Introduction
Materials and Methods
Plant Materials and Disinfection
Effects of the Medium, Initial pH of the Medium, and Type and Concentration of the Sugar Added
Growth Conditions and Growth Measurements
Extraction and Calculation of the Total Chlorophyll Content
Statistical Analysis
Results
Effects of the Culture Media on the Growth of S. cuspidatulum
Effects of the Initial pH of a Medium on the Growth of S. cuspidatulum
Effects of the Sugar Type and Concentration on the Growth of S. cuspidatulum
Discussion
Conclusion
Introduction
Sphagnum, commonly known as peat moss, is one of the most prominent moss genera, both ecologically and economically. Peatlands covered with Sphagnum play a critical role in the ecosystem by storing more global carbon than any other peatland type, primarily because this moss has slow decomposition rates (Gunnarsson, 2005; Breeuwer et al., 2008; Beike et al., 2015). Additionally, Sphagnum is pivotal in absorbing water and retaining nutrients, and these properties improve the consistency and texture of soil in which it grows naturally or to which it has been added. From an economic perspective, Sphagnum peat is highly valuable and used in several ways. Examples include fuel for heating and cooking, as a material that absorbs water, as insulation for buildings, and for the biofiltration of water. It is also beneficial in agriculture and horticulture for amending soil used for planting (Gorham, 1991; Turner, 1993; Sundberg, 2000).
While most Sphagnum species are widely distributed and commonly found in wetlands in Europe and North America (Rochefort, 2000; Sundberg, 2000), they are less common in the tropics. In Thailand, Sphagnum is generally restricted to the highlands of the north and northeastern areas. According to a checklist of bryophytes in Thailand (Sornsamran and Thaitong, 1995), eleven species of Sphagnum were reported from 1911 to 1977 throughout Thailand. Since then, the list of Sphagnum species in Thailand has been continuously revised, and it has been found that species have declined notably in local abundance (He, 2000). For example, Sphagnum has become locally extinct in Doi Suthep-Pui National Park and is gradually disappearing from many locales due to habitat modifications and global climate variability (Manachit, 2009; Kornochalert, 2009).
To counter the decline of Sphagnum in Thailand, in situ and ex situ conservation efforts should be considered as options. However, many Sphagnum habitats in Thailand are also in tourist destinations and/or agricultural areas, making in situ conservation extremely challenging. Therefore, ex situ conservation appears to be a more viable option for the conservation of Sphagnum species. In the past, in vitro micropropagation by tissue culturing was used to preserve various Sphagnum species in temperate areas (Beike et al., 2015). In marshy areas of Thailand, Sphagnum cuspidatulum Müll. Hal. is the most abundant species (Sornsamran and Thaitong, 1995). To the best of our knowledge, this species has never been formally studied in in vitroculture conditions. Thus, we chose this species for growth in an axenic culture to explore the possibility of using a tissue culture for the ex situ conservation of Sphagnum.
This study aimed to find suitable conditions for the in vitro culturing of S. cuspidatulum. Particularly, we focused on the effects of the medium, initial pH, sugar type, and sugar concentration on the dry weight and chlorophyll content of the resulting culture. Conventional media such as that of Murashige and Skoog (1962) (MS medium) were used, as well as other media that were previously employed for Sphagnum propagation. The results here have the potential to lead to better cultivation and propagation methods for this Sphagnum species and may also improve the ex situ conservation of this vulnerable plant species in the tropics.
Materials and Methods
Plant Materials and Disinfection
Gametophytes of S. cuspidatulum were collected along a nature trail from Khok Nok Kraba to Lhone Tae at 1200 to 1500 m above sea level in the Phu Luang Wildlife Sanctuary, Loei province, Thailand (17°7'16"N, 101°32'48"E). The gametophytes were kept in an icebox and delivered to the laboratory. A section 5 cm from the shoot apex of the gametophyte was cut and soaked in an 8-oz glass bottle with 10 mL distilled water and kept in a growth chamber for three months before disinfection under an 8/16 h (dark/light) photoperiod at 15°C. This pretreatment procedure was done to promote gametophyte elongation and to reduce the amount of green microalgae and fungi associated with the gametophytes. Clorox bleach (6% active NaOCl) was used as a disinfectant. Explants that had been pretreated for three months were cut to 5 mm from the shoot apex and soaked in 8% Clorox with one to two drops of Tween 20 for 5 min. The explants were then rinsed with sterile distilled water and transferred to a Petri dish containing 20 mL of the tested media.
Effects of the Medium, Initial pH of the Medium, and Type and Concentration of the Sugar Added
Nine different media types were used; they were B5 (Gamborg et al., 1968), Knop (Knop, 1865), KnopME (Knop medium with microelements; Beike et al., 2015), Sph (Sphagnum medium; Knop medium with microelements and 1.25 mM NH4NO3; Beike et al., 2015; Heck et al., 2021), MS (Murashige and Skoog, 1962), 1/2MS, 1/4MS, 1/6MS, and 1/8MS. They were chosen based on previous reports on the propagation of vascular plants and bryophytes. These media were complemented with 0.8% (w/v) agar, a pH of 4.5, and 0.5% (w/v) sucrose for culturing. The initial pH of each medium was set to 3.5, 4.0, 4.5, 5.0, 6.0, 7.0, or 8.0 in the liquid 1/4MS medium. Five replicates were used for the experiments with the media and the initial pH. Four sugar types, glucose, fructose, sucrose, and raffinose, were used at concentrations of 0%, 0.5%, 1.0%, 1.5%, and 2.0% w/v in the solid 1/4MS medium for the investigation of their effects on the S. cuspidatulum culture with five replicates. The negative control was the 1/4MS medium without sugar.
Growth Conditions and Growth Measurements
All of the treatments were cultured at 25 ± 2°C under an 8/16 h (dark/light) photoperiod under 20 µmol·m-2·s-1 from cool white fluorescent lamps for six to ten weeks. Each week, the growth of S. cuspidatulum was determined using the dry weight, which was obtained by drying in an oven at 40°C for one week. The chlorophyll content was measured in the explants at week 6.
Extraction and Calculation of the Total Chlorophyll Content
A fresh sample weighing 0.1 g was soaked in 10 mL of dimethyl sulfoxide (DMSO) in the dark for 12 h at 25 ± 2°C. After centrifugation, the supernatant was measured with a G10S UV-Vis spectrophotometer (Thermo Fisher Scientific, USA) at 645 nm and 663 nm. The total chlorophyll content (in milligrams per gram fresh weight) was calculated based on the absorbance at 645 nm (D645) and 663 nm (D663) using the formula from Alpert (1984), where the total chlorophyll in milliliters of solvent per gram of fresh weight was calculated by the equation 0.00802 x D663 + 0.0202 x D645.
Statistical Analysis
The means and standard errors of each treatment were calculated throughout the study. The statistical significance between the mean values was assessed using a one-way ANOVA, with Tukey's HSD as the post-hoc test. A p-value of 0.05 or lower was considered significant, as in work associated with RStudio ver. 2022.12.0+353 (RStudio Team, 2022).
Results
Effects of the Culture Media on the Growth of S. cuspidatulum
After four weeks, the dry weights of S. cuspidatulum cultured in a 1/4, 1/6, or 1/8 strength MS, B5, or the Sph medium were found to have increased greatly (p < 0.001). At week 6, the 1/6MS treatment induced the highest dry weight (p < 0.001; highly significant), sustaining a similar dry weight until week 10 (Fig. 1A). By week 10, the 1/4MS treatment showed the highest dry weight, followed by the Sph, B5, 1/6MS, and 1/8MS media (p < 0.001) (Fig. 1A).
The total chlorophyll content at week 6 was significantly different among the treatments. The highest chlorophyll content was observed in the MS medium, followed by the 1/2MS, 1/4MS, Sph, B5, and 1/6MS media. The 1/8MS, KnopME, and Knop media yielded the lowest chlorophyll content in the S. cuspidatulum explants (Fig. 1B). The MS, 1/2MS, and 1/4MS media showed a significantly higher total chlorophyll content than the other media. The gametophytes of S. cuspidatulum in the MS, 1/2MS, and 1/4MS media appeared to be smaller (i.e., with a lower dry weight) with a darker green color and more compact shoots when compared with the other treatments (Fig. 1A and 1D).
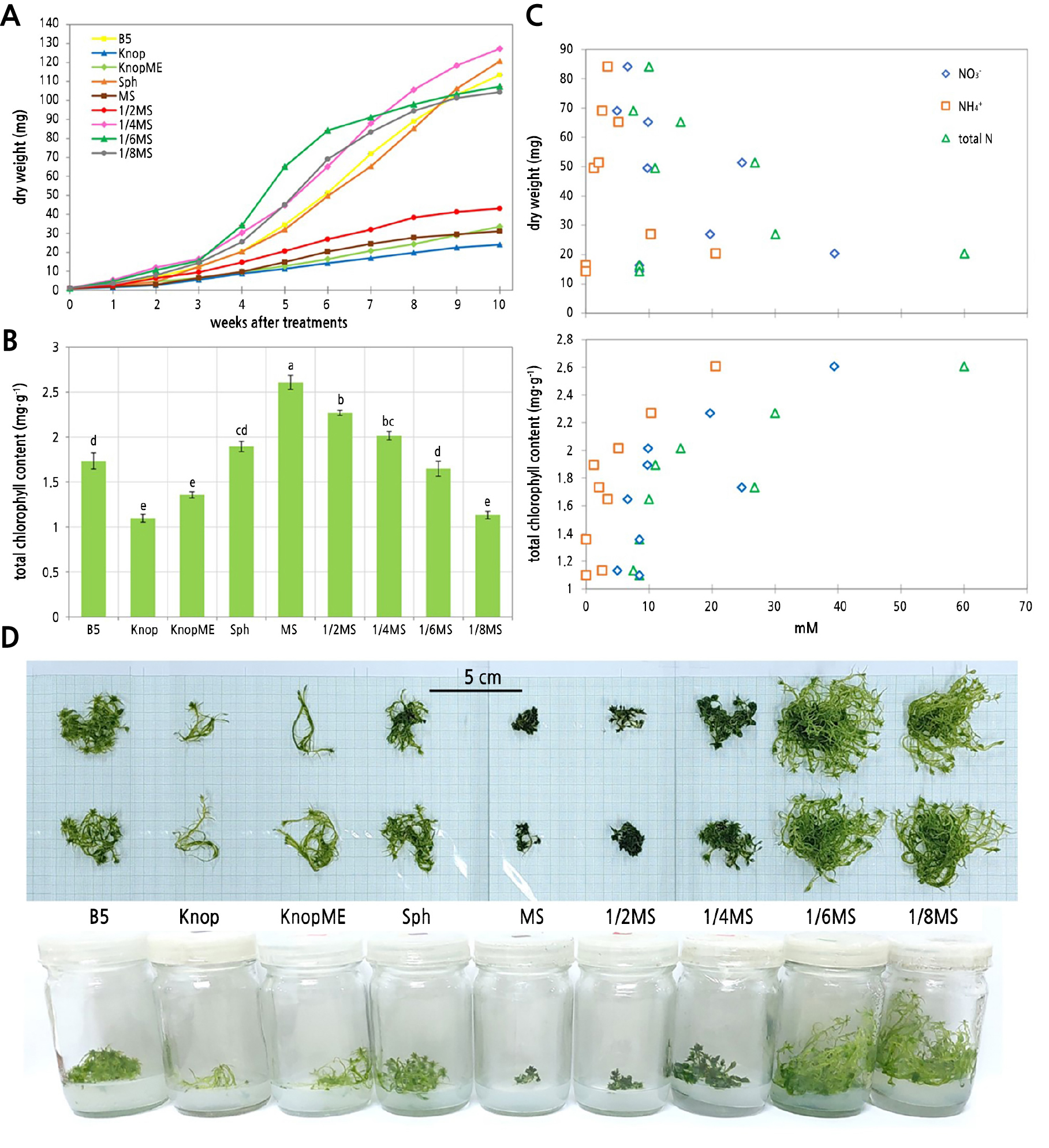
Fig. 1.
Effect of culture media on the dry weight (A) and total chlorophyll content (B) at week 6. Effect of total nitrogen, nitrate, and ammonium on the dry weight and total chlorophyll content at week 6 (C). Growth of S. cuspidatulum at week 6 in different media (D). Each point is the mean ± SE (n = 5). Bars with different letters in the same series indicate significant differences when analyzed by the Tukey HSD test (p < 0.05).
Effects of the Initial pH of a Medium on the Growth of S. cuspidatulum
The pH in all treatments gradually declined to 2.7 to 2.99 at week 6 of cultivation (p = 0.002) (Fig. 2A). The dry weight of all treatments increased with time. Cultures with an initial pH of 6.0 or 7.0 had the highest dry weight, 28.2 mg, at week 6. The dry weight continued to increase after week 4, except in the culture with an initial pH of 8, where it became stable (p = 0.387) (Fig. 2B). The total chlorophyll content was measured at week 6 and showed no significant differences among the treatments (p = 0.919) (Fig. 2C). It was found that an initial pH of a medium in the range of 3.5 to 7.0 can be used for S. cuspidatulum cultivation.
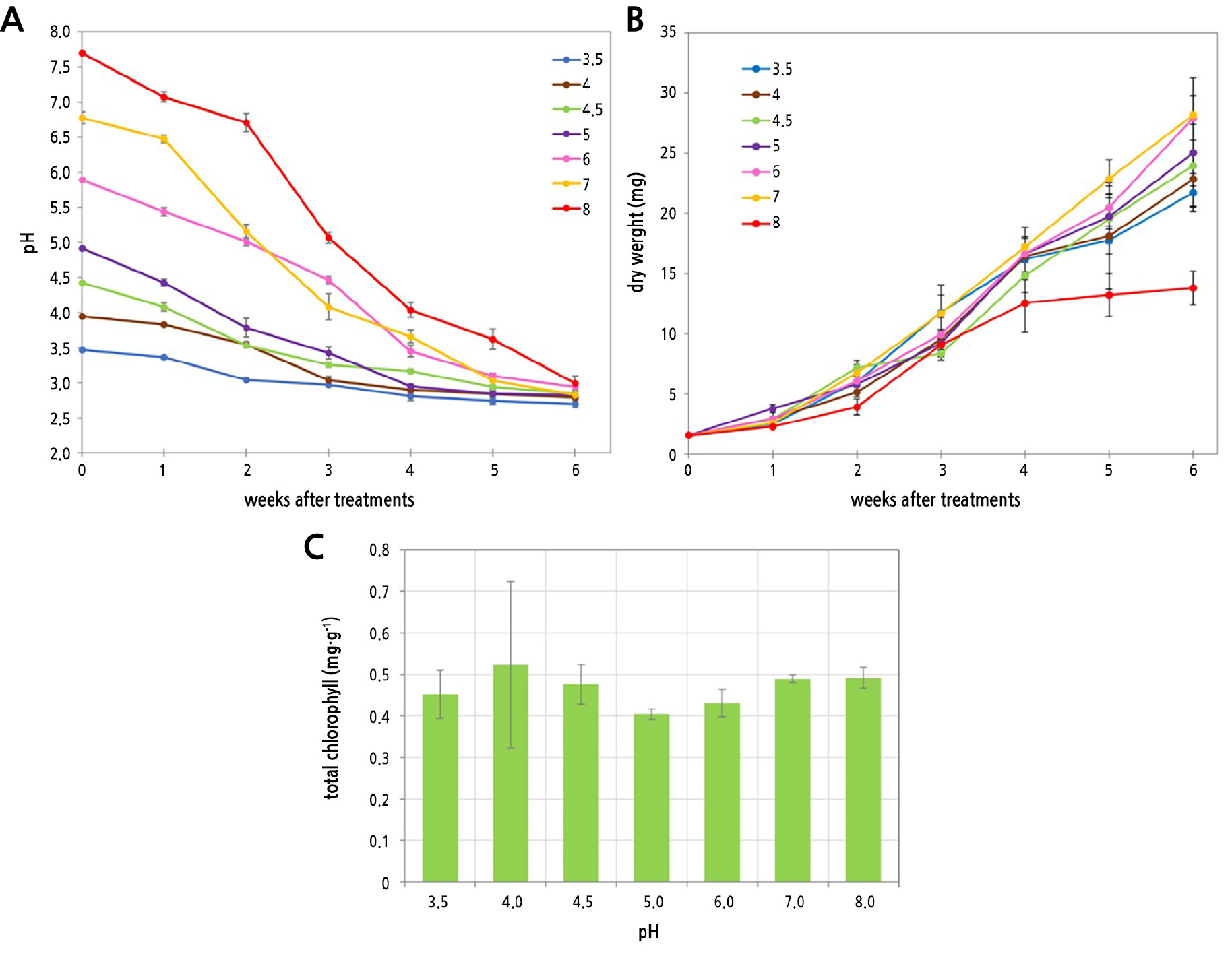
Fig. 2.
Culture pH (p = 0.002) (A) and dry weight (B) of S. cuspidatulum during six weeks of cultivation in media with different initial pHs (p = 0.387). Total chlorophyll content of S. cuspidatulum after growth in media with different initial pHs for six weeks (C). Each point is the mean ± SE (n = 5).
Effects of the Sugar Type and Concentration on the Growth of S. cuspidatulum
After six weeks of cultivation, glucose and sucrose yielded significantly higher dry weights of S. cuspidatulum than the other sugar types (p < 0.001). The dry weight of the explants grown in glucose increased substantially after the third week and continued to increase until the sixth week, resulting in a final weight of 126.32 mg. The second most suitable sugar for S. cuspidatulum growth was sucrose. In media with sucrose, the dry weight of the explants increased markedly in the fifth week and reached a final weight of 89.36 mg at the end of week 6. The final dry weights of explants grown in media with fructose or raffinose were 12.09 mg and 20.21 mg, respectively. These weights were not significantly different from the weight of the control (Fig. 3A). The dry weights and chlorophyll contents of S. cuspidatulum were compared among the media containing glucose and sucrose at different concentrations. These result showed that the 2% glucose and 1.5% glucose treatments produced significantly higher dry weights of S. cuspidatulum explants, at 170.98 mg and 166.42 mg, respectively, followed by the 1.5% sucrose (132.76 mg) and 2% sucrose (119.62 mg) treatments (p < 0.001) (Fig. 3B). The total chlorophyll content of the S. cuspidatulum explants was highest in the 0.5% glucose treatment at a fresh weight of 1.57 mg·g-1, followed by the medium without sugar (1.18 mg·g-1 fresh weight) and the medium with 1% glucose (1 mg·g-1 fresh weight). In contrast, the 2% sucrose treatment had the lowest chlorophyll content of the explant at a fresh weight of 0.15 mg·g-1 (p < 0.001) (Fig. 3C). In general, the glucose treatments yielded a higher total chlorophyll content than the sucrose treatments.
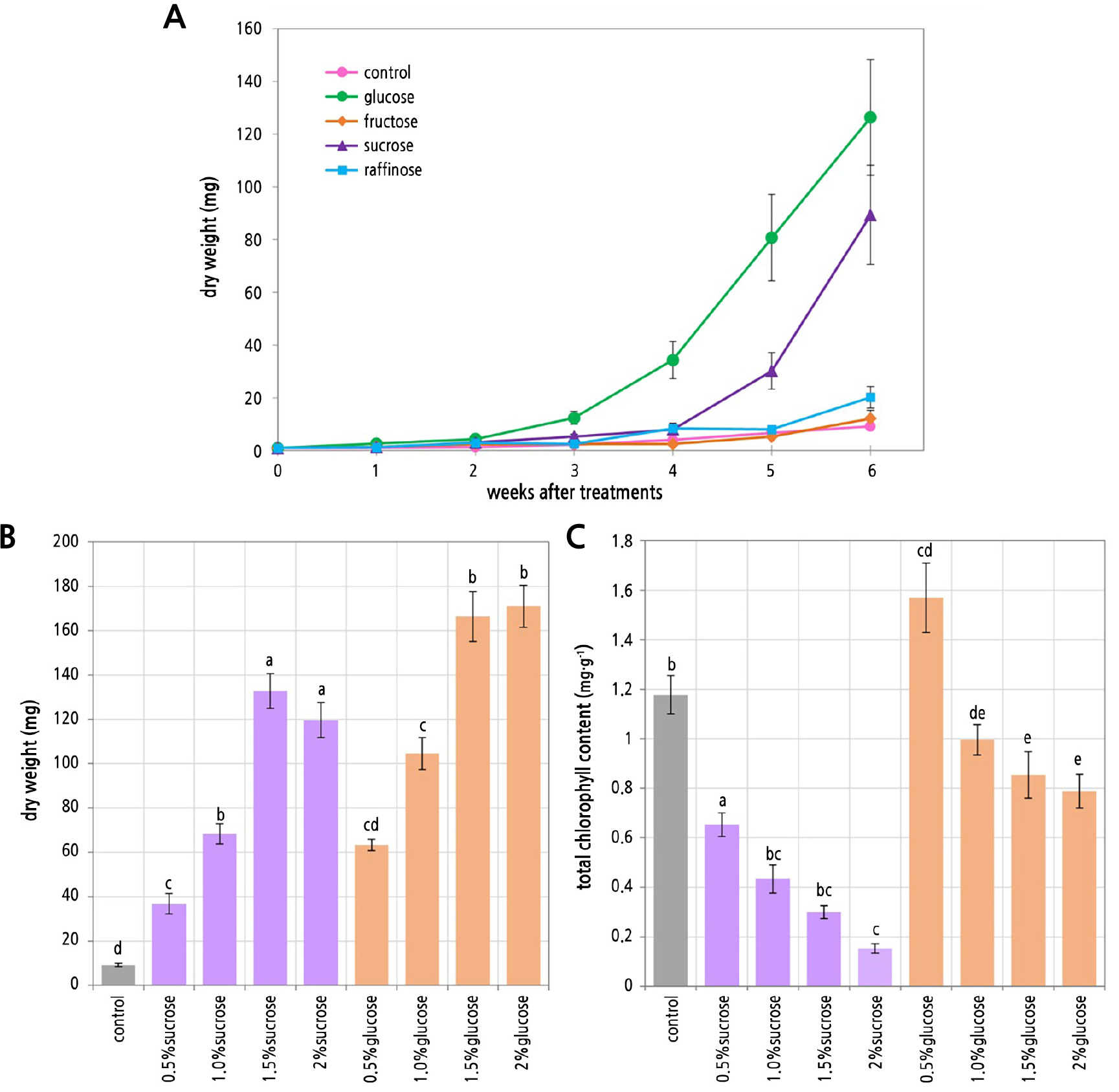
Fig. 3.
Effect of four different sugars on the dry weight of S. cuspidatulum (A). Effect of the glucose and sucrose concentrations on the dry weight of S. cuspidatulum at week 6 (B). Effect of the glucose and sucrose concentrations on the total chlorophyll content of S. cuspidatulum at week 6 (C). Each point is the mean ± SE (n = 5). Bars with different letters in the same series indicate significant differences when analyzed by the Tukey HSD test (p < 0.05).
Discussion
Explants of S. cuspidatulum were successfully multiplied during in vitro cultivation. The MS media yielded the highest dry weight and chlorophyll content after six weeks, but the results varied with the strength of the media. The full-strength (MS) and 1/2MS media produced significantly lower dry weights. This may be related to the amount of each nutrient, particularly nitrogen, in the MS and 1/2MS media, which possibly exceeded the demands of S. cuspidatulum (Fig. 1C). Previous physiological studies focusing on Sphagnum have also reported reduced growth under elevated nitrogen levels (Gunnarsson and Rydin, 2000; Chiapusio et al., 2022). In many Sphagnum species, reduced growth was associated with increased photosynthesis, although the responses differed among species in the Cuspidata section (Chiapusio et al., 2022). Therefore, it is possible that the increased nitrogen level led to an increased investment in a plant’s photosynthetic apparatus and pigments (Fig. 1C) coupled with reduced allocation of nitrogen to the structural carbon in leaves and stems and/or excess nitrogen, causing the stunted growth in the observed tissues (Limpens and Berendse, 2003).
For our in vitro culture, the MS medium contained both a nitrate and ammonia, both of which accounted for a relatively high nitrogen content about 60 mM, while the other media in this study had lower nitrogen contents. For example, the Knop and KnopME media only contained approximately 8.5 mM of nitrate, while the Sph medium contained approximately 8.5 mM of nitrate and approximately 1.25 mM of ammonium (Table 1). The effect of the increased nitrogen content in the MS medium was evident from the total chlorophyll content, as the explants grown under the MS treatments generally had a higher total chlorophyll content and a darker green color compared to the other treatments (Fig. 1B and 1D). The total chlorophyll content decreased with lower strengths of MS, which also had a lower level of nitrogen (Fig. 1B). The effect of the nitrogen content was also supported by the total chlorophyll content of the B5 treatment, which was similar to that of the 1/2MS treatment. The B5 medium contained 26.76 mM of total nitrogen, which was in the same range as the 1/2MS medium at 30.01 mM. A previous study of the in vitro propagation of Sphagnum palustre also used the Knop medium, which only contained 8.47 mM of nitrate (Beike et al., 2015). It is likely that a suitable medium for S. cuspidatulum is similar to that of other species in requiring only a small amount of nitrogen. In our case, we propose the 1/6MS and 1/8MS media to be suitable for obtaining a high dry weight in a short period, while the 1/4MS, B5, and Sph media may be more suitable for gradual growth cultivation.
Table 1.
Comparison of nutrient contents in culture media for the cultivation of Sphagnum cuspidatulum
Sphagnum cultivation often uses a buffer to maintain a stable pH in the culture media (Haraguchi et al., 2003). Without a buffer, the pH of a Sphagnum culture medium decreases over time (Beike et al., 2015). The medium's pH decreased due to acid formation by Sphagnum, similar to the process in natural bogs (Vitt, 2000; Sjörs and Gunnarsson, 2002; Hájek and Adamec, 2009; Beike et al., 2015). In another study of the in vitro propagation of Sphagnum, the pH of the medium for S. palustre L. decreased from 4.0 to 2.8 within four weeks, but by week 6, the medium’s pH had increased (Beike et al., 2015). Either the reduction of the medium’s pH did not affect the growth of Sphagnum or Sphagnum was able to grow in a wide range of pHs, from 3.6 to 6.7 (Gąbka and Lamentowicz, 2008; Wojtuń et al., 2013). Therefore, the initial pH of the culture medium did not significantly impact the outcome of the micropropagation of S. cuspidatulum.
Sugar types and concentrations significantly affected the cultivation of S. cuspidatulum. Sugar is a necessary alternative carbon source for in vitro conditions (Yaseen et al., 2013; Panathula et al., 2014). In vivo plants can obtain carbon from atmospheric carbon dioxide through photosynthesis (Yaseen et al., 2013). Sugar also is essential for maintaining the osmolality and osmotic potential in culture media. Furthermore, osmotic effects occasionally occur with an equal weight of the sugar. Glucose is a reducing sugar that generally supports better in vitro growth and organogenesis than sucrose. Sucrose is a non-reducing sugar that is usually hydrolyzed totally or partially into glucose and fructose in culture media. Although sucrose is the most popular sugar used in tissue cultures (Romano et al., 1995; Cuenca and Vieitez, 2000; Ovono et al., 2009; Yaseen et al., 2013), it sometimes inhibits chlorophyll formation and photosynthesis, making autotrophic growth less feasible (George et al., 2008; Bhatia, 2015). In comparison, fructose and raffinose were less effective for S. cuspidatulum cultivation. The reported fructose toxicity in some plants after autoclaving (Bhatia, 2015) may be related to the reduced growth of the S. cuspidatulum tissue. Raffinose is a trisaccharide that can be hydrolyzed to provide glucose, galactose, and fructose. Galactose is similar to fructose in terms of its toxicity to plant tissues (Bhatia, 2015). Therefore, we propose glucose and sucrose as optimal carbon sources for the micropropagation of S. cuspidatulum.
High sucrose concentrations appeared to inhibit the greening of the S. cuspidatulum cultures. Our study also found that 1% to 2% sucrose slightly reduced the chlorophyll content of S. cuspidatulum compared to glucose. However, if the cost of large-scale propagation is a concern, sucrose can be a good alternative, as it is almost universally used for most practical purposes during the micropropagation of many plants (Romano et al., 1995).
Conclusion
The most suitable medium for the in vitro cultivation of S. cuspidatulum was the MS medium, with the 1/6MS producing the highest dry weight at week 6. The 1/8MS and 1/4MS types can also be used to produce similar results. The initial pH of the medium did not affect the micropropagation outcome. The addition of glucose and sucrose at 1.5% to 2% to the medium increased the dry weight of the explants. Therefore, both of these sugars should be used as additions for the successful propagation of S. cuspidatulum. This set of conditions should be tested with plants from other sources as well as for other species of Sphagnum as continuing efforts to realize the ex situ conservation of this genus.


