Introduction
Materials and Methods
Plant Materials and Growth Condition
Fertigation Treatment
Physicochemical Properties of the Medium
Measurement of Growth Characteristics
Statistical Analysis
Results and Discussion
Physicochemical Properties of the Growing Medium
Root Morphology
Changes in Shoot Growth
Shoot Growth Characteristics
Conclusions
Introduction
Plug seedling nurseries in the Republic of Korea have increased in number since the early 1990s because plug seedlings have advantages, such as efficiency and automation, when producing seedlings. Many Korean seedling nurseries use grafted seedlings for production of fruit and vegetable crops, such as watermelon (Citrullus lanatus), cucumber (Cucumis sativus), tomato (Solanum lycopersicum), pepper (Capsicum annuum), and eggplant (Solanum melongena) (Lee, 2007).
Grafting has many advantages, such as yield increases, improvements in nutrition and water uptake, and resistance to soil-borne diseases and damage caused by biotic or abiotic stressors (Ruinz et al., 1997; Lee et al., 2010; Li et al., 2013; Ribeiro et al., 2016). There are many grafting methods, such as hole-insertion grafting (HIG), tongue-approach grafting (TAG), splice grafting (SG), one-cotyledon splice grafting (OC-SG), root-pruning one-cotyledon splice grafting (RPOC-SG), and cleft grafting (CG). The grafting method depends on the crop, culture type, and grower preference (Lee, 2007). RPOC-SG is used on cucumbers and watermelons, wherein root development is an important factor for seedling quality. There are many studies that detail the ideal environmental conditions for graft healing (Sainju et al., 2000; Vu et al., 2012; Vu et al., 2014). However, there is little information on the root growth of root pruning grafted seedlings.
Optimum root development is necessary for proper shoot growth and crop yield (Leskovar and Stofella, 1995). The size, morphology, or architecture of a root system may control the relative size and growth rate of the shoot (Drew and Stolzy, 1991). Because acquiring strong roots is the main purpose of grafting, it is important to understand the root development of grafted seedlings. The physical properties of a growing medium effect root development. For example, total porosity determines the water storage and air space of a growing medium (De Boodt and Verdonck, 1971; Peterson et al., 1991). When the medium has a low container capacity, absorption of water and nutrients is disrupted, which stresses the root system (Aljibury and May, 1970; Martin et al., 1970). Bulk density represents the physical properties of a medium, especially compaction of the medium particles. When a medium has low bulk density, it may be insufficient to hold the plants. (Goodman and Ennos, 1999; Lee et al., 2019). Thus, the physical properties and nutrient-holding capacity of a growing medium are important for producing high-quality crops (Beardsell et al., 1979).
Different types of growing media are used for ornamental and horticultural plants. Many criteria of growing media are considered, such as nutrient-holding capacity, physical and chemical properties, cost, and grower preference. Furthermore, during plug seedling production, the convenience of transplanting is also considered when selecting a medium. Insufficient root development of seedlings causes soil-based medium to crack, making these types of media inconvenient for transplanting and growth. Moreover, particles and dust from soil-based media can hinder hydroponic production systems. Artificial media, such as rockwool (RW), and newly developed media, such as ‘forming media’ [LC grow foam (LC), RC grow foam (RC), and terra-plug (TP)], have uniform physicochemical properties, and offer many advantages during transplanting. Media with uniform physicochemical properties are easier to water and manage nutrients (Beardsell et al., 1979). Nevertheless, there is little information about shoot growth or root development of root-pruning grafted seedlings grown in artificial or forming media.
Information on the effect of the media and fertigation on root development of cucumber seedlings is lacking. The objective of this study was to examine the root development of cucumber seedlings produced by RPOC-SG in different growing media. To this end, we investigated the shoot growth and root morphology in different growing media and the effect of fertigation on plant growth.
Materials and Methods
Plant Materials and Growth Condition
In this experiment, cucumber (Cucumis sativus L. ‘Shindong’) and bottle gourd (Lagenaria siceraria Standl. ‘Shingiwon’) were used as the scion and rootstock, respectively. Cucumber and bottle gourd seeds were sown on the same day in plug trays filled with a commercial growing medium (Plantworld, Nongwoobio Co. Ltd., Suwon, Korea). Eight days after sowing, the cucumber seedlings were grafted onto the bottle gourd seedlings using the RPOC-SG method. After grafting, the grafted seedlings were transplanted into five different media: commercial growing medium (CGM), rockwool (RW, Grodan Co. Ltd., Roermond, The Netherlands), LC grow foam (LC, Smithers-Oasis Co. Ltd., OH, USA), RC grow foam (RC, Smithers-Oasis Co. Ltd., OH, USA), and terra-plugs (TP, Smithers-Oasis Co. Ltd., OH, USA). They were then transported to a healing chamber with a temperature of 24°C, a relative humidity of 95 to 100%, a photoperiod of 16/8 h (light/dark), and a light intensity of 40 ± 5 µmol·m-2·s-1 photosynthetic photon flux density (PPFD) using LEDs (red:blue = 30:70). Five days later, the well-healed grafted seedlings were move from the healing chamber to a greenhouse. During the experimental period, the minimum, maximum, and average temperatures of the greenhouse were 19, 32, and 24°C, respectively.
Fertigation Treatment
To investigate the effect of fertigation when rooting cucumber seedlings, a multi-purpose nutrient solution (pH 6.5 and electrical conductivity (EC) 1.0 dS·m-1) was used in this experiment (Table 1). Before transplanting the grafted seedlings, the five different media (CGM, RW, LC, RC, and TP) were soaked with the nutrient solution (fertigation treatment) or tap water (non-fertigation treatment). The nutrient solution and tap water were supplied after the healing process via sub-irrigation.
Table 1.
Composition of the nutrient solution used in the experiment
Physicochemical Properties of the Medium
To measure the physical properties of the different media, such as total container capacity, air space, total porosity, and bulk density, we measured the wet weight after soaking the medium for 48 h, and the growing medium weight and drained water volume were measured after draining for 2 h at room temperature. The dry weight was measured after the medium was allowed to completely dry for 72 h. The measured value was calculated using the formula given by Fonteno and Choi et al. (Fonteno, 1996; Choi et al., 1997).
Container capacity = [(wet weight-dry weight)/volume of sample] × 100
Air space = (volume of drained water/volume of sample) × 100
Total porosity = container capacity + air space
Bulk density = dry weight/volume of sample
To measure the chemical properties, each medium was mixed at a ratio of 1:5 (v/v) with distilled water and was shaken using a shaker (KS-500, Koencon Co. Ltd., Hanam, Korea) for 3 h. Then, the pH and EC were measured using a multiparameter (8200M, GOnDO Electronic Co., Ltd., Taipei, Taiwan).
Measurement of Growth Characteristics
After the healing process, growth parameters, such as the plant height, scion/rootstock stem diameters, scion/rootstock dry weight, and root morphological traits, were measured at 4-day intervals. Next, 25 days after grafting, the plant height, scion/rootstock stem diameters, leaf length, leaf width, leaf area, fresh weight (FW), and dry weight (DW) of the shoots and roots were measured. The plant height was measured from the soil line to apical meristem. The thicknesses of the scion and rootstock were measured at a distance of 0.5 cm from the grafted point using a digital vernier caliper (CD-20CPX, Mitutoyo, Kawasaki, Japan). Leaf area was measured using a leaf area meter (LI-3000, LI-COR Inc., Lincoln, NE, USA). FW was measured using an electronic balance (EW220-3NM, Kern and Sohn GmbH., Balingen, Germany) and DW was measured after drying in an oven (Venticell-220, MMM Medcenter Einrichtungen GmbH., Planegg, Germany) at 70°C for 72 h. Root morphology parameters, such as the total root length, average root diameter, root surface area, and root volume, were measured using the WinRhizo Pro 2007a image analysis system (Regent Instruments, Sainte-Foy, QC, Canada) coupled to a professional scanner (Expression 1000XL, Epson America Inc., Long Beach, CA, USA).
Statistical Analysis
The experiment was laid out in a completely randomized design with three replications per treatment, each of which consisted of 40 grafted plants per plug tray. All the assays were performed in triplicate, and the results were averaged. To analyze the physicochemical properties of the growing medium and root morphology, three replicates were used for each growing medium. The statistical analysis was carried out using the Statistical Analysis System program (SAS 9.1, SAS Institute Inc., Cary, NC, USA). The results were subjected to analysis of variance (ANOVA) and Tukey’s multiple range tests. Graphing was performed with the SigmaPlot program (SigmaPlot 12.0, Systat Software Inc., San Jose, CA, USA).
Results and Discussion
Physicochemical Properties of the Growing Medium
The physicochemical properties of the growing media are shown in Table 2. The container capacity was highest in RW, at 80.76%, and lowest in CGM and TP, at 56.10 and 52.36%, respectively. The container capacity indicates the water retention potential of the growing medium. If the growing medium has a high container capacity, it also has high water retentivity and buffering power, thus allowing for better water management (Kim et al., 2016). On the contrary, if the growing medium has low container capacity, it has a limited ability to retain water and nutrients (Aljibury and May, 1970; Martin et al., 1970). The air space indicates the amount of oxygen in the rhizospheric zone. In this study, the air spaces of media were not significantly different. The total porosity had a similar tendency as the container capacity. The bulk density was highest in TP at 0.17 g·cm-3. The bulk density is a major contributor of the physical properties of growing media, especially their physicochemical properties (De Boodt and Verdonck, 1972; Lee et al., 2019). Souch et al. (2004) reported that compaction can impact root thickness. In general, the optimum physical properties for plant growth are total porosity 85%, container capacity 55-75%, and air space 20-30% (De Boodt and Verdonck, 1972). However, these optimum physical properties do not consider seedling production. Seedling production requires only a small volume and is contained in individual cells, so the physical properties of growing media are different.
Table 2.
The physicochemical properties of the growing media used in the experiment
| Mediumz |
Container capacity (%) |
Air space (%) | Total porosity (%) | Bulk density (g·cm-3) | pH |
EC (dS·m-1) |
| CGM | 56.10 cy | 1.47 a | 57.57 c | 0.15 b | 7.12 a | 0.27 a |
| RW | 80.76 a | 2.62 a | 83.39 a | 0.08 c | 6.80 b | 0.10 b |
| LC | 70.90 b | 1.43 a | 72.32 b | 0.05 d | 7.13 a | 0.11 b |
| RC | 75.00 ab | 2.46 a | 77.47 ab | 0.05 d | 5.88 d | 0.04 b |
| TP | 52.36 c | 1.07 a | 53.43 c | 0.17 a | 6.30 c | 0.30 a |
The optimal range of pH values in growing media was suggested to be between 5.6 and 6.5 (Nelson, 1991). Among the media used in our experiment, RC and TP were in this range. The EC was the highest in TP and CGM at 0.30 and 0.27 dS‧m-1, respectively. This may be because TP and CGM are composed of organic materials, whereas the other media are composed of inorganic materials, so their EC values were lower.
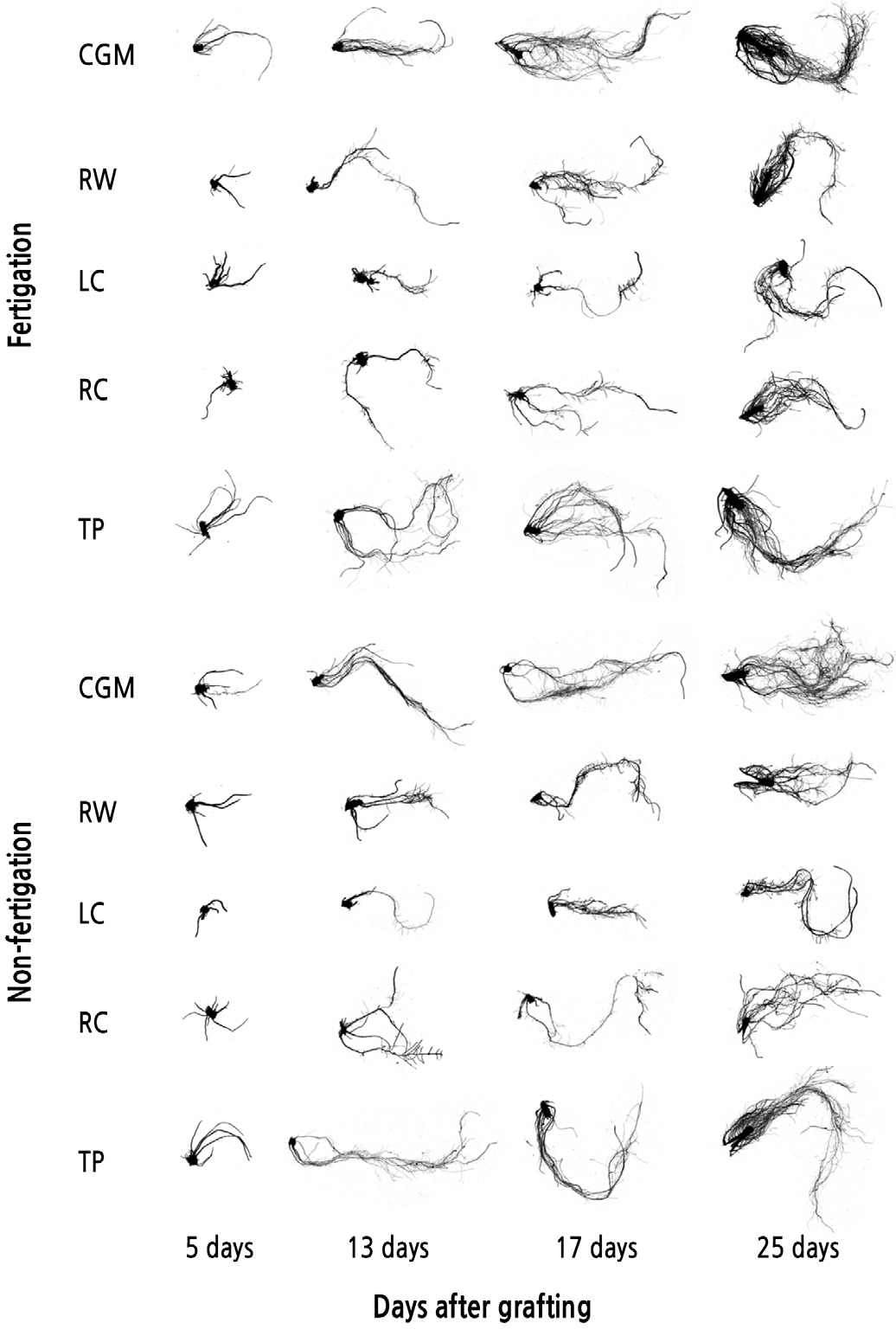
Root Morphology
The root morphology of cucumber seedlings was affected by the different media and fertigation, as shown in Figs. 1 and 2. The total root length was the longest in CGM and TP media. Except for in CGM, fertigation did not affect the total root length. The root volume was increased in the fertigation treatment but not the non-fertigation treatment. The root volume of the CGM was the highest at 0.55 cm3. The root volumes in inorganic media (RW, LC, and RC) in the fertigation treatment were 2.1, 1.5, and 2.0 times greater than in the non-fertigation treatments, respectively. However, seedlings grown in inorganic media had a lower root volume than those in CGM with the non-fertigation treatment. The root surface had the same tendency as the root volume. The root surface was the largest in CGM with fertigation at 40.1 cm2 and the lowest in LC with non-fertigation at 12.1 cm2. The average root diameter had the opposite tendency to other parameters, such as total root length, root volume, and root surface area. Except for in the CGM, fertigation increased the average root diameter. The root dry weight change was not significantly different in any medium and fertigation until 17 days after grafting. However, 25 days after grafting, seedlings in the fertigation treatments had a greater root dry weight than did those in non-fertigation treatments.

Fig. 2.
Changes in the total root length (A), root volume (B), root surface area (C), average root diameter (D), and root dry weight (E) of cucumber seedlings grown in five different media with or without fertigation. CGM, commercial growing medium; RW, rockwool; LC, LC grow foam; RC, RC grow foam; and TP, terra-plug. Vertical bars represent the standard deviation of the mean (n = 6). Different letters in the same column indicate significant differences based on Tukey’s multiple range test at p < 0.05 (n = 6). NS, **, *** Nonsignificant or significant at p < 0.01 or 0.001, respectively.
Well-developed root systems facilitate proper shoot growth and plant development. Root morphology is known to be influenced by the rhizosphere condition (Fageria and Moreira, 2011). Our results showed that root morphology parameters (i.e., total root length, root volume, root surface area, and average root diameter) were affected by the different mediums and fertigation. Seedlings are generally grown in small cells (Nelson, 1991) and are easily exposed to nutrient deficits. In this study, the fertigation treatment increased root development in all media. The average root diameter showed the opposite effect from other root morphology parameters. The seedlings in CGM and TP had lower average root diameters than did those in other media. A thin average root diameter indicates that the seedling has many fine roots and branched roots. Fine roots can easily penetrate into the soil, minimizing root system maintenance and construction costs (Forde and Lorenzo, 2001). In addition, many studies have shown that young roots exhibit the highest rates of nutrient uptake (Wells and Eissenstat, 2003). Therefore, seedlings in CGM and TP have greater advantages in terms of nutrient uptake and a more developed root system after transplanting.
Changes in Shoot Growth
The shoot growth of cucumber seedlings as affected by different media and fertigation is shown in Fig. 3. The change in shoot growth was not significantly different until 13 days after grafting. In the fertigation treatment at 25 days after grafting, plant height was highest in CGM at 15.86 cm, followed by TP (14.42 cm), LC (14.27 cm), RC (13.66 cm), and RW (11.87 cm). Compared with the non-fertigation treatment, fertigation increased the plant height by 116, 141, 170, 160, and 126% in each medium, respectively. The scion stem diameter had a similar tendency to the rootstock stem diameter. In the fertigation treatment at 25 days after grafting, the scion and rootstock stem diameters were thickest in CGM and TP; however, in non-fertigation treatments, except for CGM, there were no significant differences among the media. Shoot dry weight also increased with fertigation, especially for the CGM, LC, and TP media. Overall, fertigation increased the growth of cucumber seedlings. Previous studies reported that the nutrient solution supply increased the growth of cucumber seedlings (Hwang et al., 2006; Jang et al., 2019). Nutrients in growing media are essential for plant growth and development. Inorganic media, such as RW, LC, and RC, do not contain plant-available nutrients. Thus, shoot growth of cucumber was decreased in the non-fertigation treatments with inorganic media, and the fertigation treatment increased the growth in the inorganic media. Shoot growth did not have significant difference before 17 days. Seong et al. (2003) reported that root pruning grafted cucumber seedlings decreased plant growth 14 days after grafting, especially compared to the one-cotyledon splice and tongue approach grafting methods. In their study, seedlings resulting from the root pruning grafting method did not have sufficient root development compared with those from other non-root pruning grafting methods. In the present study, root growth was insufficient until 13 days after grafting, and nutrient and water uptake was defective in the cucumber seedlings. However, 25 days after grafting, plant growth was affected by the different media and the fertigation treatment. The change in root morphology at 17 days after grafting is considered to have affected shoot growth.
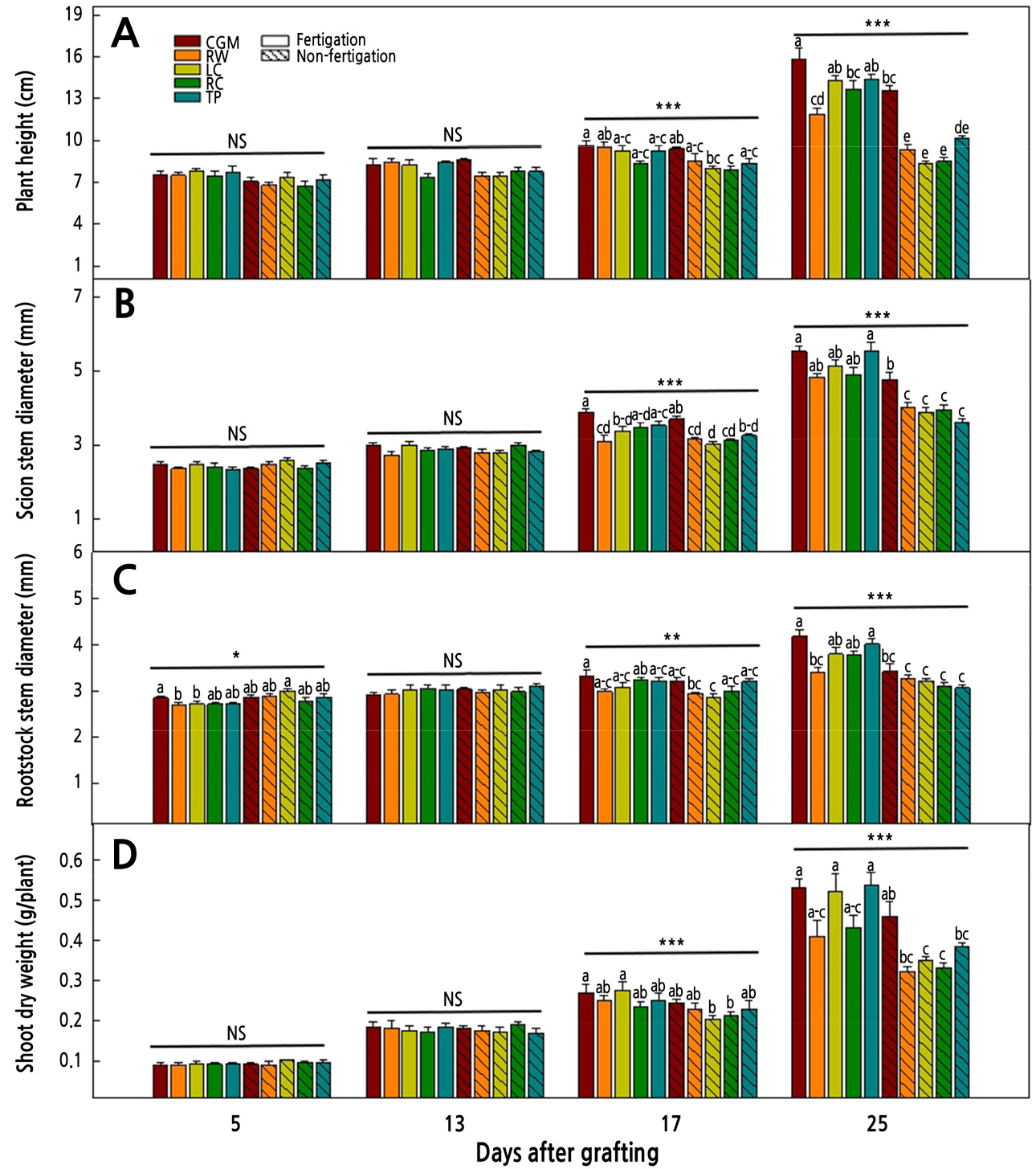
Fig. 3.
Changes in plant height (A), scion stem diameter (B), rootstock stem diameter (C), and shoot dry weight (D) of cucumber seedlings grown in five different media with or without fertigation. The vertical bars represent the standard deviation of the mean (n = 6). Different letters in the same column indicate significant differences based on Tukey’s multiple range test at p < 0.05 (n = 6). NS, *, **, *** Nonsignificant or significant at p < 0.05, 0.01, or 0.001, respectively.
Shoot Growth Characteristics
The growth of cucumber seedlings showed a similar tendency during root morphological development. CGM with the fertigation treatment increased seedling growth (Table 3 and Fig. 4). The plant height, leaf length, leaf width, and fresh weights of shoots and roots were highest in CGM with fertigation at 15.86 cm, 12.0 cm, 10.4 cm, 8.7 g, and 0.85 g, respectively. The stem diameters of the scion were highest in CGM and TP with fertigation treatments, at 5.53 mm, 5.52 mm, and stem diameters of rootstock were highest in CGM and TP with fertigation treatments, at 4.19 mm, and 4.02 mm, respectively. The CGM and TP medium had higher EC levels than did the inorganic media (RW, RC, and LC) (Table 1). Earlier studies reported that plants are impacted by eluted ions from the medium (Hamdreck, 1993; Ao et al., 2008; Choi et al., 2012). Moreover, root morphology affects cucumber seedling growth. Previous studies have reported that healthy, robust root systems can induce vigorous shoot growth (Nicola, 1998). In this study, seedling growth was increased in the CGM and TP medium because root growth was highest in these media. Traditionally, most Korean nurseries do not use nutrient solutions during seedling production. Seedling production takes place over too short a period and many nurseries use growing media that contains nutrients. However, in the present study, fertigation promoted seedling growth.
Table 3.
The growth of cucumber seedlings as affected by different media and fertigation 25 days after grafting
|
Irrigation (A) |
Mediumz (B) |
Plant height (cm) |
Stem diameter (mm) |
Leaf length (cm) |
Leaf width (cm) |
No. of leaves |
Leaf area (cm2/Plant) |
Fresh weight (g/plant) |
Dry weight (g/plant) | ||||
| Scion | Rootstock | Shoot | Root | Shoot | Root | ||||||||
| Fertigation | CGM | 15.86 ay | 5.53 a | 4.19 a | 12.0 a | 10.4 a | 4.9 ab | 206.7 a | 8.7 a | 0.85 a | 0.53 a | 0.056 a | |
| RW | 11.87 cd | 4.82 ab | 3.42 bc | 10.5 bc | 9.7 a | 4.8 ab | 132.8 cd | 6.0 d | 0.59 a | 0.41 a-c | 0.032 bc | ||
| RC | 13.66 bc | 4.89 ab | 3.79 ab | 11.2 ab | 10.2 a | 5.1 a | 162.6 bc | 7.2 bc | 0.71 a | 0.43 a-c | 0.037 a-c | ||
| LC | 14.27 ab | 5.14 ab | 3.81 ab | 10.9 ab | 10.0 a | 4.1 a | 162.8 bc | 7.4 b | 0.61 ab | 0.52 a | 0.037 a-c | ||
| TP | 14.42 ab | 5.52 a | 4.02 a | 10.9 ab | 9.7 a | 5.0 ab | 173.2 ab | 7.7 ab | 0.77 a | 0.54 a | 0.046 ab | ||
|
Non- fertigation | CGM | 13.58 bc | 4.76 b | 3.43 bc | 9.2 c | 8.0 b | 4.5 bc | 103.6 d | 6.1 cd | 0.72 a | 0.46 ab | 0.048 ab | |
| RW | 9.37 e | 3.59 c | 3.26 c | 5.1 d | 4.3 c | 4.1 cd | 29.9 e | 3.0 e | 0.37 b | 0.35 bc | 0.021 c | ||
| RC | 8.51 e | 3.88 c | 3.10 c | 5.2 d | 4.5 c | 3.7 d | 27.4 e | 2.9 e | 0.35 b | 0.32 c | 0.021 c | ||
| LC | 8.35 e | 3.95 c | 3.23 c | 4.7 d | 4.0 c | 3.7 d | 21.9 e | 2.7 e | 0.28 b | 0.33 c | 0.025 c | ||
| TP | 10.16 de | 4.00 c | 3.07 c | 5.7 d | 4.8 c | 3.9 d | 31.7 e | 3.1 e | 0.33 b | 0.39 bc | 0.037 a-c | ||
| Significantx | A | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| B | *** | *** | ** | *** | *** | * | *** | *** | *** | *** | *** | ||
| A × B | *** | ** | * | *** | *** | *** | * | *** | NS | NS | NS | ||
Conclusions
A well-developed root system induces vigorous shoot growth and plant development, and root growth is influenced by the rhizosphere condition. A robust root system increases seedling growth and quality. Moreover, seedlings with well-developed root systems will have greater survival after transplanting. In this study, we used different growing media on RPOC-SG cucumber seedlings, which differentially impacted root morphology and shoot growth. CGM with a fertigation treatment resulted in the greatest total root length, root volume, root surface area, and root dry weight, which in turn facilitated shoot growth. Among the forming media, TP increased root growth and seedling growth. Thus, a proper medium encourages a good root system for optimal seedling growth.