 47.5 mg L-1 of NO3-, PO43-, K+, Ca2+, Mg2+, and SO42, and the root media nutrients for the maximum occurrence of runner plants were 47.8, 152.8, 200.1, 16.1, 20.6, and 38.2 mg L-1 of NO3-, PO43-, K+, Ca2+, Mg2+, and SO42-, respectively (data are not shown). The nutrient concentration in the root media solution was statistically significant and exhibited the lowest value in their stress treatments while the highest value of the nutrient concentrations was different in the three cultivars with respect to the stress treatments. Fageria (2006) reported that the elevated nutrient concentrations in root media solution can enhance or reduce the nutrient availability, uptake, and plant tissue concentration. Fageria again showed that the assimilation of N and S by seedlings is closely correlated, and N can regulate S assimilation. The result is further supported by Farjana et al. (2023) who reported that nutrients in root media interact with each other and can regulate their availability to plants. They further said that nitrogen (N), phosphorous (P), potassium (K), and sulfur (S) have significant interactions among themselves, and elevation of the concentration of one nutrient in the root media solution can increase or decrease the concentration and availability of other nutrients in the plant tissue. However, a nutrient solution with adequate concentrations has no negative effect on the availability of nutrients.
Conclusions
Finally, this study emphasizes the critical importance of nutrient composition in determining strawberry plant growth and productivity. Nutrient ratios must be balanced to ensure plant health and productivity. Nitrogen deficiency, among other nutrients, has a major impact on numerous growth variables. The complicated link between nutrient availability and plant absorption is shown by analyzing tissue nutrient concentration and root media composition. Proper fertilization techniques are essential for avoiding deficits or excesses that limit growth. Nutrient levels, particularly electrical conductivity, must be monitored to ensure optimal plant nutrition. Fertilization strategies must be tailored to cultivar-specific requirements. Overall, accurate nutrition management is essential for growth of strawberry in order to improve plant quality and propagation.