Introduction
Materials and Methods
Subjects
Laboratory Environment
Materials
Analysis of Fragrance Components of the Flowers (Rose and Orchid)
Experimental Procedure
EEG Measurements and Analysis
Hedonic Evaluation of Emotions Aroused by Olfactory Stimulation
Results
EEG Changes Induced by Olfactory Stimulation with Floral Fragrances
Differences in Emotional Perceptions of Floral Fragrances
Evaluation of Olfactory Preferences Using the A1 Index
Discussion
EEG by Electrode Site
Brain-map Patterns
Emotional Descriptors Expressing Scent for Self-report Evaluation of Floral Fragrance
Comparison between the A1 Index-based Hedonic Evaluation and Questionnaire Results
Conclusions
Introduction
The human olfactory sense (Brodal, 1981) has long been neglected by researchers. A recent study on the olfactory sense noted that even though the proportion of the brain occupied by olfactory sensory neurons is small, this does not undermine the importance of the olfactory sense (Shepherd, 2004). Bushdid et al. (2014) demonstrated that human olfactory function has evolved to differentiate a trillion different odors, thus demonstrating the importance of olfaction for humans. According to one study, physiological reactions to olfactory stimulation by plant scents can affect brain activities and moods even when the scent is not consciously perceived (Lorig et al., 1990). Also, the subtle scents of flowering indoor plants used in horticultural therapy had positive effects on patients in a convalescent state (Park and Mattson, 2008), thus supporting the influence of plants on psychological and emotional states. Additionally, horticultural plants helped subjects attain emotional serenity and improved self-esteem and pride as the result of consciously enjoying the scents of plants and contemplating them (Kim et al., 2011). Furthermore, floral fragrances have been reported to increase brain activity resulting in increased locomotion, linguistics, and memory (Jo et al., 2013). Previous studies also suggest that the alpha 2 brain waves from subjects increased due to the release of valeric acid (Brauchli et al., 1995). Such results highlight the importance of the relationship between humans and plants. To gain practical insights into the therapeutic aspects of horticultural interventions and activities, the physiological and psychological aspects of human sensory organs should be measured and analyzed.
Electroencephalographs (EEGs) are usually used for clinical detection of brain diseases and have been widely applied to cognitive, psychological, and physiological diagnosis of epilepsy, sleep disorders, coma, and encephalopathy (Niedermeyer and da Silva, 2004), as well as being a mainstay in basic neuroscience research. EEG-based experimental studies have been conducted on olfactory brain regions (Jo et al., 2013; Liu et al., 2003) and on the effects of olfactory stimulation by plant essential oils (Martin, 1998) in various subjects such as animals (Oosawa et al., 2000), elderly women (Kline et al., 2000), students (Brauchli et al., 1995; Klemm et al., 1992; Van Toller et al., 1993), and newborn babies (Kendal-Reed and Van Toller, 1992). In the present study, we investigated the physiological and psychological effects of plant scents on humans using quantitative electroencephalographs (QEEGs) and questionnaire analysis to elucidate the basis of physiological and psychological theories, respectively.
Materials and Methods
Subjects
To study the effects that olfactory stimulation by floral scents has on human brain waves and moods, we recruited 44 volunteers in two different age ranges. Group 1 consisted of 11 male and 14 female college students with a mean age of 24.5 years (± 2.23) and Group 2 consisted of 10 males and 9 females with a mean age of 54.3 years (± 2.98). The two groups were selected in order to understand differences related to age and gender.
The selection criteria included right-handed, un-medicated individuals with normal olfactory functions (unaffected by conditions such as the common cold) and the absence of mental disorder, abnormal physical conditions, visual impairment, brain disorder, and heart disease. The subjects were requested to refrain from alcohol consumption for 48 hours prior to the experiments (Son et al., 1998). During the two hours prior to QEEG and olfactory experiments, smoking and the intake of drinks containing caffeine or other stimulants were prohibited. Subjects submitted their informed consent forms before beginning the experiments, after explanation of the experimental purpose and procedures.
Laboratory Environment
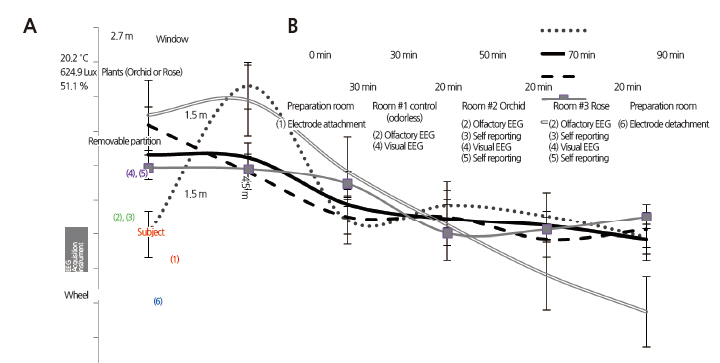
To measure EEG signals and administer the questionnaire, we used laboratory rooms at the Korea University in Seoul in April, 2013. These rooms had grayish-white walls and west-facing windows and had dimensions of 4.5 m (L) × 2.7 m (W) × 2.8 m (H). Light from the outside was shielded to prevent it from influencing the subjects’ moods and the fluorescent ceiling lights were kept on. A vase of orchids or roses was placed on a table (75 × 150 × 90 cm) at a distance of three m from the chair in which the subjects sat. To prevent visual stimuli from influencing olfactory perceptions, a partition was installed between the chair and the table, 1.5 m from the chair. The average illumination, temperature, and humidity of the rooms were held at 624 ± 17 lux, 20.2 ± 2.2°C, and 51 ± 3.4%, respectively.
Materials
The subjects underwent the experimental procedures in three different spaces with different olfactory conditions: 1) the Control space in which no olfactory stimulus was provided (control); 2) the orchid space in which orchids (Cymbidium faberi) were present as the first olfactory condition; and 3) the rose space in which roses (Rosa hybrida ‘Hera’) were present as the second olfactory condition.
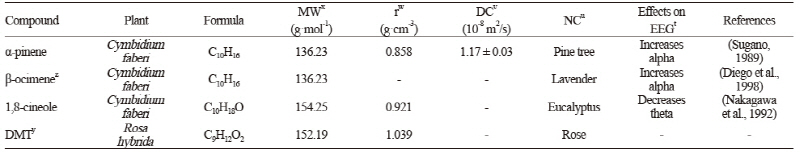
Analysis of Fragrance Components of the Flowers (Rose and Orchid)
For aroma collection, we picked a flower on the fifth day of its full bloom, when scent peaks (Hsiao et al., 2008), and analyzed the aromatic compounds with a gas chromatograph mass spectrometer (GC/MS) at the Cooperative Center for Research Facilities of Sungkyunkwan University (Table 1).
Experimental Procedure
EEG signals were measured to determine the psychophysiological effects of the floral fragrances. Electrodes were attached to each individual’s scalp in the preparation room and the experiments were conducted in the control (Room #1), the orchid condition (Room #2), and the rose condition (Room #3). Each subject, after being led into a randomly selected room, was (1) seated in a relaxed and comfortable position, (2) instructed to smell orchid and rose floral fragrances with a physically recognizable concentration for three minutes while EEG measurements were taken, and (3) asked to complete a questionnaire regarding olfactory perceptions. (4) After the subject finished the questionnaire, the partition was removed to expose the source of the olfactory stimulus and visual EEG measurements were made, (5) followed by a second questionnaire. Self-reported questionnaires were not administered in the control. Fig. 1B depicts the entire experimental procedure, which lasted about 1.5 hours per subject.
EEG Measurements and Analysis
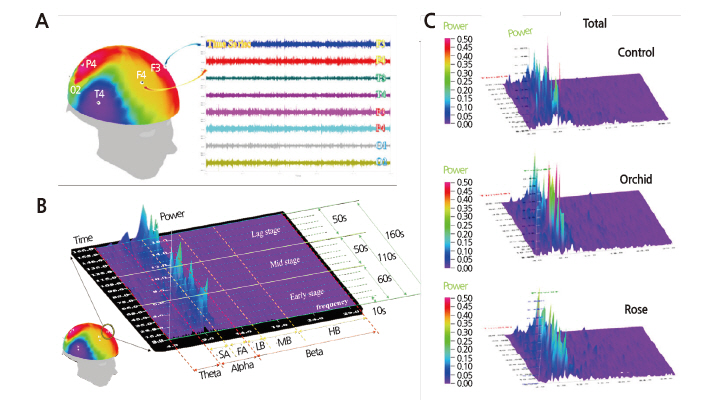
EEG signals were measured and recorded using the EEG and brain mapping system QEEG-8 (LXE3208, Laxtha Inc., Daejeon, Korea). EEG potentials were measured at eight electrode sites: the left frontal (F3), right frontal (F4), left temporal (T3), right temporal (T4), left parietal (P3), right parietal (P4), left occipital (O1), and right occipital (O2) lobes, using unipolar electrodes in accordance with the 10/20 International System of Electrode Placement (Homan et al., 1987). Electrodes used as the reference electrodes were A1 and A2, indicating the ground electrodes placed behind the left and right ears, respectively (Fig. 1B). The A1 index, which indicates the difference in the absolute alpha between F4 and F3, was reported to be significant for fragrance testing in assessing olfactory preferences (Kline et al., 2000). We used this index as an EEGbased indicator of the subjects’ olfactory preferences and compared the values of the A1 index for orchid and rose with the principal component analysis and semantic differential scores to check whether the physiological and psychological olfactory preferences coincided. The index was calculated from the following formula:
A1=ln√(AA(R) - ln√(AA(L)
AA (R) = Absolute alpha spectrum power of F4 (Right)
AA (L) = Absolute alpha spectrum power of F3 (Left)
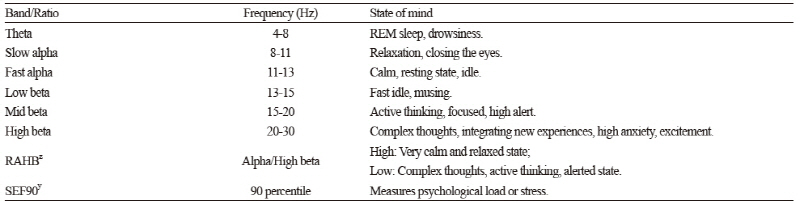
Electrode impedance was measured to be lower than 5 kΩ. The EEG signals were bandpass filtered at 0.5-50 Hz, sampled at a frequency of 256 Hz, and digitized at a resolution of 12 bits. EEG signal intensities were compared as spectral power in multiple reference bands using the fast Fourier transform (FFT) algorithm (Subha et al., 2010). The four reference EEG frequency bands were theta (4-8 Hz), alpha (8-13 Hz), beta (13-30 Hz), and gamma (30-50 Hz). Reference sub-bands were absolute slow alpha (8-11 Hz), absolute fast alpha (11-13 Hz), absolute low beta (13-15 Hz), absolute mid-beta (15-20 Hz), and absolute high beta (20-30 Hz). The ranges and characteristic features of these frequency bands are presented in Table 2. Derived frequency-domain measures were the ratio of alpha (8-13 Hz) to high beta (15-30 Hz), an indicator of the degree of calmness and relaxation, and 90% spectral edge frequency, an indicator of mental load and stress (Table 2).
Table 2. Brain states across the power spectrum used in electroencephalography (Subha et al., 2010).
| |
zRAHB: Ratio of alpha to high beta ySEF: Spectral edge frequency | |
First, from the complete EEG recording of 180 sec in eight channels, the range of 5-165 sec was selected by excluding the initial 5 and final 15 sec for noise minimization. A total of 24 graphs were output using the compressed spectral array method (Fig. 2B and 2C). The compressed spectral array data were classified into the basic EEG bands theta, alpha, and beta, and the olfactory EEG signal changes were quantified by calculating the brainwave index over three time series taken from the initial (60 sec), middle (50 sec), and late (50 sec) stages of odorant exposure. Traces were visually monitored by the experimenter for evidence of eye movement or electrode displacement. Mean values of the EEG signals of individual electrodes were calculated and used for mapping response data onto brain regions. Cortical regions with low and high electric potentials were marked blue and red, respectively. Pre- and post-processing of EEG data was performed with Telescan 2.8 software (Laxtha Inc., Daejeon, Korea). PASW software (PASW Statistics 18.0, IBM Corporation, Armonk, NY) was used for all statistical analysis.
Hedonic Evaluation of Emotions Aroused by Olfactory Stimulation
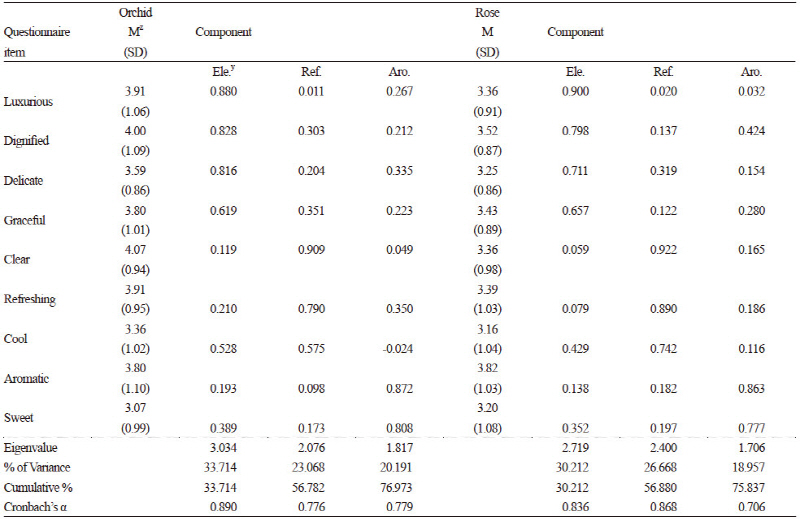
This test was compiled by adapting the semantic differential method proposed by Osgood et al. (1957). The semantic differential was used to rate the subjects’ emotional responses to rose and orchid fragrances. To create semantic differential items, descriptors expressing detailed olfactory perceptions were listed, from which 20 descriptors were selected by 26 fragrance experts. For the semantic differential rating, a five-point Likert scale was used to reduce the cognitive demands of testing, thus reducing possible confounding physiological reactions (Friborg et al., 2006). We conducted a preliminary survey with these 20 self-report questionnaire items on 151 Korea University students and evaluated the preliminary questionnaire feedback (Chung et al., 1999) applying the semantic differential technique (Osgood et al., 1957). We extracted 17 descriptors from this data, including “good,” “clear,” “cozy,” “refreshing,” “graceful,” “clean,” “crisp,” “aromatic,” “delicate,” “unique,” “sweet,” “cool,” “provocative,” “luxurious,” “satisfactory,” and “dignified,” and used them as questionnaire items. We then used factor analysis to derive the following nine basic descriptors: “luxurious,” “dignified,” “delicate,” “graceful,” “clear,” “refreshing,” “cool,” “aromatic,” and “sweet” (Table 3). These nine descriptors were subjected to a principal component analysis, which clustered components into the three largest principal components as sequentially similar descriptor groups according to the size of variance. The first principal component was termed “elegant,” and clustered “luxurious,” “dignified,” “delicate,” and “graceful.” The second principal component was termed “refreshing,” clustering “clear,” “refreshing,” and “cool.” The third principal component was termed “aromatic,” clustering “aromatic” and “sweet” (Table 3). The objectivity of the principal component analysis was verified by the concordance of the principal components derived from the pre-experiment preliminary questionnaire and those from the experiment. With these data, the reliability, validity, and suitability of the 20 descriptors were tested. Factor analysis was performed to find common factors to be used as variables. Principal components were extracted, applying an eigenvalue cutoff of ± 0.40. Varimax rotation was used to simplify the factor structure, which yielded 17 descriptors to be rated on a multiple-item scale. Cronbach’s α was calculated to verify the internal consistency, i.e., the reliability of the descriptors in terms of homogeneity. A questionnaire consisting of 17 items with these descriptors, which were derived from the preliminary sensory assessment of the emotional responses to floral scents, was administered to the subjects. The questionnaires filled in by the subjects underwent factor analysis, from which items reaching statistical significance were derived. The selected descriptors were subjected to principal component analysis to derive the principal components of the sequentially similar descriptor groups clustering similar types of moods evoked by the olfactory stimuli. We converted the results into index values through statistical analysis. We then compared the indexed values of the emotions aroused by orchid and rose fragrances using a paired t-test. In consideration of subjects’ general tendency to overestimate fragrance on a Likert scale (Laerhoven et al., 2004), we set 3.5 (instead of the median 3.0) as the reference point for plotting the quadrant charts and analyzed the data of the subjects plotted in the first quadrant. The statistical analysis was performed using PASW software (PASW Statistics 18.0, IBM Corporation, Armonk, NY).
Results
EEG Changes Induced by Olfactory Stimulation with Floral Fragrances
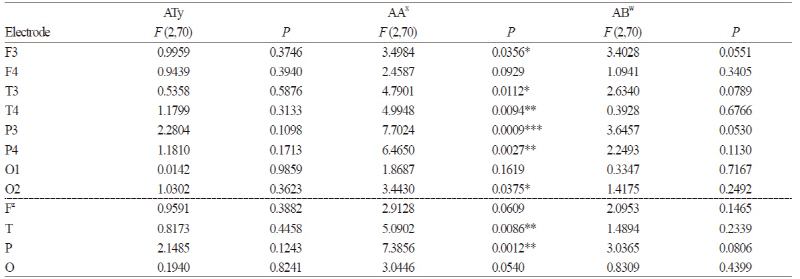
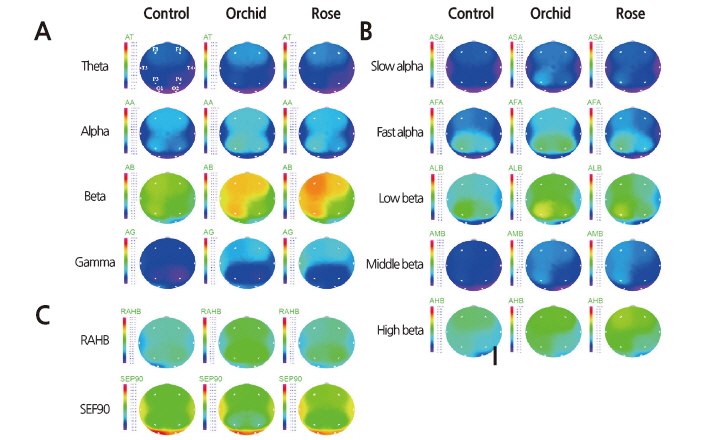
The EEG changes in different brain regions resulting from olfactory stimulation were investigated by performing EEG measurements on the subjects in an odorless room and in two rooms containing either orchids or roses with exposure times of 180 sec per stimulus condition. For the analysis, we selected the initial and middle exposure-time ranges of about 110 sec as having the best signal-to-noise ratios. The analysis revealed significant changes in absolute alpha at six of the eight electrode sites (F3, T3, T4, P3, P4, and O2; p ≤ 0.05), excepting only F4 and O1, with the greatest changes appearing at P3 (Table 4). After dividing the electrode sites into the four regions: frontal (F), temporal (T), parietal (P), and occipital (O), analysis of the QEEG measurements revealed significant spectral power increases in absolute alpha at P and T (p ≤ 0.01), especially at P (Table 4). Such changes in spectral power were not observed in other frequency bands such as absolute theta and absolute beta. The brain maps, which depict the spectral distributions of EEG activity over the eight electrode sites, demonstrated higher absolute alpha and absolute beta activations in the orchid condition compared with the control and rose conditions (Fig. 3). Absolute alpha (AA), representing serenity and relaxation, showed a significantly elevated activation in the orchid condition (Fig. 3A), especially at P3 and P4 (Table 4). Absolute beta (AB), which appears when concentrating or while executing intentional actions, showed a considerable increase in activation in the rose condition (Fig. 3A), especially at F3. Absolute theta (AT), which appears in a state of drowsiness or deep meditation, and absolute gamma (AG), associated with a state of anxiety or intense brain activity, did not exhibit significant inter-electrode differences in spectral power (Fig. 3A).
Brain maps for low frequency bands presenting a more detailed power spectrum revealed that the orchid fragrance triggered greater EEG activities than the control and the rose fragrance in the absolute slow alpha band representing relaxation and serenity, in the absolute fast alpha band representing concentration and rest, and in the absolute low beta band representing elevated concentration (Fig. 3B). Orchid showed the greatest activation at P3 and P4, while the control and rose showed similar power spectral distributions in the brain maps (Fig. 3B). As for beta power, no significant inter-condition differences were observed in absolute mid-beta (appearing in alert states or while executing intentional behaviors), with orchid showing greater activation than the other conditions. Rose provoked higher absolute high beta activation, which is indicative of anxiety or stress (Fig. 3B). In the range of absolute beta power, rose and orchid showed similar spectra, with orchid showing the greatest absolute low beta power at P3 and rose showing the greatest absolute high beta power at F3 (Fig. 3B). A high ratio of alpha (8-13 Hz) to high beta (15-30 Hz) indicates relaxation and a low ratio indicates mental load and stress. The ratio of alpha to high beta was highest in the subjects’ EEG signals when they were exposed to the orchid fragrance (Fig. 3C). Consequently, the 90% spectral edge frequency, which has an inverse or complementary relationship with the ratio of alpha to high beta, showed the lowest values in the orchid condition (Fig. 3C).
Differences in Emotional Perceptions of Floral Fragrances
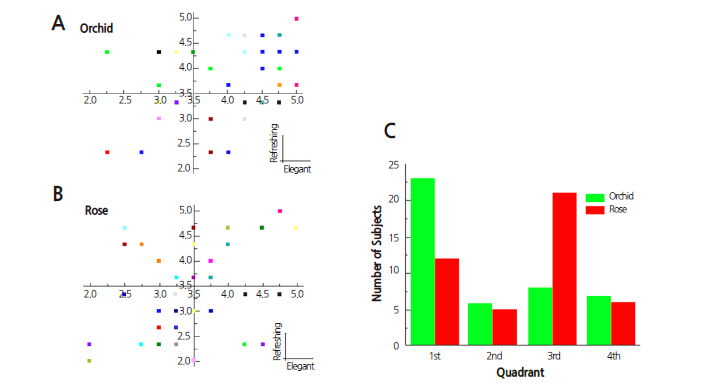
We designed a self-report questionnaire with fragrance-related descriptors to evaluate subjective perceptions of floral fragrances (Chung et al., 1999). First, we compiled a list of suitable descriptors expressing fragrance and reviewed them with 26 fragrance experts. Twenty descriptors were then selected as questionnaire items. Then we carried out a preliminary survey using a self-report questionnaire (Chung et al., 1999) and analyzed 151 questionnaires as preliminary feedback. Differential perceptions depending on the type of floral fragrance were investigated by a t-test comparing the orchid and rose conditions. The t-test revealed that orchid scored higher in the “elegant” (p ≤ 0.003) and “refreshing” (p ≤ 0.007) clusters, and the principal component “aromatic” showed no significant difference between rose and orchid (Fig. 4). Fig. 4 shows graphic representations of the principal component analysis of each subject’s questionnaire data plotted along the axes of the principal components. The analysis of the subjects’ data plotted in the first quadrant with 3.5 as the reference point yielded the finding that 23 out of 44 subjects (52%) answered that orchid is more elegant and refreshing than rose (Fig. 4A and 4C) and 21 subjects answered that the fragrance of rose is less elegant and less refreshing (Fig. 4B and 4C). No gender-dependent differences were observed, but an age-dependent difference was found in “refreshing” for rose (Fig. 4).
Evaluation of Olfactory Preferences Using the A1 Index
The A1 index calculated from the EEG data from 44 subjects was used to obtain olfactory preferences by a RMANOVA calculation. The index values indicated that orchid was preferred (p ≤ 0.05) in the mid stage (65-125 sec), as shown in Table 5. In order to determine the age-dependent differences, the results obtained were analyzed by age group (individuals in their 20s or 50s). Persons in their 50s preferred orchid to rose throughout the entire experimental time of 160 sec (p ≤ 0.05), especially in the late stage (115-165 sec) nearing the terminal phase (p ≤ 0.038). The results from the EEG-based hedonic evaluation and from the scores of the self-report questionnaire coincided in that the subjects in their 50s had higher preferences for orchid compared with subjects in their 20s.
Discussion
EEG by Electrode Site
EEG-based analysis of the effects of olfactory stimulation by floral fragrances found significant changes (p ≤ 0.05) in absolute alpha power at six of the eight electrode sites (F3, T3, T4, P3, P4, and O2) (Table 4). Pairwise EEG channel analysis also revealed significant signal increases in absolute alpha in four major brain regions (F, T, P, and O) (Table 4), consistent with reports that alpha wave power increases upon inhaling pleasant odors (Buckle, 1999) and that pleasant olfactory stimulation activates the alpha band at T and P (Ryoko et al., 2000). Previous studies have reported differences in alpha activation in the temporal and parietal lobes when subjects were presented with scents versus no scent (Ryoko et al., 2000; Peter et al., 1995; Sawada et al., 1992). According to the results of a study that employed multichannel near-infrared spectroscopy to investigate the effects of cherry blossom scent on cerebral activity, the autonomic nervous system is stimulated by inhaled floral scents, leading to elevated brain wave activity in the temporal lobe (Jo et al., 2013).
Alpha waves, which typically appear in a physically and mentally relaxed state (Ray and Cole, 1985), are usually associated with a serene and pleasant state of mind (Orne et al., 1979) and thus are especially well studied relative to the other frequency bands of human cerebral activity (Cohn, 1946; Jasper, 1936). According to a study investigating wave function (Anna, 1995), these waves bridge the conscious and unconscious realms, and the absence of alpha waves is equivalent to the absence of subconscious activity. Past experiences and memories, however vivid, cannot be remembered in the absence of alpha waves, thus highlighting the function of alpha waves as a bridge between deep and surface levels of consciousness. The results of our study, indicating that olfactory stimulation aroused by the orchid fragrance activated the alpha band, may be interpreted in this context; the elevated alpha wave activity influences the subconscious (deep consciousness) and thus evokes memories and relaxes the body and mind. This enhances our ability to deal with situations at the level of surface consciousness. This assumption is supported by the results of a study in which subjects engaged in horticultural-related activities. Subjects could enhance their serenity and self-esteem by regularly practicing contemplation and inhalation of orchid scents, which increased their selfconfidence, happiness, and satisfaction (Kim et al., 2011). Accordingly, given the effects of orchid scent in inducing pleasant moods and relaxing the body and mind by activating alpha brain waves, orchids may be used in therapeutic applications, such as gardening and horticultural activities, for inducing psychological well-being, and enhancing self-esteem.
Brain-map Patterns
Our EEG brain maps showed that absolute alpha waves had the greatest spectral power when stimulated by the orchid scent (Fig. 3), especially at P3 and P4 (Fig. 3A). This result is consistent with a previous study that found that a pleasant scent activated the alpha band in the parietal lobe (Ryoko et al., 2000). The values of the absolute slow and fast alpha waves, the ratio of alpha (8-13 Hz) to high beta (15-30 Hz), and the 90% spectral edge frequency (Fig. 3B and C) all indicated that orchid scent boosts alpha activity. Previous studies reported that orchid scent activated theta and alpha waves, which are low-frequency bands (Sugano, 1989; Diego et al., 1998; Nakagawa et al., 1992). In our study, however, orchid scent considerably stimulated absolute low- and mid-range beta waves as well (Fig. 3B). This is consistent with a previous study, which showed that pleasant olfactory stimulation with phenylethyl alcohol induced an increase in absolute alpha and in absolute beta waves in all brain regions (Peter et al., 1995). This may be assumed to be due to the activating effect of orchid-scent stimulation on alpha and mid-range beta waves, resulting not only in emotional relaxation and lower mental load, but also in enhanced concentration, thus improving cerebral functioning.
Absolute beta waves were activated most intensely when subjects were exposed to the rose scent. According to one study, rose scent is associated with the absolute beta waves typically present in an alert state and accompanying conscious activities (Subha et al., 2010). Low-range beta waves are called active waves and are conducive to enhanced concentration and learning capacity; they appear during concentrated mental activities such as learning, memorization, and calculation. However, the brain maps presenting more detailed power spectra revealed that the rose scent also enhanced absolute high beta (Fig. 3B). In a study comparing the scents of rose, lemon tree, jasmine, and lavender flowers, rose was reported to have the most intense and unpleasant scent (Min et al., 1999). Previous studies have noted that absolute high beta is indicative of stress or anxiety (Hutchison, 1996; Schacter, 1977; Vernon et al., 2004). Taking the results of our study together with those of previous studies, we infer that while a moderate amount of rose scent can contribute to enhanced concentration and learning efficiency, excessive use of roses in flower arrangements or bouquets in closed spaces may provoke anxiety and stress.
Emotional Descriptors Expressing Scent for Self-report Evaluation of Floral Fragrance
The analysis of the self-report questionnaire results revealed that subjects exposed to orchid and rose scents found the orchid scent more elegant and refreshing than the rose scent (Fig. 4). In the principal component analysis, “elegant” was identified as the first principal component that clusters the odor-related descriptors “luxurious,” “dignified,” “delicate,” and “graceful,” and “refreshing” was the second principal component clustering the descriptors “clear,” “refreshing,” and “cool.” These descriptors indicate the subjects’ positive sensory perceptions of the orchid scent. Orchid scent has long been appreciated as being superior, as demonstrated by metaphors such as national fragrance, prime fragrance, world’s number one fragrance, and princely fragrance (Lee, 2006). In the analyses of the preliminary self-report sensory assessment questionnaires, similar olfactory preferences were found after combined visual and olfactory stimulation by the flowers, and after olfactory stimulation only, with the flowers hidden behind a partition. Although odors are difficult to define and classify, as depicted by the expression “subjective representation of an objective event” (Candau and Jeanjean, 2006), the consistent results found in our study may be interpreted as the collective sociocultural olfactory perception and definition of the orchid’s scent, the product of shared perceptions of certain odors of high emblematic value engraved in the collective memory. The elegant and refreshing fragrance of orchids increases alpha brain waves, inducing a calm and comfortable mood, relieving stress, and relaxing tension, thus improving cerebral functioning and mental health. Descriptors associated with the rose scent were “sweet” and “aromatic”. According to the brain maps, the rose scent appears to be associated with absolute beta waves, leading to the assumption that the aromatic rose scent can contribute to enhancing the efficiency of activities executed at the surface level of consciousness and requiring alertness and concentration (Anna, 1995).
Comparison between the A1 Index-based Hedonic Evaluation and Questionnaire Results
The subjects’ overall olfactory preferences for orchids vs. roses were evaluated using the A1 index. The orchid scent preference was markedly higher in the mid-range of the measurement time (65-125 sec) (p ≤ 0.05) (Table 3). Brauchli et al. (1995) stated that olfactory stimulation with valeric acid led to different EEG activities depending on the measurement time range, and this finding is supported by the report that cerebral activity was clearly differentiated by the time range after being exposed to a fragrance, with the alpha-band activity greatly increased (Hummel et al., 2007). When comparing the hedonic evaluations by age group (individuals in their 20s vs. 50s), subjects in their 50s were found to prefer the orchid scent to the rose scent throughout the entire experimental time of 160 sec (p ≤ 0.043), with the difference increasing towards the terminal time range (115-165 sec) (p ≤ 0.038) (Table 3). This result may support the results of previous studies showing that persons in their 20s can easily distinguish scents and perceive them, whereas persons in their 50s tend to recall past olfactory experiences and stimuli aroused by the present odor (Royet et al., 1999). Favorite scents elicit old memories stored in the brain (Ehrlichman and Halpern, 1988), reactivate the emotions associated with long-dormant memories, and produce clear behavioral changes (Ehrlichman and Bastone, 1992). Considering these previous results, the results of our study, in that persons in their 50s preferred the fragrance of orchids, especially in the late time range of olfactory stimulation (115-165 sec), may be interpreted as the result of older subjects exhibiting slower recognition of a scent and a greater time requirement for defining the scent or for triggering the mental images and memories associated with the scent.
Studies on the fragrance and appearance of orchids conducted in the Asian cultural zone show tendencies to relate to orchids from a cultural perspective rather than from a botanical aspect. The orchid’s image and fragrance are emblematic of the attributes of a nobleman (Jung, 2005) in this population, thus symbolizing noble human attributes (Lee, 1983). This may be rooted in familiarity with the long cultural history of orchids in this region (Rouby et al., 2009).
The EEG-based hedonic evaluation (A1 index) and the self-report questionnaire results (principal component analysis and semantic differential scores) agreed in showing that persons in their 50s have a greater preference for the orchid scent than do persons in their 20s. This agreement between the A1 index and the questionnaire results verifies the A1 index as an efficient measure of olfactory preference, consistent with the results of previous research.
Conclusions
To determine the psychophysiological effects of floral fragrances, we exposed subjects to three odor conditions (odorless, orchid, and rose fragrances) and analyzed their EEG signals and self-reported olfactory preferences. Differences in absolute alpha wave activity were observed in temporal and parietal lobes, where the orchid scent produced the greatest activation. Brain-map patterns revealed that the orchid scent enhanced absolute alpha and midrange beta wave activities, which is conducive to relaxing mental tension, relieving stress, and facilitating concentration, thus enhancing brain function and triggering positive changes in mental health. The rose scent was found to enhance absolute beta activity, which is associated with alertness, conscious behavior, and elevated concentration, thus conducive to enhancing the efficiency of activities carried out at the surface level of consciousness. Comparative analysis of subjects’ emotional responses to orchid and rose scents showed that the orchid scent was perceived as being elegant and refreshing, and the rose scent as aromatic.
The purpose of this study was to investigate the psychophysiological changes that take place when persons are exposed to the fragrances of flowers in our daily lives. The results suggest that the orchid scent brings relaxation by activating alpha brain waves, inducing a positive mental state. Based on the results of this study, orchid and rose scents may find use in a variety of complementary and synergistic application areas, such as meditation and gardening as well as horticultural activities and therapeutic interventions. It is hoped that the findings of this study will contribute to improving the quality of life of many people by helping them maintain a pleasant and clear state of mind. This study is significant in that it determined the olfactory properties of Cymbidium faberi orchids and Rosa hybrida, ‘Hera’ roses aside from their aesthetic aspects. In a future study, we plan to extend this study to the olfactory and visual effects of orchids and other flowers in association with EEG activity.