Introduction
Materials and Methods
Indoor Experiment
Outdoor Experiment
Data Analysis
Results and Discussion
Indoor Experiment
Outdoor Experiment
Conclusion
Introduction
More than 800 species have been reported for the genus of Eucalyptus (Eucalyptus spp.), which are evergreen trees of the family Myrtaceae; more than 500 species Eucalyptus spp. occur indigenously in Australia (Coppen et al., 2002; Kellison et al., 2013). Eucalyptus spp. are the most common tree species planted along the streets of many countries worldwide, particularly Oceania, owing to their rapid growth and high value as a shelterbelt (Cromer et al., 1981; Pohjonen and Pukkala, 1990; Cotterill and MacRae, 1997; Coppen et al., 2002; Dirr and Warren, 2019). The biomass of Eucalyptus spp. also has various industrial applications, mostly for paper pulp, energy wood and timber for coal-fired power as well as medicinal applications, particularly for vaccine adjuvants, aromatic resources, soap, and perfume (Coppen et al., 2002; Sillett et al., 2015; Dirr and Warren, 2019). Potted Eucalyptus spp. are very popular for interior decorations, flower arrangements, and air purifiers in South Korea, where they have not been commercially planted as ornamental trees in backyards and are receiving attention in horticultural therapy and healing.
Eucalyptus trees are mostly found between 10°N and 44°S latitude, with 100 to 3,750 mm of precipitation in tropical to warm temperate zones, including of Brazil, India, China, and some countries in the Mediterranean area (Coppen et al., 2002; Teulières et al., 2007; Booth, 2013; Kellison et al., 2013); such conditions could allow the trees to expand to new growing regions worldwide. Global warming has reduced seed germination, tree survival, and suitable areas for planting trees in South America due to heat stress, increased evapotranspiration, and limited water availability (Booth, 2013; Martins et al., 2022). However, climate warming of 2–4°C would contribute to strong vegetative growth of temperate and boreal trees of relatively cool origin when compared with warm-origin trees (Feeley et al., 2007; Way and Oren, 2010; Bowman et al., 2014; Drake et al., 2015).
South Korea is located in a temperate area between 33° and 43° north latitude, with an average temperature of 2.7°C and precipitation of 116.6 mm from December to February in the cold winter season of 2022 (KMA, 2022). Some Eucalyptus spp., such as gunnii and pauciflora, can tolerate between ‒14°C and ‒18°C although the trees do not undergo endodormancy during winter (Teulières et al., 2007); they could be established in an outside field after hardening from the seedling stage. However, little research has been conducted on the adaptability of Eucalyptus trees grown in indoor and outdoor environments in a north temperate zone, with no research performed in S. Korea. E. pulverulenta, E. gunnii, and E. parvula comprise approximately 91% of Eucalyptus species sales, and they are mostly used as interior design materials in S. Korea (aT, 2023). The aim of this study was to expand the choice of Eucalyptus species by evaluating germination and survival under greenhouse conditions and to select the best species on the basis of their performance after transplantation into a field.
Materials and Methods
Indoor Experiment
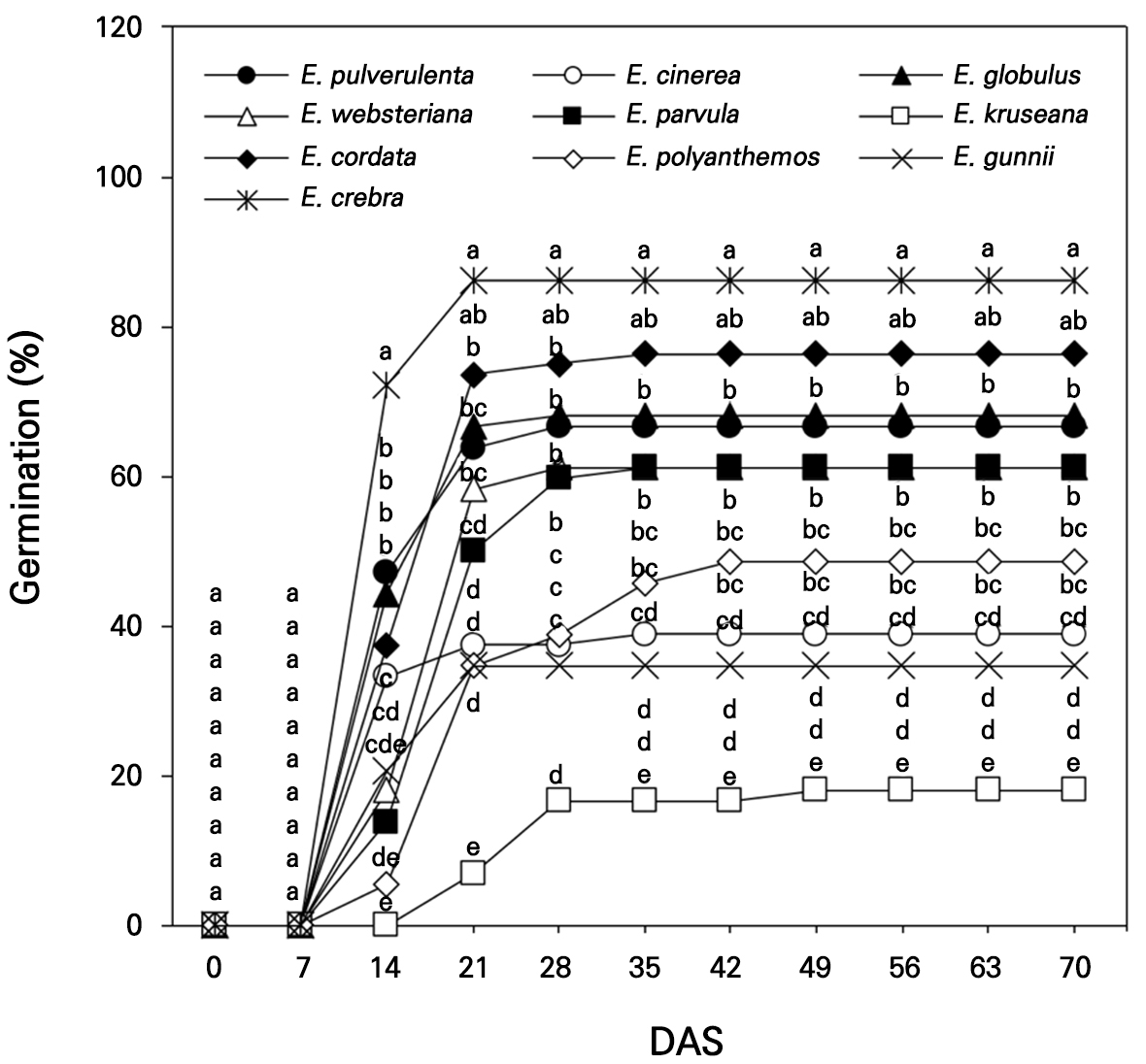
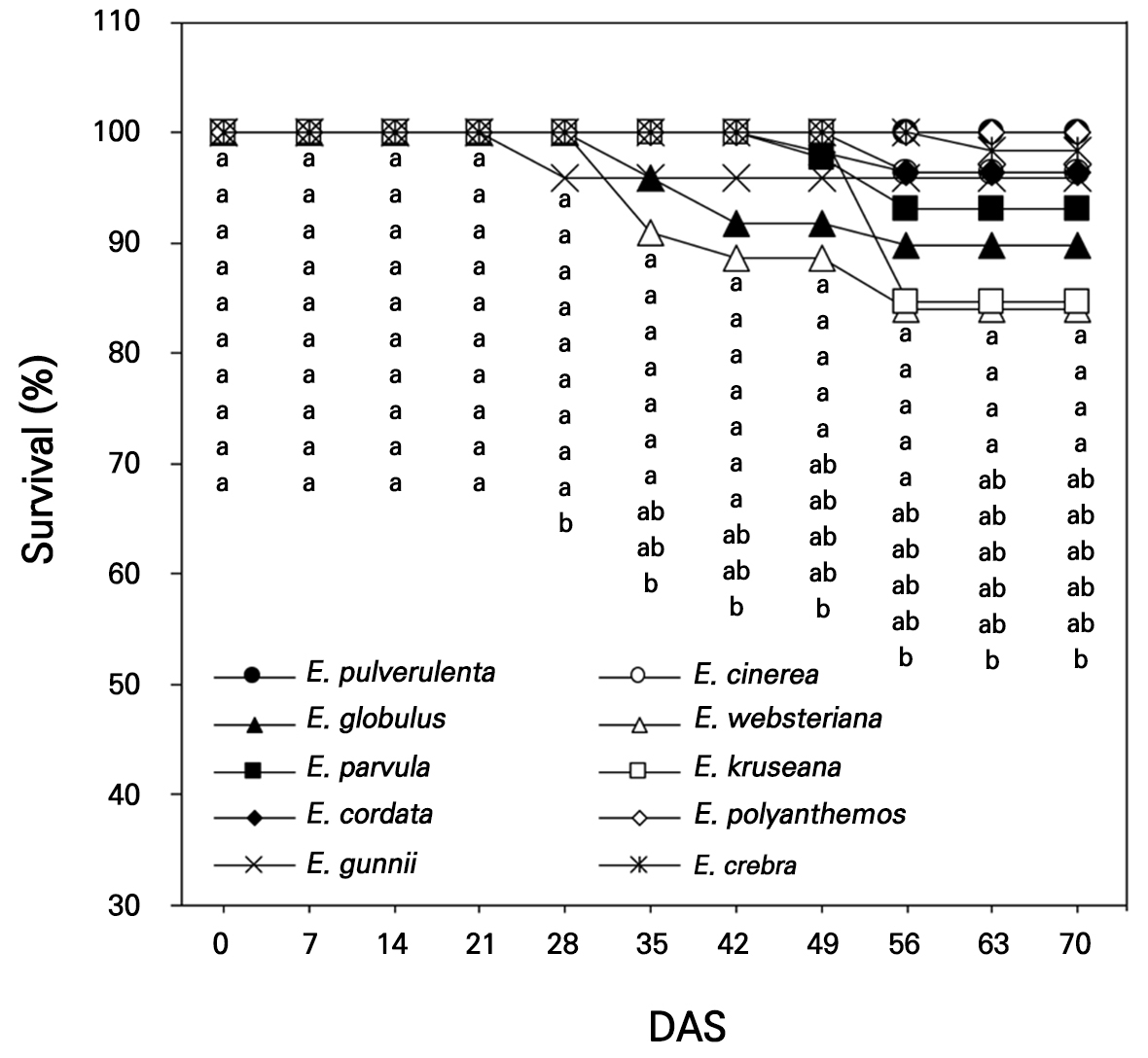
The seeds of ten Eucalyptus spp., E. pulverulenta, E. cinerea, E. globulus, E. websteriana, E. parvula, E. kruseana, E. cordata, E. polyanthemos, E. gunnii, and E. crebra, were collected for germination in an indoor experiment in a small greenhouse (2.4 m × 2.4 m) in Gyeongju-si, South Korea (35°N, 129°E) on April 3, 2022. Hundreds of Eucalyptus seeds per species were sown in 72-cell seedling trays with growth medium and drain holes. The growth medium (Olaeolae, Seoul Bio Co., Eumseong-gun, S. Korea) consisted of a mixture of components: 15% peatmoss, 68% coco peat, 7% perlite, 7% zeolite, and 3% vermiculite, which contained 0.65 EC dS·m-1, 300 mg·L-1 NO3-N, 250 mg·L-1 NH4-N, 350 mg·L-1 P2O5, and 13 cmolc·kg-1 CEC, with a water content of 55%, bulk density of 0.22 Mg·m-3, and pH of 6.2 (1:5, v/v). In the greenhouse, the lowest relative humidity (RH) and air temperature were 13% and 0.3°C, respectively, with the corresponding highest RH and air temperature being 99% and 48.6°C recorded in April (KMA, 2022, Fig. 1); an open-roof greenhouse system was used to decrease the temperature during the rest of the experimental period (May and June). The seed germination and survival rates were visually observed 14 days after sowing (DAS, Fig. 2) and were measured once a week from 0 to 70 DAS in April–June 2022 (Figs. 3 and 4).

Fig. 2.
Seedlings of ten Eucalyptus species before transplantation from a glass greenhouse to the field after nine weeks of sowing in Gyeongju-si in 2022. E. pulverulenta (A), E. cinerea (B), E. globulus (C), E. websteriana (D), E. pavula (E), E. kruseana (F), E. cordata (G), E. polyanthemos (H), E. gunnii (I), and E. crebra (J).
Outdoor Experiment
An outdoor study was conducted in an experimental field of a university farm in Gyeongsan-si, S. Korea (35°N, 127°E) on June 10, 2022, with 167 g of manure compost (poultry 30%, cow 30%, pig 5%, saw dust 30%, and zeolite 5%; Taesan Farming Corp., Gyeongju-si, S. Korea) per tree to improve soil fertility. The field soil nutrition before transplanting of Eucalyptus seedlings was 0.04 dS·m-1 EC, 12.7 g·kg-1 organic matter, 3.5 mg·kg-1 NO3, 658.3 P2O5, 0.18 cmolc·kg-1 K2O, 6.6 cmolc/kg CaO, and 1.4 cmolc·kg-1 MgO at pH 6.8. Two hundred Eucalyptus seedlings (20 per Eucalyptus species) were transplanted with 2.0 m between rows and 0.5 m between the trees from a small greenhouse for the outdoor experiment (Fig. 5). None of the Eucalyptus species were trained, and they were naturally grown with plastic tree support. The trees in the soil, a mixture of silt loam, were irrigated using a hand-held hose whenever the dry period exceeded five days in the field.
Tree height, trunk thickness 10 cm above the soil surface, and number of shoots and leaves were measured 30 and 150 days after transplanting (DAT). Leaf chlorophyll concentrations were estimated for fully expanded leaves of the mid-positioned shoots of each tree by means of SPAD-502 measurements (Minolta Co., Tokyo, Japan) at 30 and 150 DAT.
Leaf browning in each tree was recorded on the basis of any browning symptoms visually detected at 30 and 150 DAT.
The trees, including the roots, were destructively harvested at 150 DAT and separated into roots, leaves, and shoots. Each organ was dried in an oven at 65°C for seven days and the dry weight was then measured. The dry mass was partitioned into that of a whole tree considering its top-to-root ratio.
Data Analysis
One hundred replicates (seeds) per Eucalyptus species were used for the indoor experiment, with ten replicates per species for the outdoor experiment. All data were analyzed with ANOVA in SAS Version 14.1 (SAS Inc., Cary, NC, USA). The significance of the means was determined using Duncan’s multiple range test (DMRT) at p < 0.05 level of probability.
Results and Discussion
Indoor Experiment
Seed germination of ten Eucalyptus spp. increased sharply from 7 DAS and mostly peaked between 21 and 28 DAS, except for the seed germination of E. polyanthemos, which peaked by 42 DAS (Fig. 3). The maximum germination studied in Irish and British climates was rapidly reached by 3 to 10 DAS for 15 Eucalyptus species collected from alpine forests in Australia, mostly due to the suitable growing conditions at these locations although this metric varied significantly for each species (Afroze et al., 2021). Germination at 0–7 DAS was not significantly different among the species at p < 0.05. Germination was significantly higher for E. crebra at 14–70 DAS, at approximately 86.1% at 70 DAS, followed by E. cordata (76.4%), E. globulus (68.1%), and E. pulverulenta (66.7%). E. crebra seedlings in a pot showed increased photosynthetic efficiency and stomatal conductance under water-limited conditions when compared with other Eucalyptus species (Silva et al., 2016). In contrast, the germination rates of E. cinerea and E. gunnii were less than 40.0%, with the lowest germination rate (18.1%) obtained for E. kruseana mostly from 14 DAS to 70 DAS.
Seed survival was recorded as 100.0% for all the Eucalyptus species at 0–21 DAS but it decreased slightly to approximately 90.1% for E. websteriana and 96.0% for E. gunnii and E. globulus at 35 DAS (Fig. 4). E. cinerea and E. polyanthemos maintained 100.0% seedling survival at 70 DAS, which differed from seed germination. The lowest survival rate was observed for E. websteriana at approximately 84.1% at 70 DAS and a considerable decline in the survival of E. kruseana occurred at 49–56 DAS. E. websteriana is more sensitive to heat stress than other ornamental shrubs during hot summers (Suleiman et al., 2007), which may have led to a decrease in species survival in the horticultural growth medium during the warm and humid season in this study (Fig. 1). In addition, the seeds contain lower carbohydrate and nutrient levels, implying that the viability and vigor of E. cinerea and E. kruseana seeds were less enhanced (Harrison et al., 2014).
Outdoor Experiment
The minimum temperature after 120 DAS in 2022 and 2023 was lower than those of the 30-year average with occurred in heavy rainfall (Fig. 6).
E. globulus and E. crebra were significantly taller (Fig. 7A) and thicker (Fig. 7B) at 30 DAT. The tallest trees were E. globulus (141.7 cm), followed by E. polyanthemos (80.4 cm) and E. crebra at 150 DAT; the slowest growth was observed in E. kruseana at 33.7 cm and E. parvula at 38.6 cm. E. globulus had a thicker trunk (13.4 mm), followed by E. polyanthemos (9.2 mm) and E. crebra (6.5 mm) as compared to E. pulverulenta (4.9 mm), E. cinerea (4.9 mm), E. websteriana (4.4 mm), and E. kruseana (4.0 mm). E. globulus has high amounts of fiber compounds from byproducts in the stem wood, resulting in a fast growth rate; it is the most widely planted species in forests in the Mediterranean area and in subtropical areas worldwide (Cromer et al., 1981; Pohjonen and Pukkala, 1990; Cotterill and MacRae, 1997). E. globulus, E. crebra, and E. pulverulenta can be cultivated in an open field and adapted to a temperate climate with an arid spring, warm and humid summer, and cold winter, such as in S. Korea by adjusting the osmotic potential and maintaining high turgor pressure (Merchant et al., 2007).
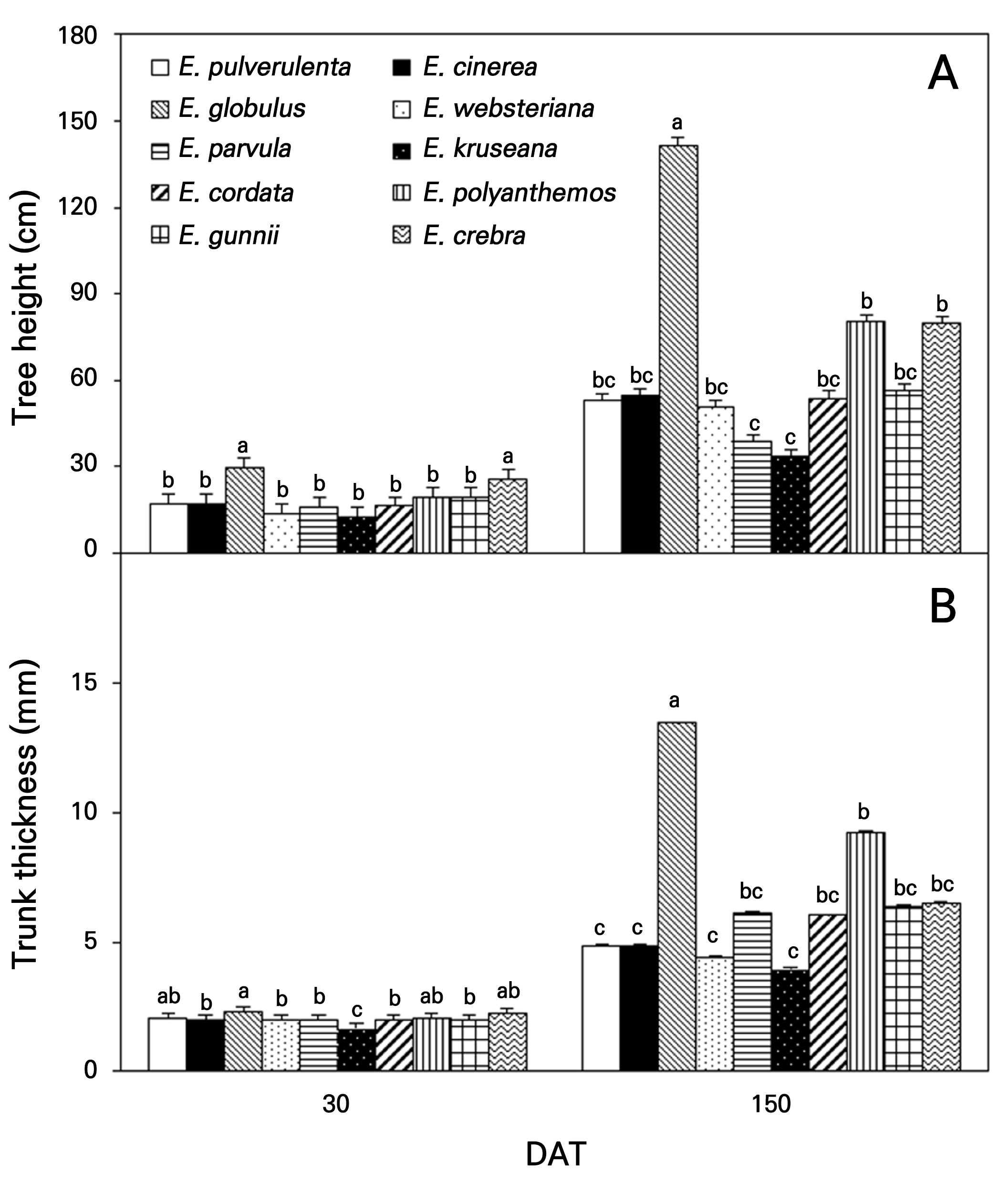
Fig. 7.
Tree height (Panel A) and trunk thickness (Panel B) of ten Eucalyptus species at 30 and 150 days after transplanting (DAT) in Gyeongsan-si in 2022. Different lowercase letters on each phase indicate significant differences determined using Duncan’s multiple-range test at p < 0.05. Bar represents error of the means.
There wer fewer than ten shoots per tree for all Eucalyptus spp. recorded at 30 DAT, and not many shoots per tree were produced by E. pulverulenta and E. polyanthemos at 150 DAT. Other amounts were produced by E. globulus (31) and E. cordata (27),but these decreased (Fig. 8A). The number of leaves ranged from 40 to 60 per tree at 30 DAT, and the highest number was found in E. parvula (596), followed by E. globulus (460), E. cordata (402), E. crebra (388), and E. gunnii (336) at 150 DAT (Fig. 8B). E. parvula grows round with low-branched and multiple stems and a compact trunk size, generating a high number of leaves and providing high value as an ornamental shrub (Dirr and Warren, 2019). In contrast, the vigorous E. polyanthemos produces a low number of shoots and leaves. E. crebra and E. polyanthemos were similar in terms of the vigorous tree structure and volume but were in terms of the tree arrangements based on their shoots and leaves, which can provide different performance levles of ornamental shrubs. Foliar SPAD units, indicating chlorophyll contents and nitrogen concentration (Ribeiro et al., 2009; Ferreira et al., 2015), for all Eucalyptus spp. were between 40 and 60 at 30 DAT. This metric was highest for E. gunnii and relatively low for E. cinerea, E. globulus, and E. polyanthemos, with light bluish and whitish-green foliage (Fig. 8C). The SPAD units for all Eucalyptus spp. increased at 150 DAT, except for E. globulus, most likely due to the indirect dilution effect as a contributor to the stimulation of vegetative growth (Dirr and Warren, 2019; Wujeska‐Klause et al., 2019). However, SPAD between 35 and 45 in the E. globulus case could be an optimum range for young evergreen trees as these levels would indicate an adequate nitrogen status to satisfy their growth (Ribeiro et al., 2009). E. gunnii showed the highest SPAD values at 150 DAT, maintaining dark green foliage during the summer and fall seasons and indicating a high foliar nitrogen content available for annual tree growth.

Fig. 8.
Number of shoots (Panel A), number of leaves (Panel B), and SPAD (Panel C) of ten Eucalyptus species at 30 and 150 days after transplanting (DAT) in Gyeongsan-si in 2022. Different lowercase letters on each phase indicate significant differences determined using Duncan’s multiple-range test at p < 0.05. Bar represents error of the means.
Leaf browning was negligible for all Eucalyptus spp. at 30 DAT but occurred considerably at 150 DAT during early winter for E. kruseana at approximately 45%, with E. polyanthemos showing a rate of 21% and E. parvula leaves showing a similar rate of 20% (Fig. 9). The brown leaves were caused by the synthesis of anthocyanins related to the photoinhibition of E. nitens and E. globulus seedlings transplanted from the greenhouse to the field at a low temperature (Close et al., 2000; Close and Beadle, 2003). The minimum temperature between 140 DAS and 150 DAS was recorded as 4.48°C (Fig. 6) and may have affected the photoinhibition of E. kruseana trees, resulting in the discoloration of leaves. However, the SPAD values were not reduced among the species during those periods.
The dry mass of leaves, shoots, and roots of each species was highest for E. globulus (107.7 g), and 64.3% of the dry mass was shoot dry mass. This was followed by E. polyanthemos (58.7 g) and E. crebra (46.7 g), with the lowest dry mass for E. cinerea (13.5 g) and E. websteriana (13.9 g) (Table 1) after all the trees, including roots, were harvested (Fig. 10). The highest top:root values were observed for E. kruseana (6.6), E. websteriana (6.5), and E. cordata (6.3), indicating shallow root systems and a reduced fixing capacity of the soil. However, the top-to-root values were relatively evenly distributed for E. pulverulenta (2.3), E. cinerea (2.5), E. parvula (2.2), E. polyanthemos (2.2), and E. crebra (2.2).
Table 1.
Dry mass of leaf, stem, root, and top:root ratio of ten Eucalyptus tree species at 200 days after transplanting in Gyeongsan-si in January 2023

Fig. 10.
Photographs of ten Eucalyptus species harvested at 200 days after transplanting in Gyeongsan-si in January of 2023. E. pulverulenta (A), E. cinerea (B), E. globulus (C), E. websteriana (D), E. pavula (E), E. kruseana (F), E. cordata (G), E. polyanthemos (H), E. gunnii (I), and E. crebra (J).
A thick trunk significantly increased tree height (r2 = 0.778), total dry weight (r2 = 0.799), root dry weight (r2 = 0.719), and number of leaves (r2 = 0.196), with little effect on SPAD (r2 = 0.022) and tree survival (r2 = 0.089) (Fig. 11). The trunk thickness of Eucalyptus spp. was strongly correlated with the above-ground biomass and canopy volume of the trees, which has been used as a common estimator of tree biomass, particularly for young-growth trees (Harrington, 1975; Sillett et al., 2015). Only one E. polyanthemos did not appear to show an increased number of leaves, with fewer shoots produced from the bark despite the increase in trunk thickness (Figs. 7 and 8).

Fig. 11.
Linear regression analysis between trunk thickness and tree height (Panel A), and total dry weight. (Panel B), root dry weight (Panel C), number of leaves (Panel D), SPAD (Panel E), and survival (Panel F) of ten Eucalyptus tree species in January of 2023. ** and *** adjacent to each dot point for trunk thickness indicate significant differences as determined by Duncan’s multiple-range test at p < 0.01 and 0.001, respectively. ns denotes not significantly different.
Conclusion
In summary, E. globulus, E. crebra, and E. polyanthemos are suitable Eucalyptus species for comprehensive cultivation in S. Korea given the country's hot summers, broadening the selection of Eucalyptus species from E. pulverulenta, E. gunnii, and E. parvula in S. Korea (aT, 2023). E. kruseana is not recommended for transitioning indoor seedlings to outdoor environments, as previously shown in a cold stress study of Eucalyptus species (Teulières et al., 2007). Eucalyptus species should be continuously studied for acclimation through a cold winter (decreasing by ‒15°C to ‒20°C) with subsequent growth in the following season in S. Korea to investigate low-temperature stress tolerance. A long-term field study is also required to evaluate the physiological responses of various Eucalyptus species to a wide range of climate conditions during global warming and provide information on indoor and outdoor suitability in the early tree growth stage.