Introduction
Materials and Methods
Plant Materials and Cultivation Conditions
Production of Cuttings in Relation to the Environmental Conditions
Growth, Fruit, and Yield Characteristics
Analysis of Root Activity
Statistical Analysis
Results and Discussion
Effects of the Collection Time of Cuttings on Transplant Production and Survival
Effects of the Collection Time of Cuttings and Propagation Methods on the Transplant Quality
Effects of the Collection Time of Cuttings and Propagation Methods on Fruit Characteristics and Yield
Conclusion
Introduction
Strawberry (Fragaria × ananassa Duch.) is an economically important horticultural crop in Korea, with domestic production reaching a value of 1.48 trillion won in 2021. It is the best income crop with respect to vegetables, accounting for 11.2% of the total vegetable production in Korea (MAFRA, 2022). With regard to strawberry cultivation, obtaining high-quality transplants accounts for 80% of the total proportion, a substantial level that also acts as a decisive factor of fruit yield and quality outcomes (Jun et al., 2014). ‘Pinning’ is the traditional propagation method of strawberry transplants in Korea and is customarily conducted from March to September. This propagation method involves complex steps, such as planting mother plants, developing runners, anchoring the runners, separating the runners from the mother plants, and inducing flower bud differentiation (Na et al., 2014; Park and Choi, 2015).
With cutting propagation, only three to four months are required to complete the steps described above (Kim et al., 2018). Recently, in Europe, cutting propagation has been attempted for strawberries to shorten the nursery period and produce uniform transplants (Kim et al., 2018; Hwang et al., 2020). This method uses cutting propagates produced from mother plants in the cultivation house under high temperatures during the year following the harvest (Kang et al., 2011). Although cutting propagation can reduce labor requirements and considering that uniform transplants can be produced in a relatively short period simultaneously, environmental and water management issues are essential to increase the survival rate and root activation of seedlings between one to two weeks after the planting of the cuttings (Kim et al., 2018). Environmental control technology has been studied extensively in relation to strawberry cutting propagation, including characteristic tests according to the conditions under which runner tips are collected for the ‘Chandler’ cultivar and comparisons of reactions during the application of a photo-selective net for the ‘Festival’ cultivar (Takeda and Hokanson, 2001; Takeda et al., 2010).
However, sufficient research has not been conducted regarding the effects of cutting propagation and the collection time of cuttings compared to the pinning propagation method for domestic strawberry cultivars. Therefore, this study was conducted to determine the effects of different collection time of cuttings on the transplant quality, post-transplanting growth, fruiting, and yield of ‘Sulhyang’ strawberry plants in comparison to the pinning propagation technique.
Materials and Methods
Plant Materials and Cultivation Conditions
‘Sulhyang’ strawberry runner cuttings were collected from mother plants growing in a greenhouse at the Jeollabuk-do Agricultural Research and Extension Services, located in Iksan, on May 8, June 5, and July 3, 2019. Collected cuttings were refrigerated at 3°C until planting on July 10, 2019 in a strawberry nursery pot (24 holes pot, 60 cm × 34 cm × 10 cm, Hwaseong Industrial Co. Ltd., Okcheon, Korea) filled with an artificial soil medium (Myung Jin Joo, Homan Industrial Co. Ltd., Jeongeup, Korea). Planted cuttings were placed in a small plastic tunnel of an elevated bed in the nursery greenhouse for 10 days. The tunnel was covered with nonwoven fabric, vinyl, and 35% shading film to block excess direct sunlight and to maintain the temperature and humidity.
Transplants obtained from pinning propagation were considered as controls to verify the effectiveness of cutting propagation according to the cutting collection timing. In this method, mother plants were planted on March 20, 2019, and the runner was induced from June 1. In both propagation methods, transplants were grown in the same nursery greenhouse with a supply of a Yamazaki nutrient solution of EC 0.8 dS·m-1 and pH 6.0 until one month before transplanting, after which, to induce flower differentiation, only water was supplied until the transplanting. On September 20, 2019, the established transplants were planted in an elevated vinyl greenhouse for hydroponic cultivation. The transplants were planted in pots filled with an artificial soil medium (The Zone, Gungon Geotec Industrial Co. Ltd., Jincheon, Korea) in two-row arrangements with 23 cm × 23 cm spacing and were allowed to grow until February of 2020. During the cultivation period, the plants were irrigated using the Yamazaki nutrient solution and managed using standard strawberry cultivation practices. Pesticides and insecticides were applied throughout the experiment to control major diseases, such as anthracnose and powdery mildew, and pests, such as aphids and mites.
Production of Cuttings in Relation to the Environmental Conditions
The average number of cuttings produced per mother plant was counted from April 1st to each collection time using 120 mother plants per treatment. Environmental conditions in the cultivation house were investigated from the time of runner initiation to the collection time of the cuttings. The cumulative solar radiation, air temperature, and relative humidity were measured every 30 min using a data logger (WatchDog 1000 Series, Encosys Co. Ltd., Anyang, Korea).
Growth, Fruit, and Yield Characteristics
The survival rate was examined six times at three-day intervals after tunnel cultivation from July 20 to August 4, 2019. The growth characteristics of the plants were investigated according to the standard manual for strawberries (RDA, 2017). The leaf chlorophyll content was measured using a SPAD meter (SPAD-502, Konica Minolta Inc., Osaka, Japan). The dry weight of the plants was measured after drying them in a constant-temperature dryer (DH-2009H, Korea Dry Tech., Jincheon, Korea) at 60°C for 72 h.
The fruit quality characteristics and marketable yield were determined during the harvest period from December to February. Fruit firmness was measured using a firmness meter (TA1 Texture Analyzer, AMETEK Inc., Largo, FL, USA) with a 4 mm probe. The soluble solids content was measured using a hand refractometer (PAL-1, ATAGO Co., Ltd., Tokyo, Japan). The marketable yield of the fruits was investigated by measuring the fruit weight and the number of fruits. Decayed, malformed, and small fruits that weighed less than 10 g were discarded.
Analysis of Root Activity
The activity of fresh roots collected from the transplants was measured on August 28 by extracting the formazan content using the TTC (triphenyl-tetrazolium chloride) method (Yoshida, 1966). The supernatant of the extracted formazan was decanted and the absorbance was measured at 470 nm using a microplate reader (Cytation 3, BioTek Instruments Inc., Winooski, VT, USA). For the standard curve, TPF (triphenyl formazan) was dissolved in ethyl acetate to prepare standard solutions with concentrations of 1, 2, 5, 10, 20, and 50 µL·L-1. The root activity was expressed as follows:
Root activity (mg·g-1·h-1) = Amount of formazan (mg) / [Root weight (g FW) × reaction time (h)]
Statistical Analysis
During the nursery period, 72 plants per replicate of treatment were placed in a completely randomized design with three replications. During the cultivation period, the established transplants were transplanted according to a randomized block design, with three replicates of 24 plants each. Statistical analyses were conducted using R software (version 4.0.3). The means were calculated from three replicates per treatment, and significant differences between treatments were evaluated using Duncan’s multiple-range test and the LSD (least significant difference) test at p < 0.05. The relationship between the number of produced cuttings and the cumulative solar radiation in the greenhouse was analyzed through a nonlinear regression analysis with SigmaPlot 12.5 (Systat Software Inc., San Jose, CA, USA).
Results and Discussion
Effects of the Collection Time of Cuttings on Transplant Production and Survival
The climatic conditions in the cultivation house from which the runner cuttings were collected from the mother plants are presented in Fig. 1. Strawberry plants grow well at 17–23°C in the daytime; however, they stop growing at temperatures higher than 30°C and suffer high-temperature stress at approximately 37°C (RDA, 2019). When collecting cuttings in May and June, the greenhouse temperature was appropriate for strawberry growth. However, the cutting conditions in July were not suitable, as the maximum temperature in the greenhouse reached 37.9°C.
There were 2.0, 5.3, and 6.7 runner cuttings formed per mother plant in the May, June, and July collections, respectively (Fig. 2). In general, approximately 10,000 transplants were transplanted in an area of 1,000 m2 for cultivation, and approximately 52,500 and 37,500 transplants were produced by the elevated pinning and soil pinning propagation methods, respectively (RDA, 2015). Based on this, 20,000 transplants could be obtained per 1,000 m2 in the May collection, whereas 53,000 and 67,000 transplants were collected for the June and July collections, respectively. Therefore, producing an appropriate number of transplants from the May collection using the cutting propagation method was difficult compared to the pinning propagation method. In contrast, in the June and July collections, the desired number of transplants could be procured for subsequent strawberry cultivation.
However, although the appropriate number of transplants was achieved in July, a few problems were observed in this collection. First, air circulation around the plants was not sufficient due to the crowding of runners that occurred as the fruits were produced. Moreover, the risk of pests and diseases increased, and the agricultural work was challenging owing to the extended runners on the floor and the need to manage the environmental conditions in the greenhouse (data not shown). Therefore, during the cutting propagation of ‘Sulhyang’ strawberry, June was considered the most appropriate time to collect a sufficient number of transplants and reduce the risks associated with pests and diseases while also decreasing the required amount of labor.
Crops respond differently to environmental conditions for their growth (Von Amim and Deng, 1996; Kang et al., 2010). In particular, light is involved in photosynthesis and morphogenesis in crops and plays a decisive role in crop yield (Clouse, 2001). As the relationship between the cumulative amount of solar radiation in the greenhouse and the number of runner cuttings is important when raising transplants, a regression analysis was conducted to examine this relationship (Fig. 3). The relationship between the two variables is expressed as the following logarithmic function:
y = 4.4113 ln (x)–24.4090 (R2 = 0.9920***),
where x denotes the cumulative solar radiation (MJ·m-2) and y represents the number of cuttings.
The number of cuttings produced in the cultivation greenhouse was closely related to the light conditions during the cutting propagation of strawberries.
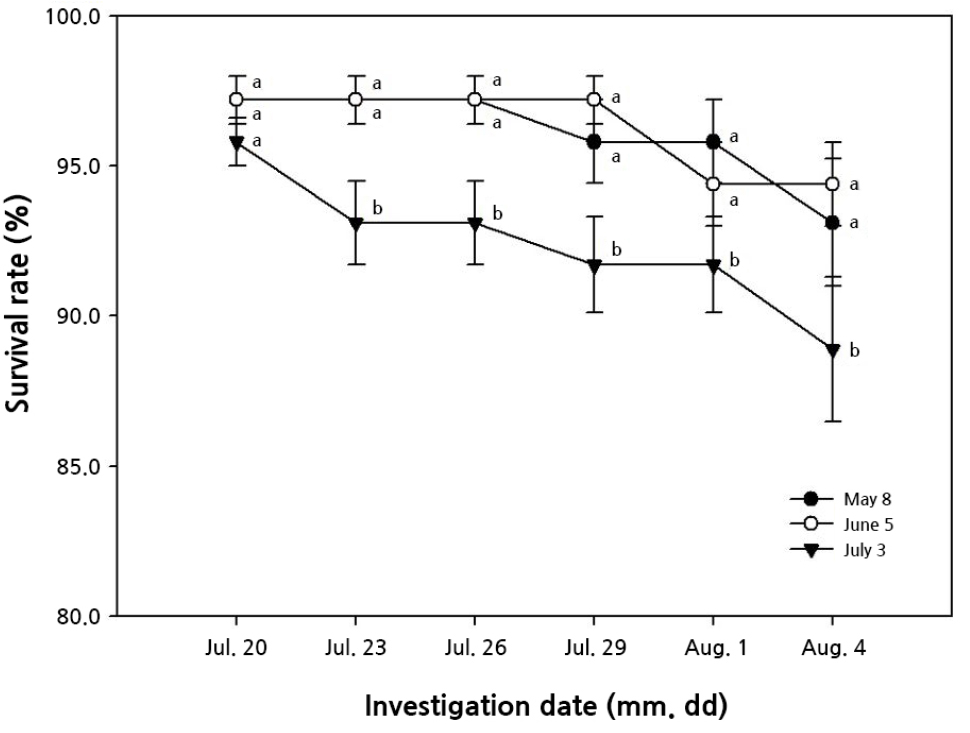
The survival rate exceeded 95.0% in all treatments at 10 days after tunnel cultivation; however, it tended to decrease slightly over time (Fig. 4). The highest proportion of surviving individuals was obtained in the June collection. In contrast, the rate was lowest in July, when cuttings were collected at the highest temperature. This result was consistent with a study by Rho et al. (2009), as the transplants withered over time and acted as a factor that lowered the plant activity rate when very numerous runner plants were collected under high-temperature conditions. Moreover, the coincidence observed in earlier work by Takeuchi and Sasaki (2008) stemmed from the failure to maintain continuous growth through nutrient contention between the generated cuttings when the number of cuttings occurring on one plant was the highest.
Effects of the Collection Time of Cuttings and Propagation Methods on the Transplant Quality
The timing of cutting collection influences root development in Alnus maritima (Schrader and Graves, 2000). In this experiment, forty days after planting, the shoot fresh weight was significantly lower in the May collection (Fig. 5). It was thought that because runner cuttings were separated from the mother plants earlier than in other treatments, nutrients from the mother plants were relatively less distributed to the transplants in the May collection. However, the root weight was significantly lower in July. Owing to the late collection of the cuttings, aging roots that occurred earlier led to reduced root weight outcomes.
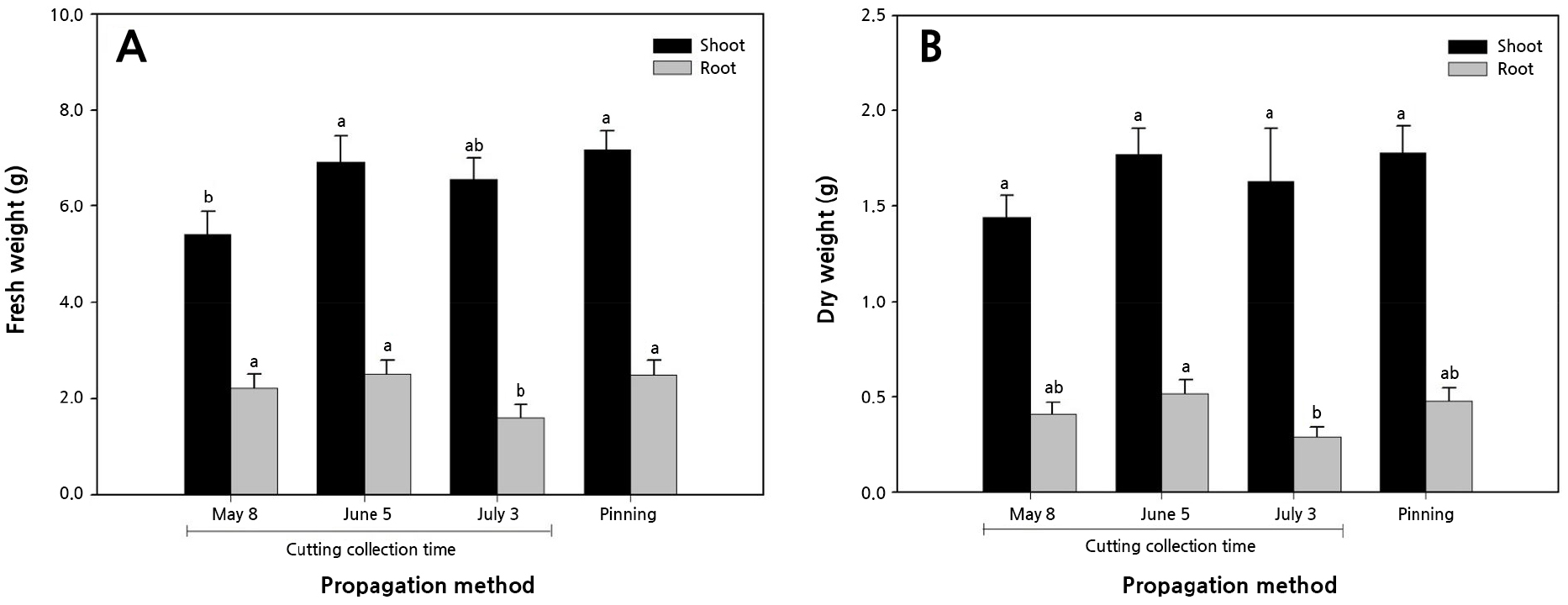
Fig. 5.
Effects of different propagation methods and cutting collection time on the fresh weight (A) and dry weight (B) of transplants at 40 days after planting (Aug. 20, 2019). Vertical bars represent the SE (standard error). Different letters at the top of the vertical bar indicate a statistical difference according to Duncan’s multiple-range tests at p < 0.05 (n = 10).
At that time, root vitality, expressed as the formazan concentration, was significantly higher in May and June (Fig. 6). For deciduous trees, owing to the competitive consumption of nutrients for shoot growth, rooting becomes more challenging with older cuttings (Hartmann et al., 1990). In the July collection here, vitality was relatively low as the temperature was higher than that most appropriate for the growth of strawberries. These experimental results were consistent with those of a previous report showing a relatively small incidence of rooting when using an old branch.

Fig. 6.
Effects of different propagation methods and cutting collection time on the root activity of transplants. Vertical bars represent the SE (standard error). Different letters at the top of the vertical bar indicate a statistical difference according to Duncan’s multiple-range tests at p < 0.05.
Transplants in the June collection were heaviest in terms of the root weight and also had the highest formazan concentration. In contrast, the concentration of formazan was lowest in the July collection, which had the lowest root weight. Moreover, the concentration of formazan was also low in transplants from pinning propagation with a relatively low root dry weight than in the June collection. Thus, it was confirmed that root activity tends to increase with an increase in root weight. These results suggest that root vitality is better when using cutting propagation compared to pinning, particularly when cuttings are collected in May and June.
At the transplanting date, the plant height, petiole length, and chlorophyll content were not significantly different among the treatments (Table 1). In contrast, the number of leaves was lowest when using the pinning treatment and highest in the May and June collections. For strawberries, high-quality transplants are determined by the appropriate crown diameter, the root weight, the number of primary roots, the T/R ratio, the degree of root vitality, and by flower differentiation without pest infection (Uematsu, 1998). When a transplant with a crown diameter of 8 mm or more is used as a cultivated plant, the growth and root activity are excellent, the production of whole fruits increases, and harvests are faster compared to when a transplant with a small crown diameter is used (Durner et al., 2002; Cocco et al., 2010).
Table 1.
Effects of different propagation methods and cutting collection time on the growth characteristics of transplants on the transplanting date (Sept. 20, 2019)
|
Propagation method |
Collection time of cuttings |
No. of leaves |
Plant height (cm) |
Petiole length (cm) |
Leaf length (cm) |
Leaf width (cm) |
Chlorophyll content (SPAD) |
Crown diameter (mm) |
T/R ratioy |
| Cutting | May 8 | 5.3 az | 26.5 a | 17.3 a | 8.1 ab | 6.6 ab | 39.0 a | 9.1 a | 3.5 ab |
| June 5 | 5.6 a | 26.2 a | 17.1 a | 8.0 ab | 6.4 ab | 38.9 a | 8.8 a | 3.0 b | |
| July 3 | 4.9 ab | 27.4 a | 18.7 a | 7.8 b | 6.1 b | 36.3 a | 8.9 a | 4.4 a | |
| Pinning | 4.4 b | 27.7 a | 17.6 a | 8.7 a | 7.0 a | 38.9 a | 8.8 a | 3.5 ab | |
In this experiment, the crown diameter was greater than 8 mm in all treatments, and there were no significant differences among the treatments. An examination of the T/R ratio to compare the integrity of the plants revealed that the value was greatest in the July collection and lowest in the June collection. Ahn et al. (1988) found that the lower the T/R ratio, the faster the differentiation of the flower, and that if the T/R ratio increases, shoot growth is greater than root development, making the transplants vulnerable or even weak. Accordingly, transplants from the July collection here showed the lowest integrity, whereas the transplants from the June collection showed excellent integrity.
Effects of the Collection Time of Cuttings and Propagation Methods on Fruit Characteristics and Yield
The fruit length, fruit diameter, and SSC did not differ significantly between the treatments (Table 2). These results were consistent with those of Kang et al. (2020), who revealed non-significant differences in the fruit length, fruit diameter, and SSC among different nursery methods during hydroponic and soil cultivation. Fruit firmness was hardest in the May and June collections, whereas it was softest when the fruit were cultivated via pinning propagation.
Table 2.
Effects of different propagation methods and cutting collection time on the fruit quality levels and yield
|
Propagation method |
Collection time of cuttings |
Fruit length (mm) |
Fruit diameter (mm) |
Fruit firmness (N, ∅4 mm) |
Soluble solids content (%) |
No. of fruits per plant |
Average fruit weight (g) | Marketable yield | |
|
Per plant (g) |
Per 10 a (kg) | ||||||||
| Cutting | May 8 | 46.7 az | 29.6 a | 1.9 a | 11.6 a | 16.1 ab | 20.9 ab | 337.3 a | 1,753 ± 46 ay |
| June 5 | 43.6 a | 29.9 a | 2.0 a | 11.6 a | 15.9 b | 22.0 a | 349.8 a | 1,819 ± 29 a | |
| July 3 | 42.7 a | 29.5 a | 1.8 ab | 11.5 a | 16.6 a | 20.1 b | 333.8 a | 1,736 ± 44 a | |
| Pinning | 45.5 a | 29.7 a | 1.7 b | 11.6 a | 15.3 c | 21.3 ab | 325.8 a | 1,694 ± 68 a | |
The number of fruits per plant was highest in the July collection and lowest when the fruit were cultivated with the pinning propagation method. Pinning has many disadvantages, such as a high plant height owing to the long nursery period or high density in the pot and uneven transplant quality caused by differences in the runner fixing period (Kang et al., 2019; Zheng et al., 2019). Therefore, the number of fruits was thought to be lowest in the transplants obtained using pinning propagation, where uneven quality levels were observed. The highest fruit weight was obtained in the June collection and the lowest was found in the July collection. In these results, the trend matched that of the T/R ratio of the transplanted plants at the time of transplanting.
The marketable yield was not significantly different among the treatments. However, the yield value was higher at 1,819 kg/10a in the June collection. The standard error was lowest in the June collection, whereas it was highest under pinning propagation, indicating greater deviation during the pinning treatment. Well-propagated transplants exhibit early fruiting and produce high-yield and high-quality fruits (Hochmuth et al., 2006; Giménez et al., 2009). In the June collection, which had excellent quality of the transplants and good root vitality, the fruit characteristics and yield were the best. By contrast, in the July collection, when the collection time was late, the growth and average fruit weight were lowest and the yield was lower than that in the June collection, possibly due to the uniformity of the transplants. These results were consistent with earlier work that found that transplants occurring after July tend to be of poor quality owing to the short nursery period, producing low yield during the winter (Uematsu, 1998; RDA, 2009). In this experiment, the mean marketable yield under pinning propagation was very low, but the deviation was the greatest. The decrease in the overall yield was thought to be caused by the decreased transplant uniformity due to differences between the orders. As a result, although no statistical difference was found, the average marketable yield value was lowest under the pinning propagation method when compared to the cutting propagation method.
Conclusion
When producing transplants, propagation methods and cutting collection time in cutting propagation differentially affected plant growth characteristics, fruit properties, and the marketable yield of ‘Sulhyang’ strawberry. Cutting propagation of strawberries could make the transplants more uniform and of better quality, and could produce fruits in a more stable manner compared to the pinning propagation method. Among the three collections here, collecting cutting in early June more effectively increased survival rates, improved transplant quality, and led to the harvesting of a stable fruit yield. Therefore, these findings suggest that cutting propagation in the early June can be applied for the cultivation of ‘Sulhyang’ strawberry to improve the transplant quality and stabilize the marketable yield more efficiently compared to pinning propagation. These results will help with the cultivation of high-quality transplants as a complementary method to the conventional pinning propagation method used for strawberries.