Introduction
Current Status of Grafted Vegetable Seedling Production and Use
Grafted Vegetable Seedling Production and Use
Challenges and Perspectives of Current Seedling Production Technology
Conclusions
Introduction
A seedling in vegetable crop production industry is defined as a young plant from a seed. The main purpose of commercial vegetable seedling production is to produce healthy seedlings year round for growers at low production costs by promoting seed germination and protecting young plants from diseases, pests, and extreme weather conditions (Kim and Lee, 2000).
Commercial vegetable seedling production emerged in the early 1990s in the Republic of Korea and has advanced with the development of the vegetable greenhouse industry. Vegetable greenhouse production technology has improved and the cultivation area has increased by 7.6 fold since the 1980s to adapt to the increasing year-round consumption of vegetables (Lee et al., 2013). Therefore, it became necessary for professional vegetable seedling producers to provide healthy seedlings year-round to vegetable growers, and it was important to consider a standard design of greenhouse structure and layout, seedling production processes, and facilities for commercial seedling production (Min and Park, 1993; Park and Oh, 1993; Park, 1994).
Since the establishment of the first commercial vegetable seedling production business, Associated Korea-America Seed and Seedling Co., LTD, in 1992, the commercial vegetable seedling industry in Korea has developed continuously in terms of market size. About 300 commercial seedling producers were operating 195 ha of seedling production area in 2013, and its market size was expected to reach approximately 242 billion won in Korean currency (Park et al., 2014). In addition to the market size, grafted vegetable seedling production has also increased dramatically in response to cropping production problems such as soil-borne diseases (Lee et al., 2013). The use of grafted seedling has advantages in adverse cultivation situations, such as resistance to soil-borne diseases, plant vigor improvement under abiotic stress and improvement of fruit yield and quality (Lee et al., 2010; Rouphael et al., 2010; Bie et al., 2017; Casals et al., 2018).
Grafting of vegetable seedlings has been practiced mainly in East Asia; about 60% of Japanese farmers used grafted seedlings of muskmelon, watermelon, cucumber, tomato, and eggplant in the 1990s, and 81% in Korea (Lee et al., 2010). At present, the use of grafted seedlings in vegetable crop production has become popular not only in Asian countries such as Korea (Lee et al., 2010; Rouphael et al., 2010; Bie et al., 2017), China (Huang et al., 2015), and Japan (Oda, 2007), but also in other regions (Lee et al., 2010; Bie et al., 2017) such as North America (Kuboda, 2008), Turkey (Yetisir, 2017; Seymen et al., 2021), and Israel (Koren and Edelstein, 2004) due to the prohibition of methyl bromide as a soil disinfectant (Ricárdez-Salinas et al., 2010). Grafted seedling production is labor intensive and costly (Leonardi and Romano, 2004; Rivard et al., 2010; Bie et al, 2017). In Korea, commercial grafting nurseries have improved the efficiency of producing high-quality grafted seedlings, and the technologies for the production of grafted seedlings have advanced rapidly over the last 30 years.
Here, we discuss how current grafted seedling practices are used in commercial grafting nurseries in the Republic of Korea. We also review the current status of grafted vegetable seedling practices based on previous research, and address the emerging challenges such as seedling quality improvement, cost savings, and mitigating environmental impacts at each stage in the grafted seedling production process.
Current Status of Grafted Vegetable Seedling Production and Use
Grafted Vegetable Seedling Production and Use
According to Jang (2013), Korean vegetable growers in 2011 used about 99% grafted seedlings in watermelon, 89% in cucumber, 98% in Korean melon, 69% in tomato, 41% in eggplant, 22% in pumpkin, 10% in pepper, and 9% in melon, and the main reason to use grafted seedlings was to enhance resistance to soil-borne diseases, especially Fusarium wilt and bacterial wilt for cucurbit and solanaceous crops, respectively.
In 2018, the annual amount of vegetable seedlings needed for the industry was analyzed by considering planting density and production area of vegetable crops, based on the principal horticultural and herbal crop production calendar (Kim, 2019) and the status of vegetable greenhouse facilities and production (MAFRA, 2019). The amount of grafted and non-grafted seedlings needed was calculated based on the percentage of grafted seedlings used by vegetable growers (Jang, 2013). Considering the amount of vegetable cultivation area in 2018, the maximum requirement of vegetable seedlings was estimated to be about 1,675 million for 71,718 ha of production area. Grafted seedlings accounted for 583.5 million, which was about 35% of the total vegetable seedlings needed (Table 1). Tomato, watermelon, cucumber, and pepper were the major crops to use grafted seedlings and represented 82% of the total amount of grafted seedlings, at 29, 19, 18, and 17%, respectively.
Table 1.
Production area, number, and proportion of grafted vegetable seedlings grown in the Republic of Korea, 2018
| Crop |
Cultivation area (ha) |
Number of seedlings required (thousand) |
Maximum number of seedlings (million) |
Maximum number of grafted seedlings (million) |
Proportion of grafted seedling used (%)z |
| Watermelon | 11,814 | 7.4-9.5 | 112.2 | 111.1 | 99 |
| Korean melon | 3,614 | 15.9-18.5 | 66.9 | 65.6 | 98 |
| Cucumber | 5,326 | 13.9-22.2 | 118.2 | 105.2 | 89 |
| Tomato | 6,058 | 25-40 | 242.3 | 167.2 | 69 |
| Eggplant | 616 | 25 | 15.4 | 6.3 | 41 |
| Pumpkin | 9,206 | 11.1-14.8 | 136.2 | 30.0 | 22 |
| Pepper | 33,630 | 25-28.6 | 961.8 | 96.2 | 10 |
| Melon | 1,454 | 11.4-15.6 | 22.7 | 2.0 | 9 |
| Total | 71,718 | 1,675.8 | 583.5 |
zProportion of grafted seedlings used was followed by Jang (2013).
Compared to the maximum number of grafted seedlings needed, 766.3 million, in 2005 (Lee at al., 2010), the estimated total amount of grafted vegetable seedlings decreased because of a reduction in the vegetable production area. However, the use of grafted seedlings dramatically increased from 2005 (Lee et al., 2010) to 2013 (Jang, 2013), from 95 to 99% for watermelon, 75 to 89% for cucumber, and 25 to 69% for tomato. The increase in the use of grafted seedlings is because grafted seedlings provide an alternative to overcome the continuous cropping obstacle in a greenhouse (Jang et al., 2009). As Korean vegetable production systems have become more specialized and skilled labor is lacking in rural areas, vegetable growers have become more dependent on commercial grafting nurseries (Park et al., 2011; Park et al., 2014).
Challenges and Perspectives of Current Seedling Production Technology
Despite the advantages of grafted seedlings in vegetable production, grafted seedling production is more complicated than non-grafted seedling production in that grafting and acclimation are required (Rouphael et al., 2010). To produce grafted vegetable seedlings, various technologies are required such as the grafting method and graft healing with special facilities like a graft healing bed or chamber (Lee et al., 2010; Bie et al., 2017). Also, skilled technicians and environmental control chambers for grafting and healing, respectively, are essential to produce high quality grafted seedlings.
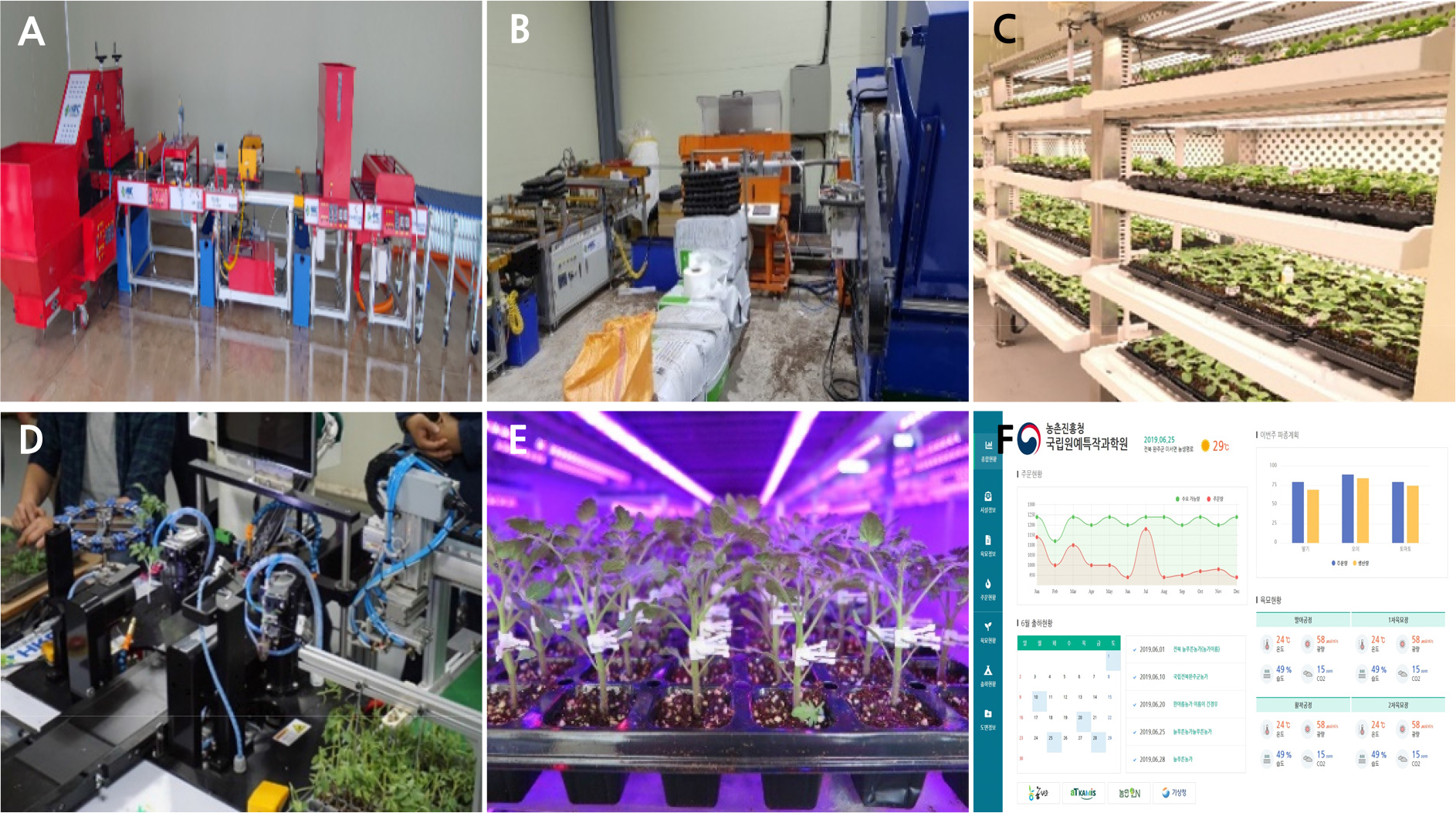
Therefore, tremendous efforts by researchers and professional grafted seedling growers have contributed to the establishment of production practices in Korea by developing technologies such as the use of rootstock cultivars, plug trays, mixing growing media, seed sowing machines, grafting methods, light-emitting diode (LED) graft healing chambers (LHCs), and seedling growth control (Lee, 2007; Lee et al., 2013). Recently, the development of a fully automatic seedling production system, a plant factory with artificial lighting and an image-based grafting robot have shown great progress (Fig. 1).
Seed Sowing Rate
The seed sowing rate is very important for commercial grafted seedling production in that the sowing rate is directly related to productivity and profit. Seed cost makes up the highest proportion (30.1%) of the operating cost for commercial grafting nurseries in Korea (Park et al., 2011). In commercial vegetable nurseries, the seed sowing rate was excessive compared to the number of seedlings ordered from vegetable crop growers. According to An et al. (2017) and RDA (2019), there were different seed sowing rates between non-grafted and grafted seedling production. The seed sowing rate of grafted seedlings (125.6%) was about 12% higher than that of the non-grafted seedlings (113.6%) in Korea.
The excessive sowing rate generally accounted for the loss of seedlings during germination, grafting work and seedling production management due to the failure of disease, pest, and growth control, and also to provide 3 to 5 % of spare seedlings for vegetable growers (Park et al., 2014). The higher seed sowing rate for the production of grafted seedlings is due to the failure rate of grafting-take and non-uniformity of seedling growth in plug trays. Kim and Hwang (2015) reported that the success rate of grafting and the survival rate after grafting by grafting experts were 100 and 99.7%, respectively. In commercial grafting nurseries, the loss of seedlings during grafting work is minimal. The main cause for the excessive sowing rate in commercial grafting nurseries is careless management during seedling production. Unfavorable and non-uniform environmental conditions (i.e., temperature, light, irrigation etc.) could result in non-uniform seedlings that are at different growth stages, and the seedlings with retarded growth will be removed from a plug tray (Fig. 2).
To reduce the loss of seedlings during seedling production, the development of facilities and techniques to precisely and uniformly control environmental conditions in a greenhouse is required. Additionally, the application of a plant factory with artificial lighting can reduce the loss of seedlings.
Cultivars of Rootstocks for Grafted Vegetable Seedlings
Rootstock selection is one of the most important factors for successful grafted seedling production by considering scion and rootstock compatibility and the performance of grafted seedlings after establishment in soils with disease and adverse environmental conditions (Lee et al., 2010; Bie et al., 2017). More than 500 rootstock cultivars for vegetable grafted seedlings are registered in Korea (KSVS, 2020). It was reported that commercial grafting nurseries use 342 scion cultivars and 85 rootstocks for tomato and 230 scion cultivars and 51 rootstocks for cucumber in Japan (Zhao and Kubota, 2015).
However, according to RDA (2019) one or two major cultivars were mostly used for grafted tomato, pepper, cucumber, and watermelon seedlings in Korea (Table 2). Also, most of the rootstock cultivars of solanaceous crops were the same species as their scion and mainly used for resistance to soil-borne diseases, whereas the rootstocks used in cucurbit crops were different species from the scion and showed both soil borne disease resistance and environmental stress tolerance. Most of the rootstock cultivars of tomato were Solanum lycopersicum L. and showed resistance to bacterial wilt. The most used rootstock cultivars of tomato were ‘B-Blocking’ (30%) and ‘Connection’ (10%). For pepper, ‘Tantan’ (33.3%) and ‘Anseongmatchum’ (22.1%) were the major rootstock cultivars belonging to Capsicum annum L. and showing resistance to Phytophthora blight. Major rootstock cultivars of cucumber were ‘Heukjong’ (53.8%), Cucurbita ficifolia Bouché, and ‘Sintojwa’ (23.1%), C. maxima Duch. × C. mochata Duch., showing resistance to Fusarium wilt and cold. Similar to cucumber rootstocks, the major rootstock cultivars of watermelon, ‘Bullojangsaeng’ (38.5%) and ‘RSdongjanggun’ (15.4%) of Lagenaria siceraria L., were resistant to Fusarium wilt and cold. Although various rootstock cultivars are available, continuous breeding efforts are necessary to develop new rootstock cultivars, because many existing rootstock cultivars provide weak protection against current soil borne diseases, especially resistance of bottle gourd rootstock to Fusarium wilt (Kim et al., 2020).
Table 2.
Major rootstocks of grafted seedlings in tomato, pepper, cucumber, and watermelon in the Republic of Korea, 2018
Grafting Method
Grafting methods are generally determined by considering the species of scions and rootstocks, the success rate of grafting take and the efficiency of the grafting work (Lee et al., 2010; Bie et al., 2017). Since the introduction of modernized vegetable seedling production systems in the early 1990s, various grafting methods such as hole insertion grafting, tongue approach grafting, splice grafting, pin grafting, and cleft grafting have been applied (Rivard et al., 2010).
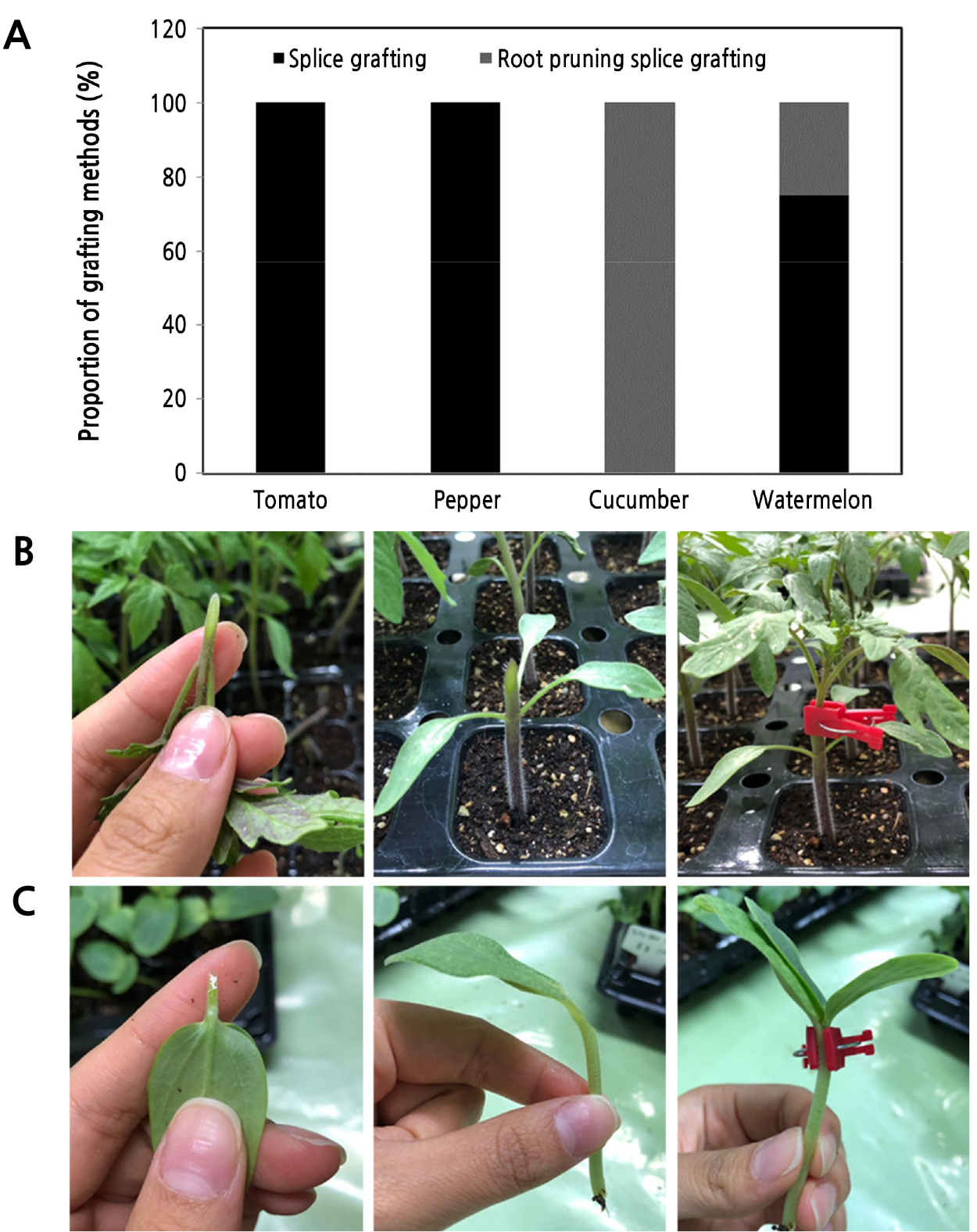
Although there are many grafting methods available (Leonardi and romano, 2004; Lee et al., 2010), the grafting method used currently in Korea for tomato, pepper, cucumber, and watermelon grafted seedling production is mostly the splice grafting method (Fig. 3) (RDA, 2019). With respect to solanaceous vegetables, the splice grafting method without root pruning of rootstock seedlings was used for all grafted seedlings of tomato and pepper. However, in cucumber and watermelon, the root pruning splice grafting method, in which the root of the rootstock seedling is cut, was used in 100 and 25% of seedlings, respectively. According to Lee et al. (2010) and Bie et al. (2017), splice grafting is one of the most common grafting methods and can be applied to most vegetable seedlings by hand and with a grafting robot. Splice grafting can use a smaller size of scions and rootstocks than other grafting methods. Therefore, the days from sowing to grafting can be reduced and more plants can be placed into the healing room after grafting (Oda, 2007).
Cell Size of Plug Tray
Selection of plug tray cell size is highly dependent on the crop species, grafting method, and seedling production period (Yu et al., 2002; Yaping and Diankui, 2005; Babaj et al., 2012; Kim et al., 2019). Also, commercial nurseries tend to use plug trays with more cells and smaller cell size to increase the number of plants produced, to decrease the space for seedling production and to reduce propagation costs per plant (Dufault and Waters, 1985; Oh et al., 2014). The physical volume of the plug tray is relatively small and it directly affects seedling growth. Using plug trays with more cells would be economical to produce more seedlings, however, the root growth of seedlings would be restricted by the smaller size of cells.
Plug trays used for grafted seedling production showed a diverse range of cells per tray from 32 to 200 cells depending on the vegetable crop species, seedling growth stage, and grafting practices (Table 3) (An et al., 2017; RDA, 2019). In general, scion seedlings used smaller sized cell trays from 72 to 200 cells than the rootstock seedlings (from 32 to 162 cells) and the grafted seedlings (from 32 to 72 cells). For grafted tomato seedling production, scions, rootstocks, and grafted seedlings were mostly grown in trays containing 128 cells (45.5%), 40 cells (63.6%) and 40 cells (100%), respectively. Pepper scions, rootstocks and grafted seedlings used trays containing 128 cells (50%), 50 cells (50%) and 50 cells (50%), respectively. The scions, rootstocks and grafted seedlings of cucumber were grown in trays containing 162 cells (100%), 128 cells (60%) and 40 cells (100%), respectively. Watermelon scion, rootstock and grafted seedlings were grown in trays containing 162 cells (60%), 40 cells (50%) and 40 cells (75%), respectively. As the roots of scions are cut before grafting, plug trays with 105-200 cells have been used for scion production. In rootstock production, the cell size of plug trays is affected by root cutting before grafting and temporary planting after grafting. Cucumber seedlings were grafted by the one cotyledon splice method after root cutting, therefore, cucumber rootstocks are produced in plug trays with 105-162 cells. In tomato, the cell size of the plug tray used for rootstock production was determined considering whether to add the process of temporary planting after grafting.
Table 3.
Proportion of plug tray size of scion, rootstock, and grafted seedlings in tomato, pepper, cucumber and watermelon in the Republic of Korea, 2018
| Crop | Proportion of plug tray use (%)z | |||||||
| 32 cells | 40 cells | 50 cells | 72 cells | 105 cells | 128 cells | 162 cells | 200 cells | |
| Tomato | ||||||||
| Scion | 0 | 0 | 0 | 9.1 | 36.4 | 45.5 | 9.1 | 0 |
| Rootstock | 27.3 | 63.6 | 0 | 0 | 0 | 36 | 0 | 0 |
| After grafted | 27.3 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pepper | ||||||||
| Scion | 0 | 0 | 0 | 0 | 25 | 50 | 0 | 25 |
| Rootstock | 0 | 0 | 50 | 50 | 0 | 0 | 0 | 0 |
| After grafted | 0 | 0 | 50 | 50 | 0 | 0 | 0 | 0 |
| Cucumber | ||||||||
| Scion | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 |
| Rootstock | 0 | 0 | 0 | 0 | 20 | 60 | 20 | 0 |
| After grafted | 60 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Watermelon | ||||||||
| Scion | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 40 |
| Rootstock | 25 | 50 | 0 | 0 | 0 | 25 | 0 | 0 |
| After grafted | 25 | 75 | 0 | 0 | 0 | 0 | 0 | 0 |
LED Healing Chamber (LHC)
Although the grafting union and acclimatization process has been traditionally accomplished under a tunnel type bed by using polyethylene and light-shading film (Fig. 4), the commercial grafted seedling growers who have installing and use LHCs have increased since the first LHC was applied in 2012 (An et al., 2016; IPET, 2016). Park et al. (2020) reported that a LHC was used by about 20% of commercial grafted nurseries in 2018.
In Korea, the LHC was developed in response to grafted seedling grower’s needs. Splice grafting is an effective grafting method for most vegetables and the procedure for splice grafting is easier and more convenient than other grafting methods. However, after grafting the plants have to be managed carefully since scions easily fall off at an early stage and it requires a longer period for healing and rooting as compared to other grafting methods (Lee et al., 1998; Davis et al., 2008). To address these disadvantages, LHCs which can control the environmental conditions have been used widely in commercial grafting nurseries.
In the comparison between a tunnel type healing bed and a LHC for grafted tomato seedlings, the success rate of graft-take was greater and the period of graft union formation and acclimatization was shorter in a LHC than in the tunnel type healing bed. According to IPET (2016), the graft-take success rates in a tunnel type healing bed and in a LHC were about 80 and 93.6%, respectively. Also, LHCs required less days for graft union formation and acclimatization (5.3 days) than the tunnel type (7.1 days).
Many studies on healing chambers with artificial lighting have been conducted to optimize the temperature, humidity and light quality and intensity to improve the success rate of graft healing and acclimatization (Kim and Park, 2001; Jang et al., 2009; Jang et al., 2011; Kang et al., 2013; Kang et al., 2015; Kim et al., 2018). Commercial nurseries have maintained LHCs at air temperatures from 24 to 26 °C and under 20.6 ± 5.2 µmol·m-2·s-1 of photosynthetic photon flux (PPF) with LED (Red : Blue = 2 : 1) lighting. Although commercial grafting nurseries have used LHCs with high satisfaction compared to the traditional tunnel type healing bed; the light intensity, about 20 µmol·m-2·s-1 of PPF, was much lower than the results of previous LHC studies. Kang et al. (2015) recommended environmental conditions in an artificial light healing room as follows; increase of light intensity from 0 to 90 µmol·m-2·s-1 of PPF, and decrease of relative humidity from 95 to 85% during 7 days of watermelon graft healing and acclimatization. Jang et al. (2011) set three PPF treatments (0, 142, and 237 µmol·m-2·s-1) and found that an increased PPF during healing and acclimatization positively affected the growth and quality of grafted cucumber seedlings. The light condition initially used in LHCs has been fixed due to several reasons (electricity bill, cost of LEDs, manufacturer etc.) and has been applied until now without improvement; therefore, the light conditions in LHCs should be improved for promoting graft healing, growth and quality of grafted seedlings.
Seedling Size and Scheduling
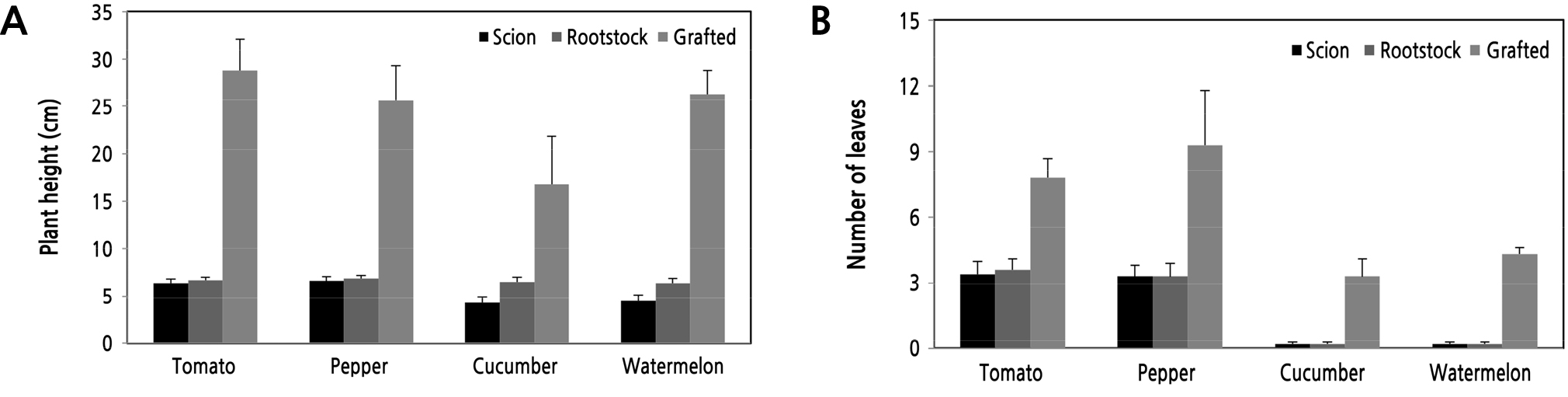
Depending on vegetable crop growth characteristics, there were diverse sizes of scions and rootstocks when grafting and also of their grafted seedlings when transporting (Fig. 5) (An et al., 2017; RDA, 2019). Overall scions of tomato, pepper, cucumber, and watermelon were smaller in plant height and leaf number than their rootstocks. For tomato and pepper, plant heights and leaf numbers of scions and rootstocks were similar to each other from 6.4 cm to 6.9 cm and from 3.3 leaves to 3.6 leaves. However, plant heights of cucumber and watermelon scions were smaller than the rootstocks. Although the plant heights of grafted tomato seedlings (28.8 cm) were higher than for pepper (25.6 cm), grafted pepper seedlings (9.3 leaves) showed a higher number of leaves than tomato (7.8 leaves). Grafted seedlings of cucumber and watermelon showed plant heights of 16.8 and 26.3 cm, respectively, and leaf numbers of 3.3 and 4.3 leaves, respectively. The periods of grafted seedling production varied depending on the species, seedling growth stage and season (Table 4). The periods of grafted seedling production were 43-56 days for tomato, 49-73 days for pepper, 23-32 days for cucumber and 37-43 days for watermelon, depending on the season.
Table 4.
Days of the seedling production process of scion and rootstock in tomato, pepper, cucumber, and watermelon in the Republic of Korea, 2018
| Crop | Days of grafted seedling production process | ||||
| Germination | Germination to grafting | Graft healing | Graft healing to shipment | Total | |
| Tomato Scion Rootstock | 2.7 ± 0.5z 2.7 ± 0.5 | 19.5 ± 4.5y (23.8 ± 5.0)x 20.2 ± 3.7 (26.4 ± 1.8) | 5.3 ± 0.5 | 16.3 ± 2.2>y (24.2 ±5.0)x | 43.8 ± 6.1y (56.0 ± 4.5)x |
| Pepper Scion Rootstock | 3.7 ± 0.6 3.7 ± 0.6 | 22.3 ± 2.5 (34.3 ± 0.6) 23.7 ± 3.2 (36.7 ± 2.1) | 5.7 ± 0.6 | 17.7 ± 2.5 (29.7 ± 0.6) | 49.3 ± 3.8 (73.3 ± 0.6) |
| Cucumber Scion Rootstock | 1.3 ± 0.6 1.7 ± 0.6 | 7.7 ± 2.1 (13.3 ± 0.6) 8.3 ± 1.2 (13.7 ± 0.6) | 5.7 ± 0.6 | 8.7 ± 1.5 (12.3 ± 2.1) | 23.3 ± 1.5 (32.7 ± 0.6) |
| Watermelon Scion Rootstock | 2.3 ± 0.5 3.3 ± 0.5 | 10.5 ± 1.7 (11.8 ± 2.4) 9.8 ± 2.2 (10.8 ± 2.6) | 5.5 ± 0.6 | 19.5 ± 0.6 (23.8 ± 1.5) | 37.8 ± 1.5 (43.5 ± 2.1) |
Production of scions and rootstocks with a standardized size is important for use of automation in the grafting process. The lack of uniformity among seedlings causes an increased failure rate of grafting robots (Kubota et al., 2008). The size of grafted seedlings can affect the cost of transportation and have a potential effect on the quality after transportation (Cantliffe, 1993). Additionally, scheduling of the grafted seedling production process is very critical to produce high quality seedlings with proper and uniform size for grafting and for transportation of grafted seedlings (Lee et al., 2010; Bie et al., 2017). Although there were certain sizes of seedlings for scions, rootstocks, and grafted seedlings, the production period at each stage was different depending on the seedling growth stage and season. Korea has four distinct seasons and it is difficult to cultivate uniform seedlings and fix production scheduling regardless of season. In the hot summer months, the growth rate of seedlings increases due to high temperature and irradiation, however, the growth rate in the cold winter is delayed by low temperature and irradiation. Therefore, there is a large range in the period required for seedling production between summer and winter seasons. To produce uniform seedlings and fix production scheduling throughout the year, facilities and cultivation systems that can control the environmental conditions for seedling production regardless of the season must be developed.
A plant factory with artificial lighting (PFAL) is one solution for the uniform and stable production of high quality seedlings. Studies on the production of scions and rootstocks in a PFAL have been conducted since 2000 in Korea, and the appropriate environmental conditions (light, temperature) and irrigation were studied for scions and rootstocks produced in a PFAL (Kwack et al, 2014; An et al., 2020; Hwang et al., 2020). Recently, PFAL facilities and technologies adequate for the demand of commercial grafting nurseries were developed and the field test has been conducted in a few commercial grafting nurseries.
Fertigation and Wastewater Management
Nutrient and water management is important for producing high quality seedlings. Seedlings produced in plug trays have limited root volume, therefore, fertilization and irrigation must be conducted more precisely. According to RDA (2019), 76.9% of grafted seedling growers followed fertigation management considering the seedling growth stage with a standard fertigation plan, and wastewater was drained out without any physical or chemical filtration process in 100% of grafted seedling growers (Table 5). Although approximately 70% of commercial seedling growers controlled fertilization based on plant growth stage, most seedling growers conducted fertigation based on their experience without consideration of the scion and rootstock cultivars, container size, medium, and growth stage before/after grafting. While to improve grafted seedling productivity, it is also important to consider the environmental impacts of fertilizer and irrigation (McLaughlin et al., 1996; Khalid et al., 2018). There is limited data on nutrient load in the environment from seedling production, however, increasing the number and area of commercial nurseries since 1990 in Korea has undoubtedly increased the amount of leachate and nutrient discharge. Therefore, precise management practices for fertigation in seedling production considering not only seedling growth but also the environmental impact must be investigated and leachate recycling systems should be applied to the field of seedling production.
Table 5.
Seedling production practices of fertigation and wastewater management in the Republic of Korea, 2018
Conclusions
Grafted seedlings production methods are completed in a short production period associated with high complexity, and the process must be precisely managed to optimize productivity and minimize environmental impacts regardless of weather conditions. Today, commercial grafting nurseries in Korea are struggling to produce high quality seedlings, due to the difficulties caused by climate change, increasing production cost and lack of labor. From the previous studies, we found several key ways to enhance the production of high quality grafted seedlings with lower production costs and less environmental impacts. The development of new rootstock cultivars, facilities, and cultivation systems, the application of plant factories, the improvement of light condition in LHCs, and environmentally friendly wastewater management are important factors to consider in order to manage and improve the seedling quality and the efficiency of seedling production. The Korean government, researchers, and commercial grafting nurseries will need to work together to develop effective technologies for sustainable vegetable seedling production in Korea.