Introduction
Materials and Methods
Experimental conditions and management of seedlings
Calibration and working procedure of the load cells
Preparation of the treatment solutions
Experimental methods and analysis procedures
Results and Discussion
Conclusion
Introduction
In modern agriculture, the pursuit of sustainable and efficient cultivation practices has led researchers to explore innovative techniques that optimize plant growth and yield. Among these techniques, controlled irrigation stands as a pivotal factor in enhancing crop productivity and water resource management (Marchin et al. 2020). As water scarcity becomes an increasingly pressing concern, it becomes imperative to investigate irrigation strategies that not only ensure optimal plant development but also minimize water wastage (Kojima et al. 2016; Bogena et al. 2017). In this context, the manipulation of irrigation frequencies using load cell technology has emerged as a promising avenue, particularly in the context of high-value horticultural crops such as strawberries (Meena et al. 2015). This study delves into the effects of controlled irrigation frequencies, monitored through load cell technology, on the growth and premature deterioration of shoot apical meristems in different strawberry varieties, offering insights into the potential for improved cultivation practices in the face of changing agricultural landscapes.
Water scarcity, driven by climate change and escalating population demands, has prompted a re-evaluation of traditional irrigation methods. Over-reliance on conventional irrigation practices has led to inefficient water utilization, reduced crop yields, and environmental degradation (Masseroni et al. 2020). Controlled irrigation strategies, rooted in precision agriculture, provide a means to address these challenges by delivering water directly to plants when and where they need it the most (Yu et al. 2021). Load cell controlled irrigation systems offer precise water management benefits but come with notable drawbacks. The initial cost can be prohibitive for smaller farms due to expenses for load cells, sensors, and automation. Regular maintenance is essential to ensure accuracy, requiring calibration and software updates, adding to operational complexity. Setup and calibration are intricate, demanding expertise in both agriculture and technology, potentially discouraging adoption. Dependency on electricity poses reliability concerns in areas prone to power outages. Sensor accuracy may fluctuate due to environmental factors, necessitating adjustments. Despite these challenges, such systems enhance water efficiency and plant growth, making them advantageous for farms with resources to invest and maintain them effectively. Load cell controlled irrigation systems offer several benefits. They measure the weight of seedling pots or trays, providing real-time feedback on soil moisture levels for precise irrigation control. This accuracy helps in conserving water by preventing both under- and over-irrigation, crucial for sustainable agriculture. Improved irrigation management fosters healthier root development and overall plant growth, vital during the seedling stage. Integration with automated systems reduces manual labor, cutting operational costs (De-Pascale et al. 2019). Moreover, load cell technology, capable of measuring subtle changes in plant weight, offers a dynamic approach to irrigation management. By continuously monitoring plant weight, the load cell can provide real-time data on water uptake and loss, enabling growers to tailor irrigation frequencies to match plant requirements precisely (McCauley et al. 2021).
In the intricate hierarchy of plant growth and development, the apical meristem holds a pivotal role. The apical meristem is responsible for initiating the growth of new tissues. It is the source of primary meristems that give rise to various plant organs, and thus, its growth and shedding significantly influence overall plant morphology and productivity. The regulation of apical meristem growth is intricate, influenced by a multitude of internal and external factors, including hormonal signals, environmental conditions, nutrient availability, and water deficiency (Philipson 1990; Kwiatkowska 2008). Irrigation is a fundamental component for strawberry production, having a pivotal role in providing plants with the necessary water for growth and sustenance (Cahn and Johnson 2017; Gavilán et al. 2021). However, the frequency and timing of irrigation can significantly influence plant physiology, and an improper irrigation regimen may lead to various controlled responses (Souza et al. 2020), including apical meristem abscission (Schussler and Westgate 1995; Andersen et al. 2002). This phenomenon, characterized by the premature termination of the apical meristem’s growth, can result in reduced crop yields, altered plant architecture, and diminished overall crop quality (Oury et al. 2016). To better understand and address the complex relationship between irrigation frequencies and apical meristem abortion, researchers have turned to innovative technologies, such as load cells. By integrating load cell technology into controlled irrigation systems, scientists aim to optimize irrigation management practices, ensuring that plants receive the right amount of water at the right time to mitigate shoot apical meristem deterioration.
Strawberries (Fragaria ananassa) have captured global attention due to their economic significance, nutritional value, and delicate flavor profile. Cultivating strawberries poses specific challenges due to their susceptibility to water controlled and sensitivity to environmental changes, diseases and pests (Dávalos-González et al. 2022). However, the availability of diverse strawberry varieties with varying attributes, such as yield, taste, and disease resistance, presents an opportunity for targeted cultivation practices. Through a comprehensive exploration of the current scientific literature and experimental data and analysis, this study seeks to shed light on the intricate relationship between the irrigation patterns and apical meristem health of strawberry plants. Ultimately, the findings of this investigation could hold significant implications for the field of horticulture, offering valuable insights into strategies to enhance strawberry yield, quality, and sustainability. Understanding how controlled irrigation frequencies affect the growth and abscission of apical meristems across different strawberry varieties can guide growers in selecting optimal irrigation strategies tailored to specific genotypes.
This study investigated the effects of substrate moisture content using load cell technology on the growth and premature deterioration of shoot apical meristems in various strawberry varieties. By examining how different irrigation frequencies influence on the shoot apical meristem dynamics, researchers can gain insights into the physiological responses of strawberry plants. The study also sought to identify potential correlations between irrigation regimes and varietal attributes, providing a foundation for precision irrigation practices that optimize yield and resource use efficiency.
Materials and Methods
Experimental conditions and management of seedlings
The study involving strawberry seedlings was conducted within a climate-controlled greenhouse situated at Chungnam National University (CNU) in South Korea, specifically at coordinates 36° 20' N, 127° 26' E. This investigation focused on assessing the subsequent occurrence and growth of three distinct strawberry cultivars during the spring season. For this experiment, three true leaf generated strawberry seedlings of ‘Altaking’, ‘Keumsil’, and ‘Vitaberry’ were transplanted into 32 cell plastic trays. The cells were filled using a commercially prepared root medium consisting of a blend of coco peat (52.5%), perlite (30%), peat moss (15%), and vermiculite (2.5%), followed by appropriate irrigation. The physical characteristics of the root medium were evaluated according to the methodology outlined in Choi et al. (1999), resulting in the following measurements: a total porosity of 83.9%, container capacity of 74.2%, and air-filled porosity of 9.7%. Seedlings prepared from cuttings were raised for the experiment in a separate greenhouse in different beds were irrigated properly before transplanting, and irrigation was done for 10 days after transplanting. The moisture content of the substrate was adjusted to 55, 48, 41, 38% using a load cell. The seedlings were then allowed to grow for 60 days.
After that, the treated seedlings were transferred into plastic containers, measuring 64.3 cm in length, 23.5 cm in width, and 17 cm in height, to house ten strawberry seedlings. These seedlings were transplanted in a zigzag pattern within the containers. The planting beds were established using the same root medium. After transplantation, the seedlings were irrigated twice daily. Nutrient solution application commenced seven days after transplantation and was administered three times a day (at 9 am, 1 pm, and 4 pm) via an automated irrigation system equipped with a timer, each session lasting for 3 minutes. Throughout the duration of the experiment, runners and auxiliary buds of the strawberry plants were manually restricted. On the other hand, the emergence of inflorescence was visually investigated. The internal greenhouse conditions were maintained at an average temperature of 24°C during the day and 14°C during the night.
Calibration and working procedure of the load cells
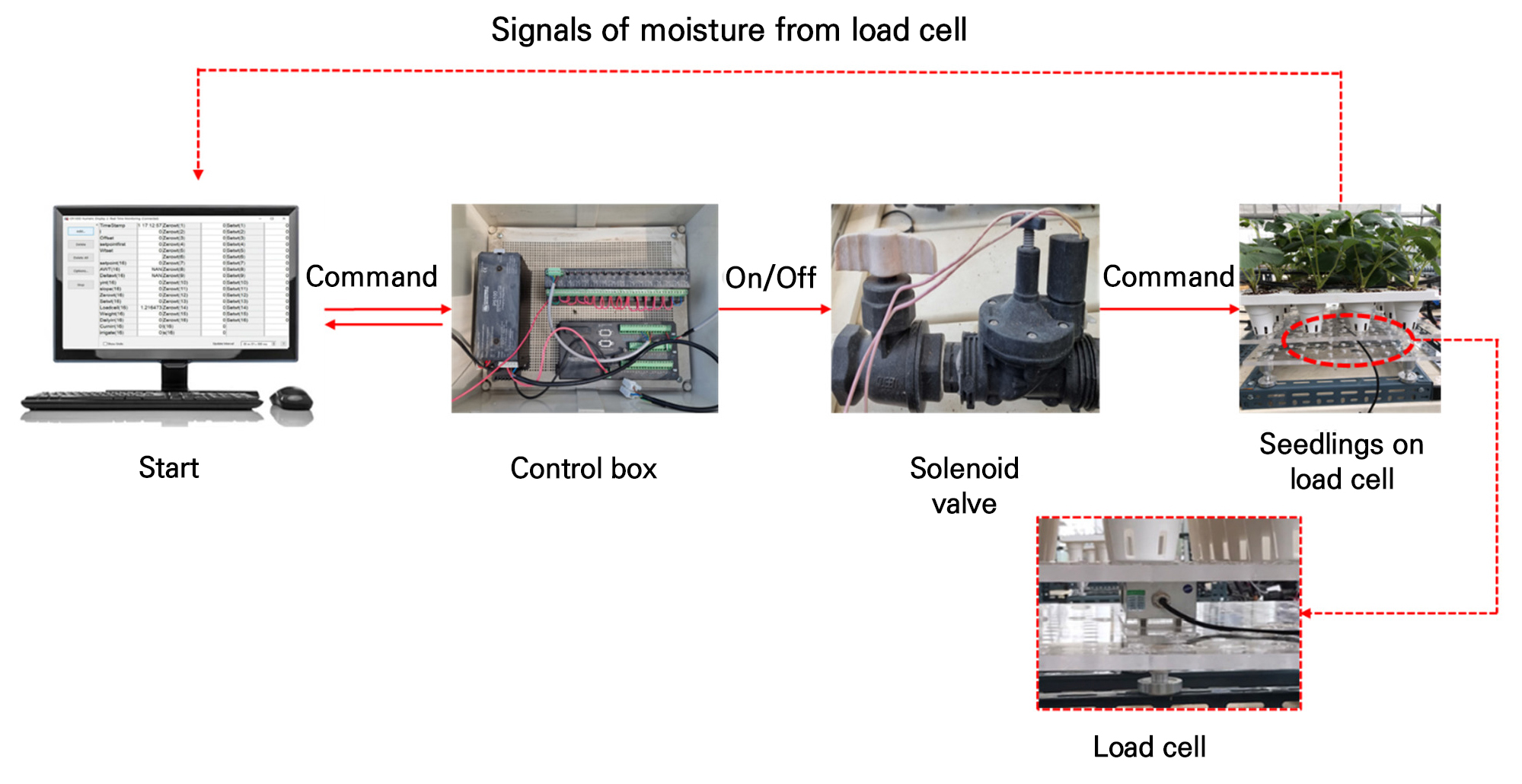
BCLS (60–150 kgf) single point load cells (CAS, Yangju, Korea) were used to perform the experiment. The load cells were calibrated with the help of computer software (Logger net). The wet setting was done by following different stage. First the weight of empty trays was measured, after which the trays were filled with rood media and the weight of trays with root media was measured again. The trays were soaked in water and kept in water for fully soaking. The trays were the taken out from the water and kept aside for 30 minutes in room temperature for the drainage of excess water from the root media. Then the weight was measured again. All the values were recorded and used to measure the substrate moisture percentages. The values were set in logger net software for wet setting and controlling the irrigation frequencies based on the presence of moisture in root media. Standardization of the software was done using a standard 10 kg weight, which was then calibrated to 0 kg by setpoint selection. Then, the seedlings trays were placed on the load cells and connected with the computer software. The irrigation frequencies were controlled by computer along with a control box, which helps to regulate the solenoid valve to fertigate the seedlings (Fig. 1). Automatic irrigation was done based on the wet setting of software, where irrigation was done immediately after the reduction of sated root media moisture.
Preparation of the treatment solutions
A combination of N-P2O5-K2O (18-18-18, provided by Haifa Chemicals Ltd., Matam-Haifa, Israel) was applied which was regulated using the load cells at the initial stage for 60 days and then via a drip irrigation system, occurring three times daily with each session lasting for 3 minutes for another 60 days. The pH levels of the solutions were adjusted to a range between 5.8 and 6.0 by incorporating hydrochloric acid (HCl) while maintaining an electrical conductivity (EC) of 0.6. Additionally, Proplex-Ca 20 (Plant Health Ltd., United Kingdom) was drenched once a week. It’s important to note that this experimental procedure was replicated three times for each treatment.
Experimental methods and analysis procedures
Measurement of plant growth
Sixty days after the transplantation of the plants, they were carefully uprooted, and the entire aboveground portion was harvested. To assess plant growth, a range of parameters was examined with 10 plants chosen randomly from each treatment group. The average value of these 10 selected plants within each treatment was regarded as the representative value for that specific nitrogen concentration.
Data were gathered on various aspects of plant growth, including plant height (in centimetres), leaf count, length and width of leaves (in centimetres), petiole length (in centimetres), crown diameter (in centimetres), and the fresh and dry weight (in grams) of the mother plants. Additionally, information was recorded on the number and length (in centimetres) of the runners and the fresh and dry weight (in grams) of both runners and daughter plants.
Observation of inflorescence
The flower bud differentiation stage was observed with a microscope (SZ2-ILST, Olympus Corporation, Japan) before transplanting. Seedlings were uprooted and stored in a refrigerator. Seedlings were dissected carefully and observed with a microscope to differentiate the stage of differentiation. The emergence of primary inflorescence, abscission of shoot apical meristems and the stoppage of secondary inflorescence was investigated visually while the plants were growing. Thirty plants from each treatment were selected and observed visually. The number of inflorescence emergence and abscission of apical meristems was counted. Then, the percentage of abscission was measured.
Nutrient contents assessment
The root media samples were collected and set aside for air drying. Those were utilized to create a saturated paste by combining them with distilled water at a ratio of 1:10 (root media to distilled water, grams per gram). This paste was left at room temperature for two hours, and 1–2 drops of a soil wetting agent were introduced to establish equilibrium. To obtain a clear solution from the mixture of the root media and distilled water, the concoction was filtered through three layers of white gauze. This solution was used for assessing the pH and electrical conductivity (EC), using a pH/EC meter (Multi meter CP-500L, Istek Co., Seoul, Korea). One drop of preservative was added, and the solution was preserved in a refrigerator for the purpose of analysing the ion concentrations within the root media, utilizing ion-chromatography (883 Basic IC plus, Metrohm, Switzerland). All these procedures were conducted with three separate replications for accuracy.
To analyze the tissue contents, fully expanded new leaves were collected from each treatment and carefully washed with detergent followed by a thorough rinsing with distilled water. The washed leaves were dried in an oven at 75°C for 48 hours. Ensuring they were completely dry, the leaves were finely ground using a pestle and mortar, and the resulting powder was passed through a 0.5 mm sieve for uniformity. 0.5 grams of leaf samples from each treatment were subjected to dry ash at 500°C for 6 hours. The ashed material was burned in a hooded chamber with the addition of 2 mL of 95% H2SO4. 50 mL of 0.5 N HCl were introduced to the darkened burnt leaf sample, and a clear liquid solution was obtained after filtration through Advantec No. 2 filter paper (Toyo Roshi Kaisha, Ltd., Japan). The concentrations of potassium (K), magnesium (Mg), calcium (Ca), iron (Fe), manganese (Mn), copper (Cu), and zinc (Zn) were determined using an Atomic Absorption Spectrophotometer (AA-7000, Shimadzu Co., Japan).
To determine the total nitrogen concentration within the plant tissue, the process was started by placing finely ground leaves (0.1 grams) into glass tubes. A mixture consisting of sulfuric acid and salicylic acid (5 mL) at a ratio of 20:1 (volume to weight) was added to these tubes. The tubes were left to settle at room temperature for approximately one hour before undergoing digestion in pre-heated digestion blocks at 270°C for 5 minutes. The hot tubes were cautiously removed from the blocks and allowed to cool. Kjeldahl digestion mixture (K2SO4, CuSeO3·2H2O, and pumice at a weight ratio of 970:19:11) was added into each tube (2 grams per tube). When the temperature of the digestion blocks reached 400°C, the sample tubes were returned for further digestion, lasting approximately 30 minutes. Properly digested samples, characterized by a light green color, were retrieved, and allowed to cool to room temperature. These samples were diluted with distilled water (20 mL). The analysis of total nitrogen content was performed following the method outlined by Eastin (1978) using a Kjeldahl Digestion and Distillation Unit manufactured in Korea.
Statistical analysis
The numerical values pertaining to vegetative growth, development, and nutrient content were subjected to statistical analysis using the Duncan’s multiple range test (with a significance level of p < 0.05) within CoStat Version 6.311, developed by CoHort Software in Monterey, California, USA.
Results and Discussion
The experiment conducted to assess the growth of seedlings in relation to the different treatments yielded intriguing results. In this study, the different irrigation frequencies showed a significant influence on the average values of growth for all the strawberry cultivars (Table 1). Among the four treatments, seedlings treated with a 34% irrigation frequency showed a lower growth, i.e., height of plant, leaf length and width, number and length of petioles, SPAD, crown diameter, and fresh and dry weight of seedlings compared to the 48% and 55% treatments. Among the various treatments applied, it was abundantly clear that the percentage of growth greatly depended on the specific treatment regimen. The most notable finding was that the seedlings subjected to a 55% fertigation treatment exhibited the greatest growth in terms of dry weight. This suggests that the fertigation frequency involved in this treatment was particularly conducive to the robust development of the seedlings. Conversely, the experiment revealed a contrasting outcome for the seedlings exposed to a 34% irrigation frequency. This group exhibited the lowest growth in terms of dry weight, highlighting the adverse effects of this lower volume of irrigation on seedling development. It is prominent that the lower percentage of irrigation frequency is responsible for the hindered growth. Scagel et al. (2012) found the same results that irrigation frequency influences plant growth and development. This result is also supported by Li et al. (2018, 2019), showing that plant growth and nutrient uptake are affected by the irrigation frequency, and a lower rate of irrigation causes lower growth of seedlings.
Table 1.
Growth and development of ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ strawberry cultivars at 60 days after transplanting influenced by different moisture content (%) at which load cell was settled to regulate fertigation
| Cultivar |
Moisture content (%) |
Plant height (cm) |
Leaf length (cm) |
Leaf width (cm) |
No. of petiole |
Petiole length (cm) | SPAD |
Crown dia. (cm) |
Fresh weight (g) |
Dry weight (g) | Inflorescence | ||
| No. |
Fresh weight (g) |
Dry weight (g) | |||||||||||
| Altaking | 55 | 43.1 az | 11.5 a | 8.9 c | 12.2 a | 31.7 a | 41.9 b | 1.6 a | 78.4 a | 13.9 a | 1.9 a | 40.2 a | 2.8 a |
| 48 | 43.2 a | 12.6 a | 9.5 b | 11.7 b | 29.7 b | 41.9 b | 1.6 a | 73.9 b | 12.9 b | 1.8 ab | 19.9 b | 2.6 b | |
| 41 | 43.1 a | 12.3 a | 9.8 a | 11.9 b | 29.5 b | 41.0 c | 1.6 a | 73.9 b | 12.7 b | 1.7 ab | 19.1 b | 2.5 b | |
| 34 | 42.7 b | 12.4 a | 9.9 a | 10.5 c | 29.6 b | 42.8 a | 1.5 a | 68.7 c | 11.6 c | 1.6 b | 18.7 b | 2.4 b | |
| F-sig. | ** | NS | ** | *** | *** | ** | NS | *** | *** | NS | *** | *** | |
| Kuemsil | 55 | 37.9 a | 9.2 a | 7.5 a | 12.2 a | 26.5 a | 50.9 b | 1.5 a | 73.1 a | 13.8 a | 1.1 a | 7.1 a | 1.1 a |
| 48 | 36.4 a | 8.9 a | 7.2 b | 11.3 b | 22.8 b | 50.0 c | 1.4 b | 67.5 b | 12.5 b | 1.0 a | 5.5 b | 0.8 b | |
| 41 | 36.4 a | 8.9 a | 7.1 b | 11.0 b | 22.6 b | 49.6 c | 1.5 b | 63.6 b | 11.8 b | 1.0 a | 5.3 b | 0.8 b | |
| 34 | 34.4 b | 9.3 a | 7.6 a | 10.8 c | 21.1 c | 52.4 a | 1.4 b | 56.5 c | 10.5 c | 1.0 a | 5.1 b | 0.8 b | |
| F-sig. | ** | NS | ** | *** | *** | ** | NS | *** | *** | NS | *** | *** | |
| Vitaberry | 55 | 33.9 a | 9.8 a | 7.9 b | 12.0 a | 23.3 a | 53.3 a | 1.5 a | 52.6 a | 10.2 a | 1.0 a | 10.1 a | 1.5 a |
| 48 | 34.9 a | 9.9 a | 7.4 c | 11.1 b | 23.7 a | 51.5 b | 1.5 a | 50.5 b | 9.5 b | 1.0 a | 8.4 b | 1.3 b | |
| 41 | 32.4 a | 9.7 a | 8.3 a | 10.7 b | 20.7 b | 47.4 c | 1.4 b | 46.9 c | 8.9 c | 1.0 a | 8.3 b | 1.2 b | |
| 34 | 32.1 b | 9.6 a | 7.5 c | 9.3 c | 21.0 b | 46.2 c | 1.4 b | 40.3 d | 7.6 d | 1.0 a | 7.5 b | 1.1 b | |
| F-sig. | ** | NS | ** | *** | *** | ** | NS | *** | *** | NS | *** | *** | |
The different irrigation frequencies resulted in different plant heights in all the strawberry varieties, and it was partially significant among the treatments. According to the experiment, the average maximum plant height was attained in ‘Altaking’ (43.2 cm) with an irrigation setpoint of 48%, while in ‘Kuemsil’ (37.9 cm), and ‘Vitaberry’ (33.9 cm) both with an irrigation frequency of 55%. There was no significant difference in plant height among the treatments of 55%, 48%, and 41%, but the lowest was with an irrigation frequency of 34%. Therefore, irrigation frequency during the growing season is responsible for plant growth (Warren and Bilderback 2002). Petiole length also showed the same result as plant height in all the cultivars. The maximum leaf length in ‘Altaking’ and ‘Vitaberry’ was observed with an irrigation frequency of 48%, and in ‘Keumsil’, the leaf length was maximum with an irrigation frequency of 34%. On the other hand, the maximum leaf width in ‘Altaking’ and ‘’Keumsil’ was with an irrigation frequency of 34% and in ‘Vitaberry’ with an irrigation frequency of 41%. Which indicates that, in case of plant height, the higher irrigation frequency gave maximum results, but the leaf width showed a better result in the lower irrigation frequency treatments. Again, the highest number of petioles in ‘Altaking’, ‘Keumsil’, and ‘Vitaberry’ was with an irrigation frequency of 55%. Furthermore, SPAD was the highest with an irrigation frequency of 34% in ‘Altaking’ and ‘Kuemsil’ but in ‘Vitaberry’ with an irrigation frequency of 55%. The crown diameter was almost the same in all the treatments of the strawberry varieties. While in all the cultivars, the leaf length and crown diameter were non-significant, and the leaf width, number of petioles, petiole length, and SPAD were partially significant. The highest growth of strawberry mother plants with regard to the data on dry weight in ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ was observed with an irrigation frequency of 55%. This result is similar to Lalk et al. (2023), who found that strawberry cultivars cultivated with irrigation two times per day gave higher growth and development compared to strawberry seedlings raised with irrigation one time a day. According to the findings, two irrigations per day were responsible for enhancing the moisture in the root zone by 44.0% compared with irrigation once a day. The experiment also confirmed that strawberry cultivars treated with maximum irrigation can produce higher shoot dry weight compared to seedlings treated with a lower irrigation frequency.
These findings underscore the significance of irrigation frequencies to optimize plant growth and development. The variance in growth between the 55% and 34% treatments demonstrates that seemingly minor adjustments can have a substantial impact on the outcome. It also emphasizes the complexity of plant physiology and the need for a nuanced approach to cultivation. By identifying the optimal conditions for growth, it is possible to maximize crop yields, reduce resource consumption, and ultimately ensure food security in a world facing increasing challenges related to agriculture and sustainability. Therefore, appropriate nutrition is required for plants to grow steadily and produce high-quality fruits; this can only be achieved by providing them with enough nutrients. Extremely low or excessive nutrients availability have a significant impact on shoot development and growth (Savini et al. 2005).
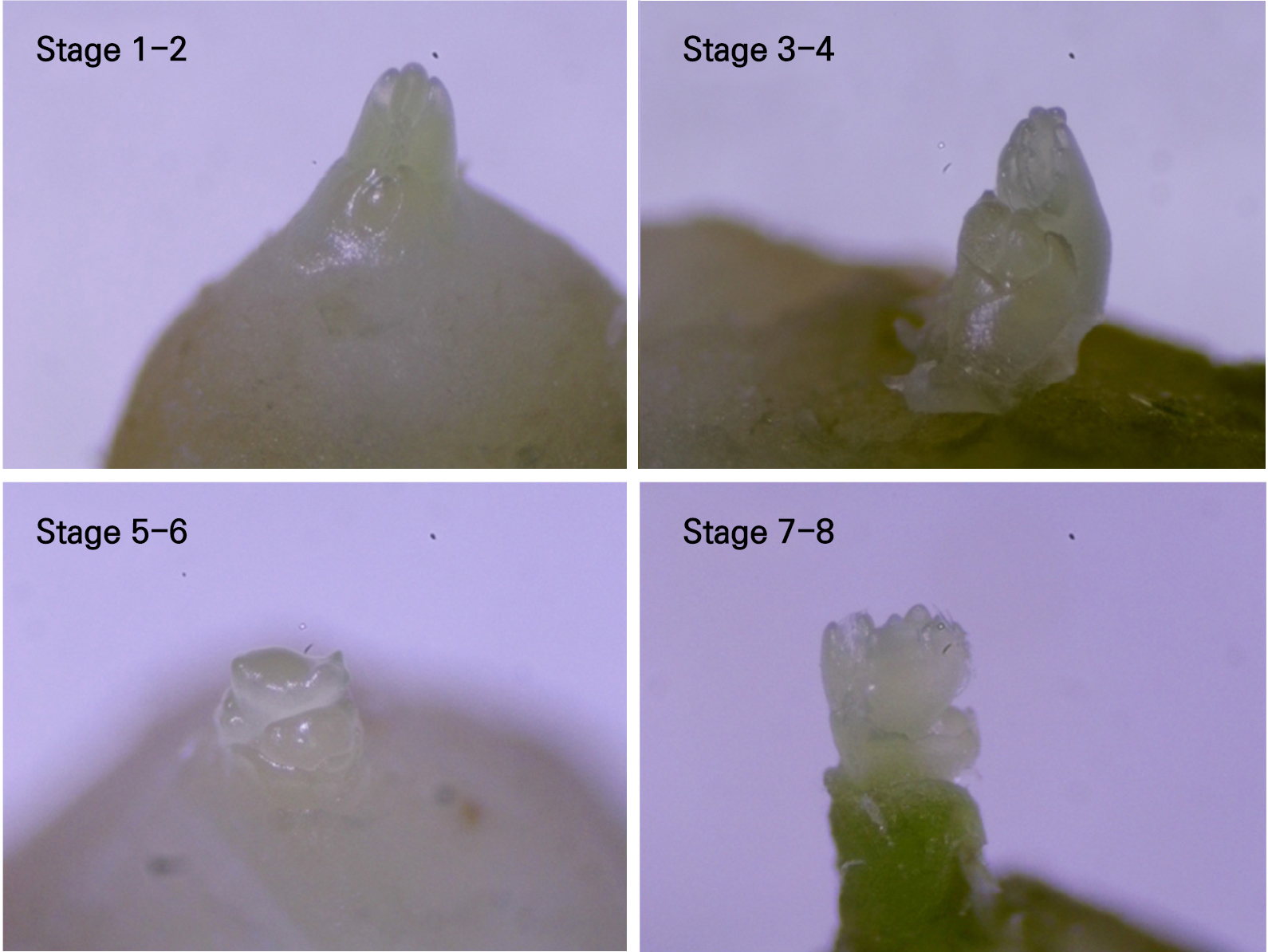
According to Hwang et al. (2020) flower bud differentiation and fruit set of strawberry seedlings depend on the amount and concentration of fertigation applied. Excessive application of irrigation water and nutrients showed a tendency to reduce flower formation and fruit yield. The flower bud differentiation stage was observed before the seedlings were transplanted (Fig. 2 and Table 2). Table 2 offers a comprehensive overview of how the moisture content influences flower bud differentiation stages across the three cultivars, ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’. The data shows variations in the developmental stages of flower buds concerning differing moisture levels within each cultivar. The stages are categorized into four sections: 1–2, 3–4, 5–6, and 7–8. Each stage denotes the progression of flower bud development, and the table displays the percentage of buds in each stage for different moisture levels within each cultivar. Across different moisture levels, ‘Altaking’ shows varying percentages of flower bud differentiation at different stages. 50–85% of the seedlings were observed in the flower bud differentiation stages 7–8. With the highest irrigation frequency (55%) treatment, 50% of the seedlings were in those stages while 60% of the seedlings were in those stages for the lowest irrigation frequency (34%). ‘Kuemsil’ showed a fluctuating percentages of flower bud differentiation across different stages; 36% of the seedlings treated with a 55% irrigation frequency were found at stages 1–2 and 5–6; in contrast, 84.6% of the seedlings treated with a 34% irrigation frequency were found at the flower bud differentiation stages of 1–2. For the ‘Vitaberry’ seedlings treated with a 55% irrigation frequency, 57.1% of the seedlings were observed with flower bud differentiation stages of 7–8, while 73.4% of the seedlings with a 34% irrigation frequency were at the same stages.
Table 2.
Flower bud differentiation of ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ strawberry cultivars influenced by different moisture content (%) at which load cell was settled to regulate fertigation
| Cultivar | Moisture content (%) | Flower bud differentiation stagez | Speculum populationy | |||
| 1–2 | 3–4 | 5–6 | 7–8 | |||
| Altaking | 55 | 1 (10%) | 1 (10%) | 3 (30%) | 5 (50%) | 10 |
| 48 | 2 (13.3%) | 13 (86.7%) | 15 | |||
| 41 | 1 (8.3%) | 1 (8.3%) | 10 (83.4%) | 12 | ||
| 34 | 2 (20%) | 2 (20%) | 6 (60%) | 10 | ||
| Kuemsil | 55 | 4 (36.4%) | 1 (9.1%) | 4 (36.4%) | 2 (18.1%) | 11 |
| 48 | 5 (41.6%) | 2 (16.7%) | 3 (25.0%) | 2 (16.7%) | 12 | |
| 41 | 9 (69.2%) | 1 (7.7%) | 1 (7.7%) | 2 (15.4) | 13 | |
| 34 | 11 (84.6%) | 1 (7.7%) | 1 (7.7%) | 13 | ||
| Vitaberry | 55 | 4 (28.6%) | 2 (14.3%) | 8 (57.1%) | 14 | |
| 48 | 4 (30.8%) | 2 (15.4%) | 1 (7.7%) | 6 (46.1%) | 13 | |
| 41 | 1 (7.1%) | 13 (92.9%) | 14 | |||
| 34 | 2 (13.3%) | 2 (13.3%) | 11 (73.4%) | 15 | ||
The highest number of inflorescences was found in seedlings treated with a 55% irrigation frequency, but there was no significant difference among the treatments tested. However, the growth of inflorescence in terms of the fresh and dry weight was statistically significant, and the maximum was found in seedlings treated with a 55% irrigation frequency. This result is also supported by the findings of Tsuchida and Jomura (2020). The number and growth of flower buds were decreased by the limited availability of water, which could have been brought on by a number of events such as the abscission of immature buds shortly before anthesis and the restriction of flower bud differentiation in the summer. These results suggest that low fertility and poor flower development were driven by inadequate irrigation. The deterioration of shoot apical meristems among the treatments was statistically significant, and the maximum was found in the plants with the 34% treatment in each cultivar (Table 3). This result indicates that the emergence of new leaves after inflorescence emergence was minimum in strawberries treated with 34% fertigation, and more than 70% of the seedlings did not have new leaves after the occurrence of inflorescence. Besides, the formation of axillary buds also was lower in this treatment. It can be caused by the absence of adequate nutrients in root media which was supplied with irrigation water. On the other hand, more than 95% of the strawberry seedlings treated with 55% fertigation showed the occurrence of new leaves after the inflorescence emerged. In general, the growth of secondary meristems, like cambium, in plants including strawberries, is influenced by several nutrients. According to Blanco et al. (2020), insufficient watering did not promote, intensify, or worsen flower growth, and fruit setting. However, deficit irrigation along with fertilizers reduced the rate of vegetative growth before and after flowering, which in turn reduced the further production of inflorescence and fruits.
Table 3.
Deterioration of shoot apical meristems of ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ strawberry cultivars influenced by different moisture content (%) at which load cell was settled to regulate fertigation
| Cultivar | Moisture content (%) | Deterioration (%)z |
| Altaking | 55 | 3.3 dy |
| 48 | 10.0 c | |
| 41 | 36.7 b | |
| 34 | 81.5 a | |
| F-sig. | *** | |
| Kuemsil | 55 | 3.3 d |
| 48 | 6.7 c | |
| 41 | 46.7 b | |
| 34 | 73.3 a | |
| F-sig. | *** | |
| Vitaberry | 55 | 3.3 d |
| 48 | 10.0 c | |
| 41 | 43.3 b | |
| 34 | 72.4 a | |
| F-sig. | *** |
The comparative analysis of the tissue nutrient content in ‘Altaking’, ‘Keumsil’, and ‘Vitaberry’ revealed intriguing findings. Statistical significance was observed among these strawberry varieties, with notable variations in the nutrient levels. Notably, the maximum nutrient content was detected in the dry matter of seedlings subjected to a 55% irrigation frequency (Table 4). This result suggests that a more frequent watering regimen can enhance the accumulation of essential nutrients within strawberry plants, potentially contributing to their overall health and vitality. In contrast, the minimum nutrient content was identified in the dry matter of seedlings exposed to a 34% irrigation frequency, signifying that reduced irrigation intervals might hinder the uptake and assimilation of crucial nutrients. These findings underscore the critical role of irrigation management in influencing the nutritional composition of strawberries and may hold significant implications for optimizing cultivation practices to attain strawberries of superior quality and nutritional value. However, the tissue contents in ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ at the highest growth of their mother plants regarding their dry weight and lowest abscission of apical meristems were 3.8, 3.7, and 3.7% of total nitrogen, 5.9, 5.4, and 6.0% of P, 3.8, 3.7, and 3.8% of K, 6.1, 7.7, and 8.2% of Ca, 3.2, 3.2, and 3.1% of Mg, 6.9, 7.1, and 6.6 mg kg-1 of Cu, 180.5, 182.8, and 183.1 mg kg-1 of Fe, 253.4, 264.6, and 249.3 mg kg-1 of Mn, and 23.1, 20.2, and 18.5 mg kg-1 of Zn, respectively.
Table 4.
Tissue contents of ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ strawberry cultivars at 60 days after transplanting influenced by different moisture content (%) at which load cell was settled to regulate fertigation
| Cultivar | Moisture content (%) | T-N | P | K | Ca | Mg | Cu | Fe | Mn | Zn |
| ------------------ (%) ----------------- | -------------- (mg kg-1) ------------- | |||||||||
| Altaking | 55 | 3.8 az | 5.9 a | 3.8 a | 6.1 a | 3.2 a | 6.9 a | 180.5 a | 253.4 a | 23.1 a |
| 48 | 3.4 b | 4.2 b | 3.4 b | 5.1 b | 2.8 b | 5.5 b | 142.6 b | 216.1 a | 15.6 b | |
| 41 | 2.8 c | 3.1 c | 2.7 c | 5.2 b | 2.5 b | 4.5 c | 133.1 b | 127.5 b | 13.5 b | |
| 34 | 2.6 c | 2.9 d | 1.8 d | 2.6 c | 1.5 c | 3.6 d | 95.1 c | 106.5 b | 9.7 c | |
| F-sig. | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Kuemsil | 55 | 3.7 a | 5.4 a | 3.7 a | 7.7 a | 3.2 a | 7.1 a | 182.8 a | 264.6 a | 20.2 a |
| 48 | 3.2 b | 5.1 b | 3.6 a | 6.3 b | 2.8 b | 5.4 b | 139.7 b | 213.1 a | 16.1 b | |
| 41 | 2.8 c | 3.1 c | 2.7 b | 4.8 c | 2.4 b | 4.6 c | 130.2 b | 139.7 b | 14.6 b | |
| 34 | 2.5 c | 2.8 d | 1.7 c | 2.9 d | 1.4 c | 3.4 d | 80.1 c | 117.5 b | 10.5 c | |
| F-sig. | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Vitaberry | 55 | 3.7 a | 6.0 a | 3.8 a | 8.2 a | 3.1 a | 6.6 a | 183.1 a | 249.3 a | 18.5 a |
| 48 | 3.4 a | 5.1 b | 3.5 b | 6.8 b | 2.7 b | 5.4 b | 140.9 b | 217.5 a | 15.8 b | |
| 41 | 2.8 b | 3.1 c | 2.7 c | 4.7 c | 2.3 b | 4.9 c | 133.1 b | 147.6 b | 15.4 b | |
| 34 | 2.6 b | 2.7 d | 1.7 d | 2.8 d | 1.3 c | 3.5 d | 83.1 c | 114.7 b | 9.0 c | |
| F-sig. | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
According to Agehara (2021), the concentration of the tissue contents in plant sap is influenced by the fertigation levels and irrigation frequency, and these have a great impact on the growth of strawberry seedlings. Additionally, based on the research of Yamasaki and Yano (2009), nutrient concentration in above ground tissue depends on the frequency of fertigation in the root media because a higher fertigation frequency increases the amount of nutrients in the root zone which Eshghi and Tafazoli (2007) showed as well.
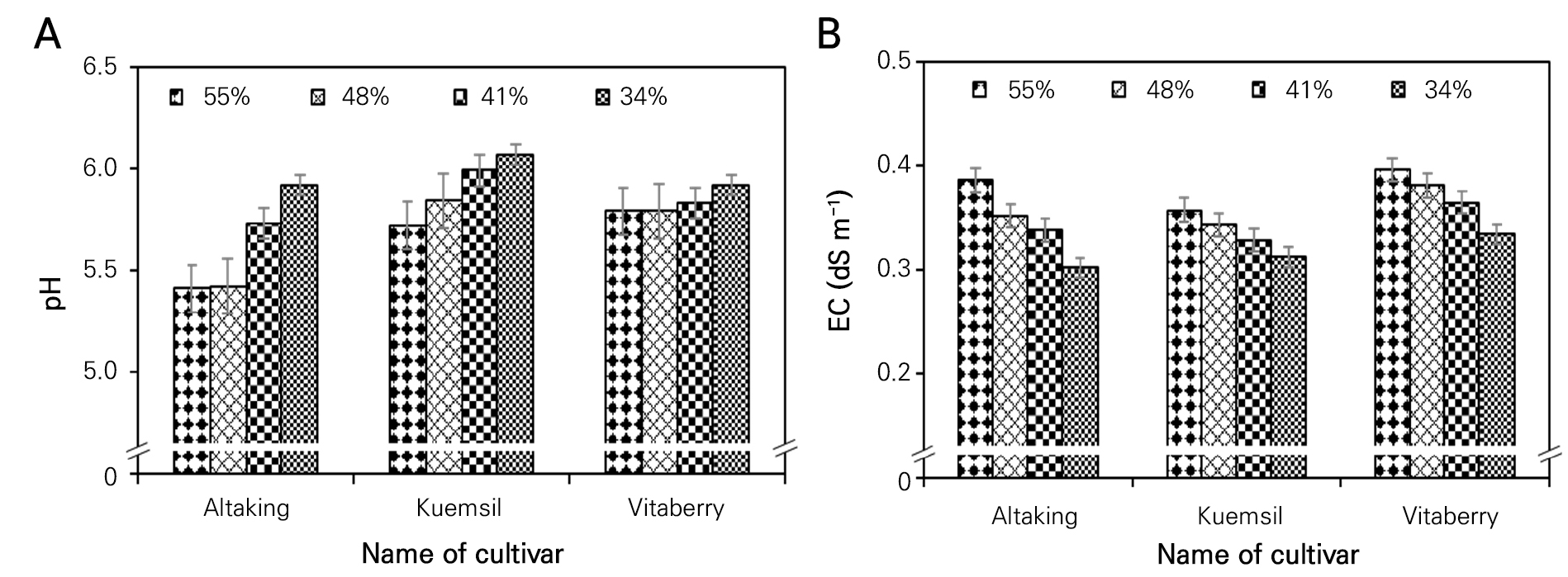
In this investigation, a notable trend emerged regarding the pH levels and electrical conductivity (EC) when varying the irrigation frequencies (Fig. 3). As the irrigation frequencies decreased, a consistent increase in the pH levels within the experimental conditions was observed. This observation highlights a clear inverse relationship between irrigation frequency and pH, implying that reduced watering intervals tend to lead to higher soil pH values. This phenomenon could be attributed to the fact that less frequent irrigation might enable a greater accumulation of alkaline substances in the soil, thereby raising the pH.
Conversely, the EC levels exhibited an opposite pattern. The highest EC values were recorded when the irrigation frequency was at its peak. This finding suggests that a more frequent watering schedule, which provides ample moisture to the soil, tends to result in elevated levels of dissolved salts and ions. As a result, the electrical conductivity of the soil solution increases under such conditions. This increase in EC can have implications for nutrient availability and plant health because excessively high levels of salts in the soil can potentially lead to adverse effects on plant growth and nutrient uptake. These findings are supported by the findings of Kang et al. (2011) who showed that the concentration of nutrients in the root zone and shoots is directly attributed to an increase in fertilizer solution, which also has a significant impact on the electrical conductivity of the root media solution. This result is also supported by Choi et al. (2016), showing that root media electrical conductivity depends on the amount of fertigation. These findings underscore the critical role of irrigation frequency in influencing the soil pH and EC, which, in turn, can impact plant health and nutrient management. Effective irrigation strategies should consider these dynamics to ensure optimal growing conditions and crop success.
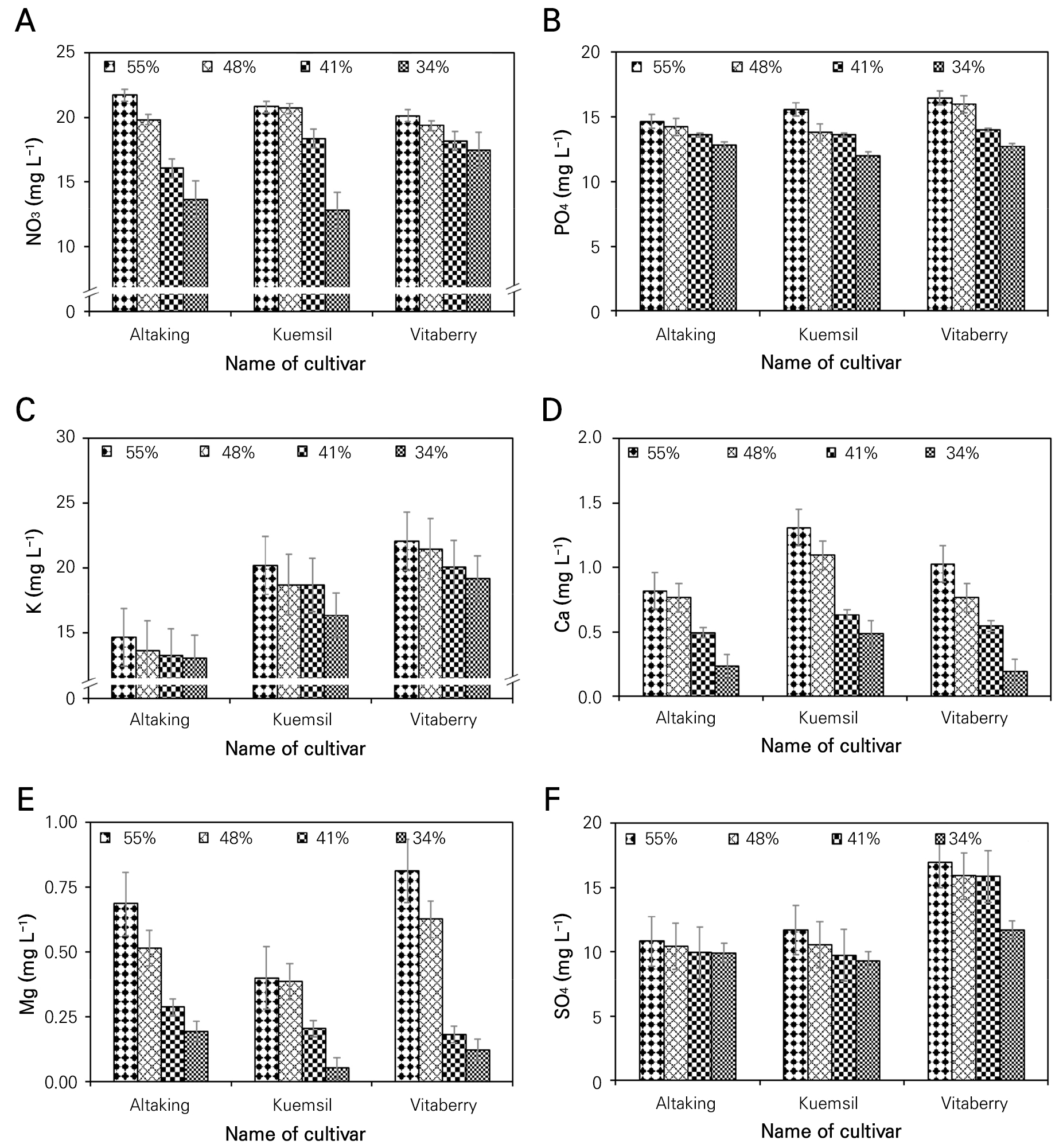
According to Silber et al. (2003), nutrients uptake depends on irrigation frequency, and a high fertigation frequency enhances nutrient uptake and increases plant growth. This study revealed a noteworthy correlation between the root medium nutrient concentration and irrigation frequency, shedding light on the crucial role that watering schedules have in nutrient management for plants. In the present study, a distinct and positive relationship was observed between these two factors, where the nutrient concentration in the root medium increased in tandem with higher irrigation frequencies. This finding implies that frequent irrigation can have a pronounced impact on the availability and uptake of essential nutrients by plants. As water is supplied more regularly, it not only replenishes moisture levels in the root medium but also facilitates the dissolution and dispersion of nutrients present in the soil, making them more accessible to the plants’ root systems. However, the root medium nutrient concentrations (Fig. 4) in ‘Altaking’, ‘Kuemsil’, and ‘Vitaberry’ at their highest mother plants growth and lowest abscission of apical meristems were 21.7, 20.8, and 20.1 mg L-1 of NO3-, 14.6, 15.6, and 16.5 mg L-1 of PO43-, 14.6, 20.2, and 22.1 mg L-1 of K+, 0.8, 1.3, and 1.0 mg L-1 of Ca2+, 0.7, 0.4, and 0.8 mg L-1 of Mg2+ and 10.8, 11.7 and 16.9 mg L-1 of SO42-, respectively (data not shown). Root medium nutrient concentration also showed a positive relation with the irrigation frequency. As the irrigation frequency increases, the concentration of nutrients in the root media increases and consequently the uptake of nutrients by plants increases (Scagel et al. 2011). These findings are also affirmed by Fageria (2006) who found that the increased concentration of nutrients in the root media solution can change the availability, absorption, and concentration of nutrients in plant tissue. This outcome is further supported by Farjana et al. (2023), showing that a higher level of fertigation yields the maximum vegetative growth of strawberry seedlings.
Conclusion
In conclusion, the results of this study shed light on the significant impact of irrigation set points on the growth and development of strawberry seedlings. This study used various strawberry cultivars and systematically varied the irrigation frequencies with the dry weights of the seedlings serving as a robust indicator of growth performance. The most striking finding was the pronounced difference in dry weights among the different irrigation frequencies. Notably, the seedlings reached their highest growth when subjected to a 55% set-point, while growth was significantly reduced when the irrigation was set at 34%. This disparity highlights the importance of precise irrigation management in optimizing the growth of strawberry plants. Furthermore, this study investigated the premature deterioration of shoot apical meristem, which has a pivotal role in the development of strawberry plants. The results show that this process was significantly affected by the irrigation frequencies, with the highest deterioration occurring at the 34% set-point and the lowest at 55%. Taken together, these findings underscore the intricate relationship between irrigation, flower bud differentiation, shoot apical meristem deterioration, and the overall growth of strawberry seedlings. The results strongly suggest that a 55% irrigation frequency is the most favorable for promoting optimal growth and development in strawberry cultivars. This information is invaluable for strawberry growers seeking to enhance their crop yields and quality through precise irrigation management.