Introduction
Materials and Methods
Plant Materials and Preharvest Conditions
Vase Life and Ornamental Quality
Water Relations
Cellular Component Contents
Petal Color and Vacuolar pH
Statistical Analysis
Results
Effects of Nocturnal Supplemental Lighting and Different Irrigation Regimes on Postharvest Performance of Cut Roses
Effects of Nocturnal Supplemental Lighting and Irrigation on Water Relations of Cut Roses
Effects of Nocturnal Supplemental Lighting and Irrigation on Cellular Components of Cut Roses
Effects of Nocturnal Supplemental Lighting and Different Irrigation Regimes on Petal Color and Vacuolar pH of Cut Roses
Discussion
Introduction
Roses (Rosa x hybrida) are one of the most economically important cut flowers and hold an important place in the florist trade. The vase life and postharvest quality are important factors that growers must consider during rose production. Postharvest quality in cut flowers is controlled by a combination of factors including multiple genetic factors and preharvest environmental conditions (Fanourakis et al., 2013). A strong relationship between the preharvest environment and variations in the vase life of cut roses has been demonstrated even when the flowers are held under optimal postharvest conditions (Slootweg et al., 2001; Pompodakis et al., 2005). Environmental factors contribute to the postharvest keeping quality of cut rose by affecting their ability to control postharvest water loss, which is closely related to their anatomical and physiological characteristics, and these are determined by growth conditions such as relative humidity (RH), vapor pressure deficit (VPD), and supplementary lighting in the greenhouse (Fanourakis et al., 2013; In and Lim, 2018). Previous studies have shown that rose plants grown under high RH and low VPD might have decreased vase life due to a reduction in stomatal responsiveness and an increase in water loss after harvest (Fanourakis et al., 2012). High air temperature in the greenhouse increased the cut flower water uptake after harvest, leading to shortening of the vase life (Moe, 1975). Velez-Ramirez et al. (2011) showed that the effect of light conditions on vase life depends on the photoperiod, light intensity, and light quality. Partial root drying was found to enhance stomatal responsiveness; however, variations in irrigation and its relationship with the vase life of cut roses has not yet been fully addressed.
Furthermore, the vase life of cut roses is most limited by water stress, especially uncontrolled water loss from the cut shoot mainly caused by high air humidity or excessive water supply during cultivation (Mortensen and Fjeld, 1998; Torre et al., 2003; Fanourakis et al., 2013, 2015). On the other hand, increasing the photosynthetic photon flux density (PPFD) during the growing period increases the vase life of cut roses (Fjeld, 1994). However, it also has been reported that growing plants under high-intensity supplementary lighting and under long days disturbs stomatal closure, resulting in increased water loss from the flowering shoot and, hence, early wilting after cutting (Slootweg and Meeteren, 1991; Mortensen and Fjeld, 1998). Treating roses with low RH for six hours before harvest resulted in significantly prolonged vase life as compared with growth under constantly high RH and continuous light (Mortensen et al, 2007). Potted miniature roses show less stress during post-production when they are watered cyclicallycompared to plants that are treated with a constant supply of water, irrespective of whether the constant water supply was adequate or not (Michelle et al., 2000). In carnations (Dianthus caryophyllus), irrigating at high water tensions (–75 kPa) reduced the longevity and quality of flowers.
Since cut roses are in high demand and have a high commercial value in the global floriculture industry, most rose growers extend the daylength by using supplemental lighting to increase rose production (Kim and Lee, 2008). Supplemental lighting increases the growth rate and decreases the percentage of blind shoots containing aborted flower buds. However, the increased growth rate under supplemental lighting requires increased irrigation, otherwise the growth rate is negatively affected by drought stress (Shi and Kim, 2014, 2015; Shi et al., 2019).
Understanding the interrelationships between preharvest environments and the variation in vase life of cut roses will greatly help not only to improve growth conditions and postharvest treatments, but also to develop methods to predict and improve the longevity of cut flowers. There have been few studies on the effects of irrigation under supplemental lighting on the postharvest life of cut roses. Therefore, the aim of this study was to investigate the effect of irrigation under day-extending supplemental lighting during the preharvest period on the vase life of cut rose ‘Charming Black’ for prolonging the cut-flower vase life.
Materials and Methods
Plant Materials and Preharvest Conditions
The experiment was conducted in an experimental glass-covered greenhouse located at the University of Seoul. Due to its high growth rate and sensitivity to environmental conditions, the Korean rose (Rosa hybrida) cultivar ‘Charming Black’ was used. The rose plants were planted from cuttings on July 8, 2011 in rockwool slabs (1 m long, 0.15 m wide, and 0.075 m deep, UR Rockwool, Pocheon, Korea) with a plant density of 5 plants·m-2. The plants were grown using the ‘arching’ method (Kool and Lenssen, 1997), which consisted of bending over the blind and thin stems which were not considered useful for flower production.
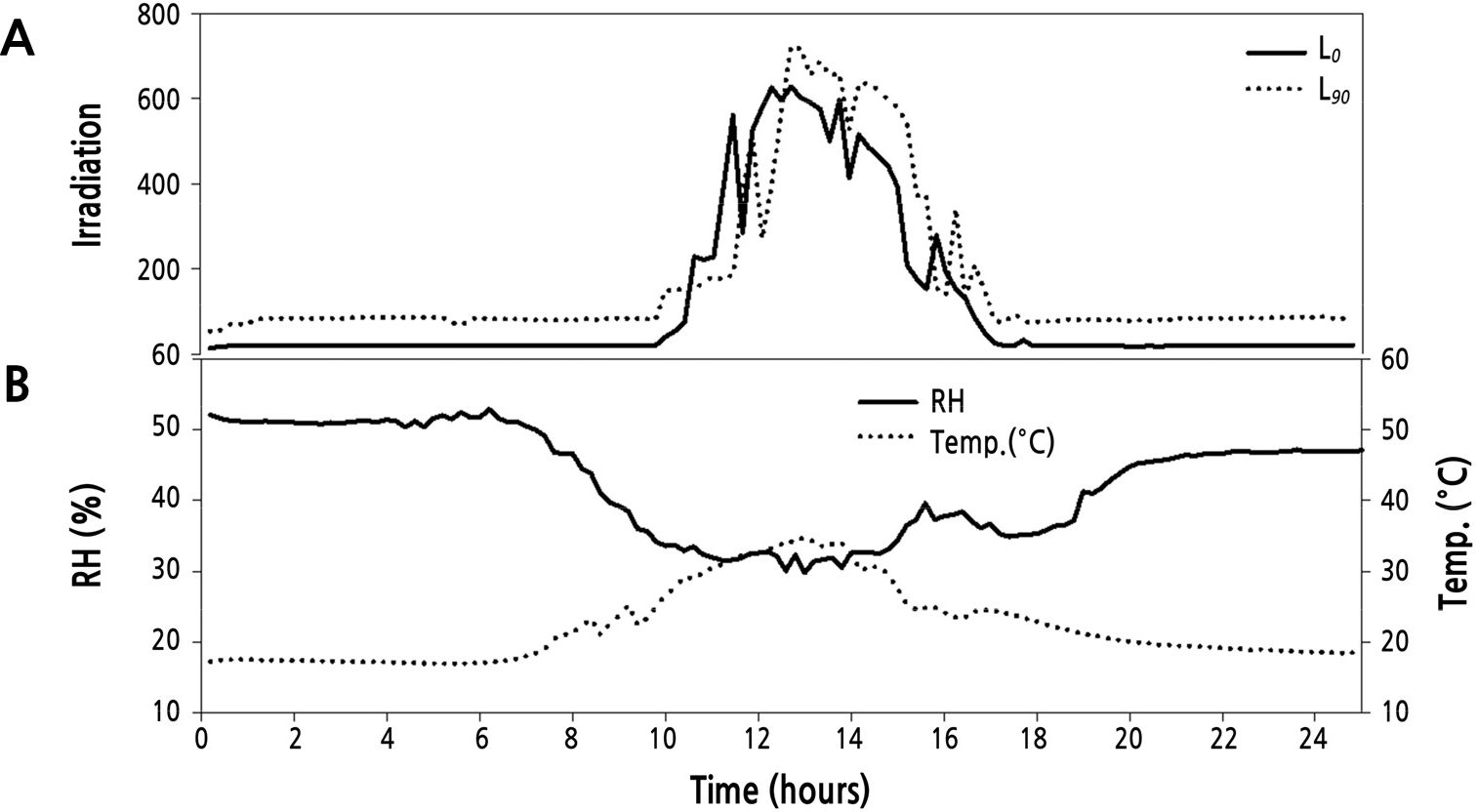
After the plants had gone through two flowering cycles, plants were divided into two supplemental light treatment groups: L0 (photosynthetically active radiation [PAR] 0) and L90(PAR 90 µmol·m-2·s-1). The supplemental lighting was programmed to come on between 16:00–24:00 h and 02:00–10:00 h, creating a night time of only 2 hours (Lee and Kim, 2015). During this experiment (2 months)the daylength averaged 22hours. When the water content dropped below 60% in the rockwool slab, drought stress occurred during the nocturnal supplemental lighting (Shi and Kim, 2014). Therefore, the plants were drip-irrigated once per hour from 10:00–16:00 h, and two water regimes were applied to the L0 and L90 groups: no irrigation (60–65% water content of rockwool slab media, L0×NI; L90×NI) and irrigation once per hour (80–85% water content of rockwool slab media, L0×I; L90×I) during supplemental lighting (16:00–24:00 h and 02:00–10:00 h) (Fig. 1). The water content of the rockwool was measured continually by the FDR (frequency domain reflectometry)(Shi and Kim, 2014, 2015) method throughout theexperiment (Coco-100, Mirae Sensor, Korea). The sensors were placed in the middle of the slab as the best representation for water content in the slabs (Abdel-Mawgoud et al., 2006). The nutrient solution was composed of Ca(NO3)2·4H2O, KNO3, EDTA-Fe, Mg(NO3)2·6H2O, (NH4)2PO4, MnSO4·5H2O, ZnSO4·7H2O, H3BO3, CuSO4·5H2O, and (NH4)6MO7O24·4H2O provided at 1,841, 2,323, 64.5, 204.8, 575, 12.05, 8.63, 9.27, 1.25, and 0.88 mg·L-1, respectively, and H2SO4 was provided at 281 µL·L-1 (EC 1.0 dS·m-1, pH 6.0 ± 0.2). A high-pressure sodium lamp (GEO-NH 400W-L/P) was used as the light source. The four treatments were: L0×NI: supplemental light intensity 0 µmol·m-2·s-1, no irrigation (63% water content of rockwool slab media); L0×I: supplemental light intensity 0 µmol·m-2·s-1 and irrigation (84% water content of rockwool slab media); L90×NI: supplemental lighting of 90 µmol·m-2·s-1 of PAR and no irrigation (61% water content of rockwool slab media); L90×I: supplemental lighting of 90 µmol·m-2·s-1 of PAR and irrigation (82% water content of rockwool slab media). The average day/night temperatures during the experiment were 26/17°C, RH at 45%, and ventilation was provided automatically from 8:00–16:00 h (Fig. 2). Pest and disease control were applied as required.
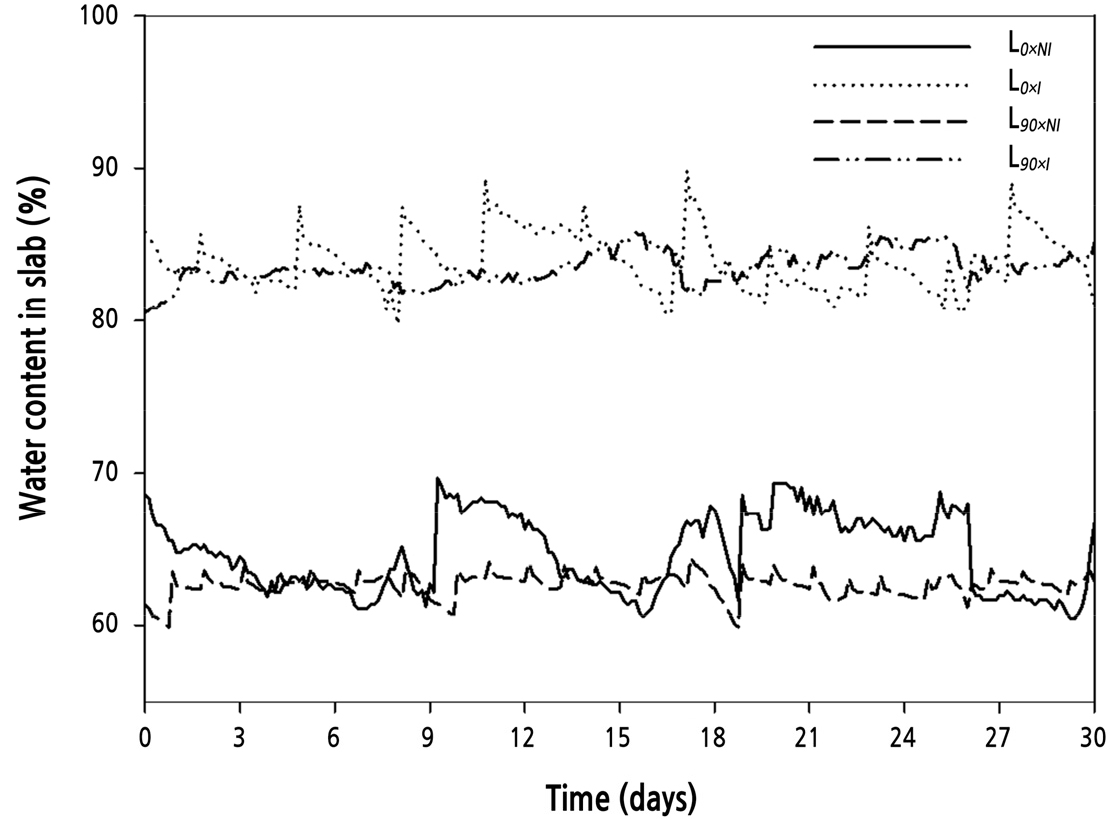
Fig. 1.
Water content of rockwool slabs used to grow ‘Charming Black’ roses. Water content was constantly measured throughout the experiment. Graph shown is representative of one month (30 D period). Treatments were: solid line, normal daylength, not irrigated (L0×NI); dotted line, normal daylength, irrigated (L0×I); heavy dash, extended daylength, not irrigated (L90×NI); and uneven dash, extended daylength, irrigated (L90×I).
Rose flowers were harvested from the greenhouse at the University of Seoul at the commercial harvest stage 2 (loose pointed bud; Fig. 3) according to In et al. (2009). Twenty flowers were cut for each of the four treatment groups: ten flowers for assessing vase life, ornamental quality, and water relations, and ten for assessing cellular component contents, petal color, and vacuolar pH. The stem length was cut to 40 cm, and all leaves but the two uppermostwere removed (some lower leaves were used as described below). The cut stems were placed in deionized water (200 mL) in 500-mL Erlenmeyer flasks. Flasks were covered with Parafilm (Bemis, WI, USA) to diminish water evaporation from the flasks and to prevent bacteria from entering from the air. The deionized water was replaced every day.The postharvest experiments were carried out in a growth chamber at the Environmental Floriculture Lab, University of Seoul. Flasks were placed in chambers at a constant temperature (Tair) of 22°C, with a RH about 65% using a daily light period of 12 h that was maintained using cool white florescent tubes (light intensity of 14 µmol·m-2·s-1) (Fanourakis et al., 2013).
Vase Life and Ornamental Quality
The visual quality of the cut roses was inspected daily while in the flasks. Vase life was defined as the period from the placement of the cut flower into the flasks in the environmentally-controlled chamber to the time when one or more of the following senescence symptoms was detected: bending of the peduncle (bent-neck; neck angle greater than 45°), wilting (≥30% petal turgor loss), bluing (≥30% blue petals), petal abscission (drop of three or more petals), and leaf abscission and yellowing (Kazemi and Ameri, 2012; In and Lim, 2018).
The ornamental quality was assessed according to Li et al. (2017) with some modifications. The opening and deterioration of the flower was classified into 5 stages (Fig. 3).The stems were harvested at stage 2, defined as loose pointed bud, and were assigned an ornamental quality value (OV) of 2. Later stages were half-open flower (stage 3; OV 3), open flower (stage 4; OV 3), and supreme bloom (stage 5; OV 1). After stage 5, 30% of petals were wilted, blackening, abscised, or the flower necks bent, and the ornamental quality score was 0. Eachflower was individually scored for its ornamental quality value for each stage listed above. Ornamental value was computed daily based on the sum of the values for the ten stems cut from each treatment group. Average daily ornamental value = Ornamental value/10.
The bent neck rate was calculated using the formula: Bent neck rate (%) = (Bn/Nn) × 100; where Bn refers to the number of bent neck stems, and Nn means the number of normal stems.
Water Relations
The fresh weights of the cut roses were measured immediately after cutting and before placing the stems in the water-filled flasks. Each flower's fresh weight was recorded at 2-day intervals while in the flask. The water uptake, water loss, water balance value, and the flower's relative fresh weight were calculated as described in He et al. (2006).
At harvest time, the 6 lowest leaves (five-leaflet) and 6 petals per flower (3 inner whorl and 3 outer whorl) from each stem were detached and weighed. These leaves and petals were set on a plastic platein the same climatic conditions as the vase life test, and their weights were measured after 6, 12, and 24 h (Mortensen and Fjeld, 1998).
Cellular Component Contents
The soluble sugar content was determined using Anthrone colorimetry (Yemm and Willis, 1954). The soluble protein content was measured using the Coomassie brilliant blue staining method (Bradford, 1976).
Petal Color and Vacuolar pH
Petal color was measured on a color reader (CR-10, MINOLTA CO., LTD. Japan) on day 7; three petals per flower (3 outer whorl petals) from each stem were measured per treatment.
To measure vacuolar pH of the petal, 6 petals per flower (3 inner whorl and 3 outer whorl) were ground to a fine powder with liquid nitrogen, mixed in a 10-mL centrifuge tube with 20 ml deionized water, and kept for 30 min at –25°C, followed by plunging into a water bath at 50°C for 45 s. The sample was centrifuged at 2000 rpm for 10 minutes at 0°C. The resulting supernatant was filtered through filter papers (Whatman No. 2; Whatman Ltd, Maidstone, UK) (Kurkdjian and Guern, 1981). The pH values of the solutions were measured with a pH electrode (Hanna Instruments, Texas, United States).
Statistical Analysis
The experiment was a completely randomized design (CRD) consisting of four treatments. There were 4 treatment combinations and twenty stems for each treatment. Eighty cut stems at the commercial harvest stage 2 were selected for uniformity of size, color, and free from any defects. Vase experiments involved 3 to 10 replicates for each treatment (see table and figures). The morphological, physiological, and biochemical data were subjected to analysis of variance (ANOVA) using Statistical Analysis System (version 9.3, SAS Institute Inc., Cary, NC, USA). Diagrams were created using Sigmaplot 14.0 (Systat Software Inc., San Jose, USA). Means were compared by the least significant difference (LSD) test at the 0.05 probability level. Data are presented as means ± standard errors (SE).
Results
Effects of Nocturnal Supplemental Lighting and Different Irrigation Regimes on Postharvest Performance of Cut Roses
Vase life was significantly affected by supplemental lighting, with the supplemental lighting (L90) treatments producing flower stems with a longer vase life than those cut from plants without supplemental lighting (L0). Cut roses grown without supplemental lighting had a vase life of only 9 days. However, there were no significant differences between the two irrigation regimes for stems cut from plants grown without supplemental lighting. Cut roses grown under supplemental lighting without irrigation (L90×NI) had a maximum vase life of 13 days, yet adding irrigation to the supplemental light regime (L90×I) decreased the vase life by >15% to 11 days (Fig. 4).
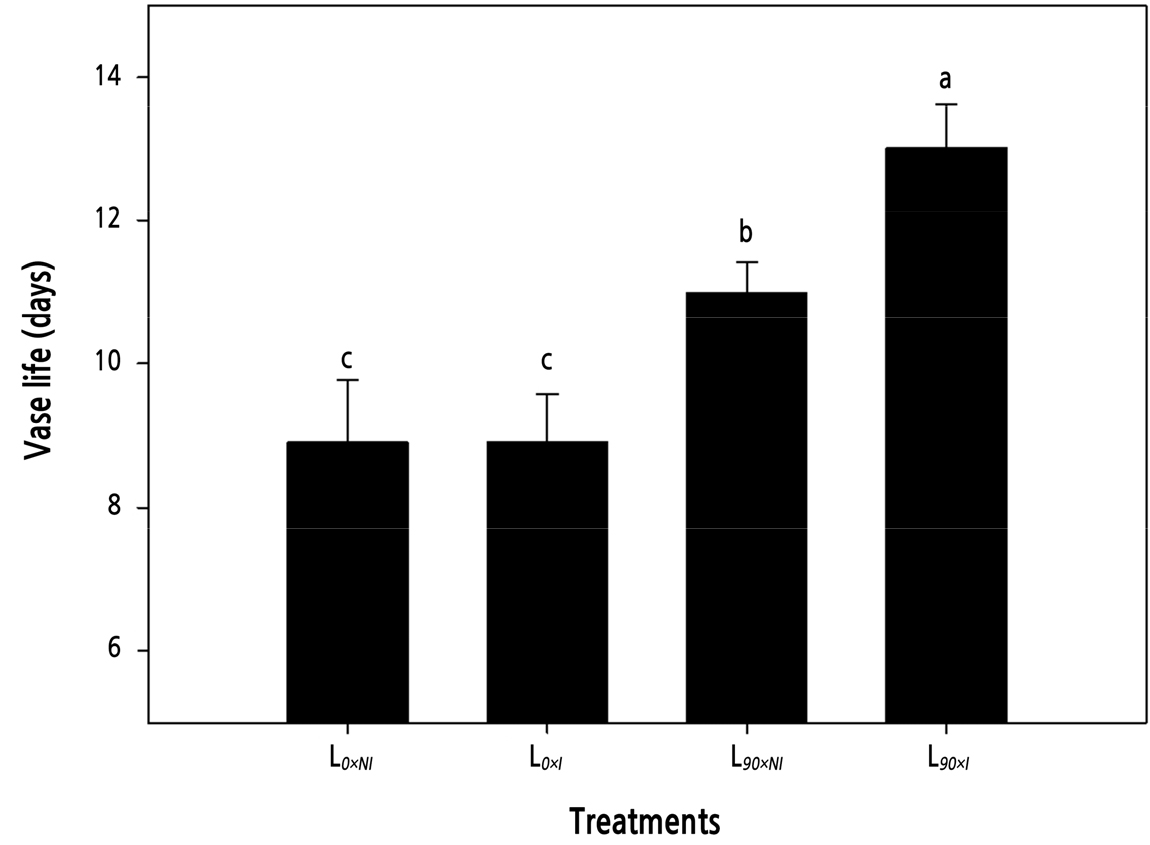
Fig. 4.
Effects of nocturnal supplemental lighting and different irrigation regimes during plant growth on the vase life of the cut rose ‘Charming Black’. L0×NI : Normal daylength, not irrigated; L0×I : Normal daylength, irrigated; L90×NI : Extended daylength, not irrigated; L90×I : Extended daylength, irrigated. Vertical bars indicate mean standard errors (n = 10). Different letters (a–c) among pretreatments indicate statistically significant differences at p ≤ 0.05 based on Duncan’s multiple range test.
The average daily ornamental values of roses cut from plants grown under supplemental lighting were significantly greater than for those grown without supplemental lighting. The highest ornamental value occurred on day 4 in the L0 group and then decreased quickly. At day 5 after harvest, 18% of the petals on roses from the L0 groups showed blueing (data not shown), a rate that increased with time. Under the L90 treatments, the flowers lasted for a longer period, and senescence was delayed by either 2 days (L90xI) or 4 days (L90xNI) compared with L0 groups. Moreover, the L90xNI treatment showed the highest average daily ornamental value (Fig. 5B) at stage 5, supreme bloom, when the roses from the L90xI plants showed blueing of their petals (Fig. 5B).
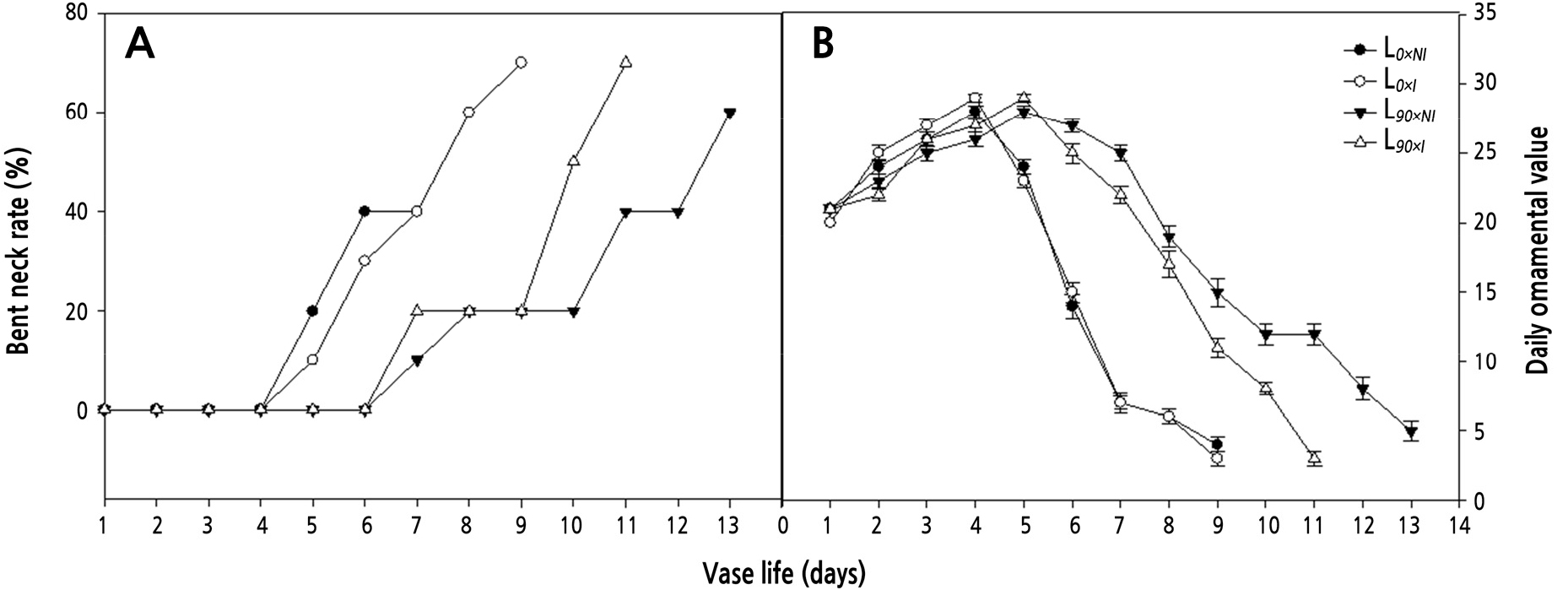
Fig. 5.
Effects of nocturnal supplemental lighting and different irrigation regimes during the growth period on the bent neck rate (A) and daily ornamental value (B) of the cut rose ‘Charming Black’ over 14 days postharvest in a vase filled with water. L0×NI : Normal daylength, not irrigated; L0×I : Normal daylength, irrigated; L90×NI : Extended daylength, not irrigated; L90×I : Extended daylength, irrigated. Vertical bars indicate mean standard errors (n = 10).
Neck bending occurred on day 5 in the L0 groups and on day 7 in the L90 groups. Supplemental lighting significantly delayed this phenomenon, but irrigating the plants grown under supplemental light did not. The lowest bent neck rate was observed in roses cut from the L90xNI group (Fig. 5A).
Effects of Nocturnal Supplemental Lighting and Irrigation on Water Relations of Cut Roses
Roses cut from plants treated with irrigation under supplemental lighting (L90×I; open triangles) showed significantly higher water loss than did those from plants grown under lighting without irrigation (L90×NI) and non-supplemental lighting treatments (L0) during most of the postharvest period. During days 2–6, there were no significant differences between L90×NI (filled triangles) and L0 (circles) groups (Fig. 6A).
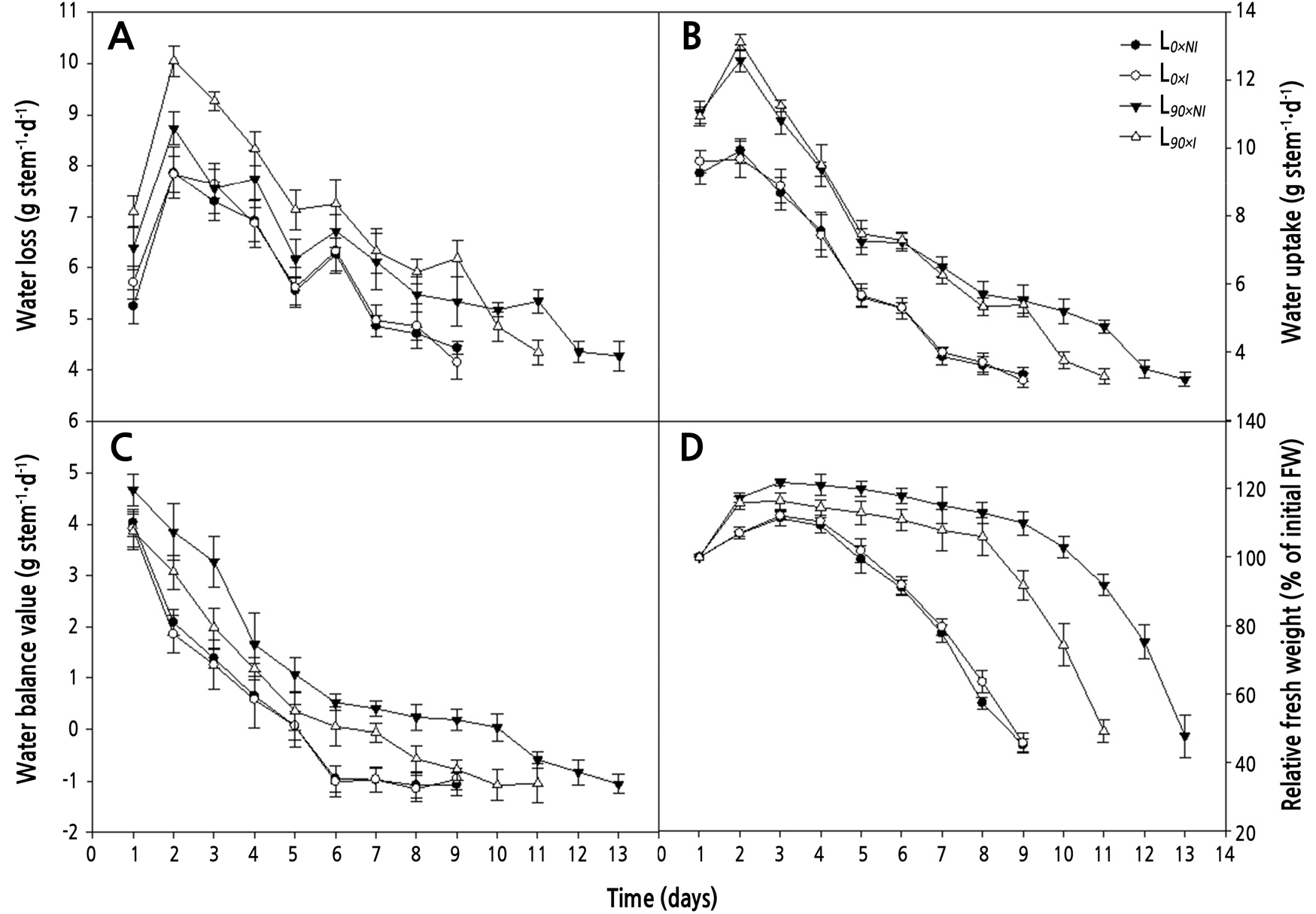
Fig. 6.
Effects of nocturnal supplemental lighting and different irrigation regimes during plant growth on water loss (A), water uptake (B), water balance value (C), and relative fresh weight (D) during vase display of the cut rose ‘Charming Black’. L0×NI : Normal daylength, not irrigated; L0×I : Normal daylength, irrigated; L90×NI : Extended daylength, not irrigated; L90×I : Extended daylength, irrigated. Vertical bars indicate mean standard errors (n = 10).
Supplemental lighting treatments significantly enhanced the water uptake of cut roses as compared to the non-supplemental lighting treatments. However, no significant differences were seen between either the L0×NIand L0×I or the L90×NIand L90×I treatments until day 9. Among the supplemental lighting treatments, the water uptake was 26.9% higher for the non-irrigated roses (L90×NI; 5.2 g per stem) than for the irrigated roses (L90×I; 3.8 g per stem) from day 10 of the postharvest period (Fig. 6B).
In both the supplemental and non-supplemental light groups, the water balance value decreased over the postharvest period, but the decline was significantly faster for the stems cut from the plants grown under shorter days. Roses from the L90×NIregime exhibited the most favorable water balance over the postharvest period. The number of days to negative water balance was significantly influenced by both supplemental lighting and irrigation. Roses from the L0 groups reached a negative water balance the soonest, at day 5. Roses from the L90×I treatment did not reach this negative value until day 7. Flowers from the L90×NI treatment retained a positive water balance for an average 10 days after harvest (Fig. 6C).
Cut flowers in all regimens showed transient increases in relative fresh weight (RFW). During the postharvest period, RFW increased until day 3 for the L0 group and until day 7 for the L90 group, before decreasing thereafter. The average RFW for roses in the L90 group was significantly higher than that for the L0 group; however, withholding irrigation under supplemental lighting (L90×NI) resulted in the highest RFW of around 16.5% until day 9 compared to the L90×I treatment (Fig. 6D).
Water loss (% of total water) of detached leaves and flower petals was tracked for 6, 12, and 24 h (Fig. 7) after harvest. Tissues from the plants treated with both supplemental lighting and irrigation showed the greatest rates of water loss. Water loss from leaves in the L90×I group increased by 25.4, 37.8, and 52.7%, respectively, at each time point (Fig. 7A). Water loss from flower petals in the L90×I group increased by 12.4, 18.8, and 30.7% at each time point, respectively (Fig. 7B). The rate of water loss was higher from leaves and flower petals from the L90×I treatment that gave a shorter vase life compared to the L90×NI treatment.
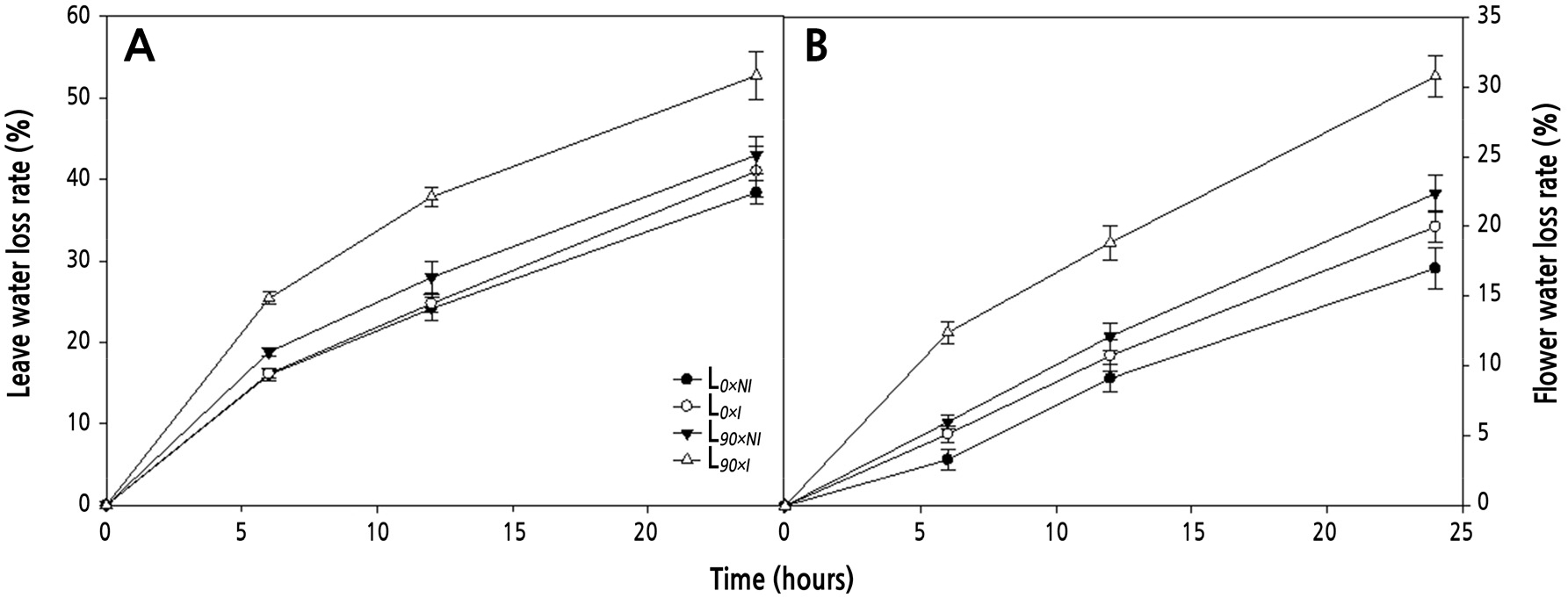
Fig. 7.
Effects of nocturnal supplemental lighting and different irrigation regimes during plant growth on water loss rates of detached leaves (A) and flower petals (B) of the cut rose ‘Charming Black’. L0×NI : Normal daylength, not irrigated; L0×I : Normal daylength, irrigated; L90×NI : Extended daylength, not irrigated; L90×I : Extended daylength, irrigated. Vertical bars indicate mean standard errors (n = 4).
Effects of Nocturnal Supplemental Lighting and Irrigation on Cellular Components of Cut Roses
Supplemental lighting led to significantly higher soluble sugar contents in the petals of cut flowers during their vase life. By day 7, soluble sugars were approximately 1.3-fold higher in roses cut from plants grown under supplemental lighting but not irrigated (L90×NI; 306.5 mg·g-1 FW) compared to plants grown under shorter days (L0; 244.8 mg·g-1 FW). Soluble sugars remained high in flowers cut from plants grown under supplemental lighting but not irrigated (L90×NI) during most of the postharvest period (Fig. 8A).
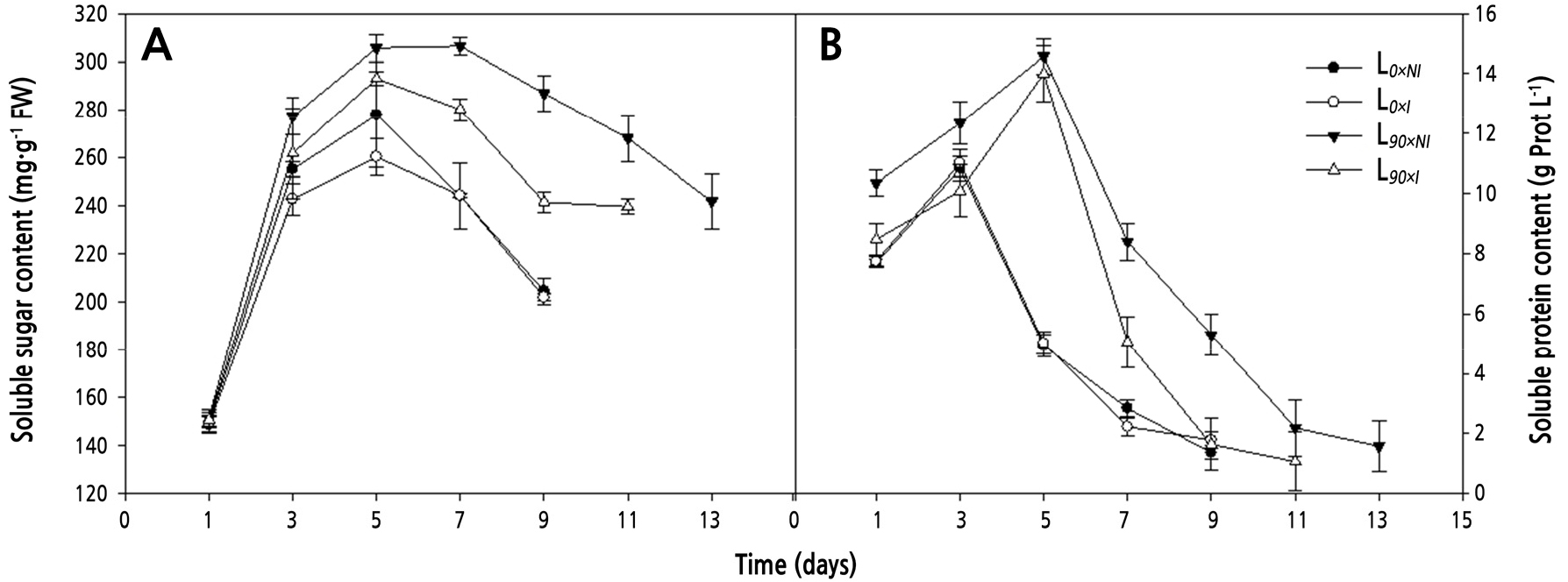
Fig. 8.
Effects of nocturnal supplemental lighting and different irrigation regimes during plant growth on soluble sugar content (A) and soluble protein content (B) in ‘Charming Black’ cut roses during vase display. L0×NI : Normal daylength, not irrigated; L0×I : Normal daylength, irrigated; L90×NI : Extended daylength, not irrigated; L90×I : Extended daylength, irrigated. Vertical bars indicate mean standard errors (n = 3).
In roses from all treatments, soluble protein contents first increased and then decreased during the postharvest period. The highest soluble protein content in roses from the shorter-day groups (L0) was found on day 3 (11.0 g prot L-1) and on day 5 in roses from the longer-day groups (L90) (14.6 g prot L-1). The soluble protein content was always lower in roses from the shorter-day groups, with the highest value of 9.6 g·prot·L-1 on day 5. Meanwhile, on day 9, cut roses from the L90×NI treatment had an increase of 3.7 g·prot·L-1 higher than that of the L90×I treatment (Fig. 8B).
Effects of Nocturnal Supplemental Lighting and Different Irrigation Regimes on Petal Color and Vacuolar pH of Cut Roses
The values of five color parameters were not different between the two shorter-day groups. Supplemental lighting delayed changes in petal color during the vase life. Cut roses grown under supplemental lighting and irrigation (L90×I) had a significantly lower L* value (lightness from black to white) (by 28.72, 24.30, and 20.02% compared to the L0×NI, L0×I, and L90×NI treatments, respectively), indicating that the petal was darker, although the value was not significantly different than the standard. The a* value (from green to red), representing redness, in the L90×I group was lower by 47.58, 47.89, and 44.15% compared to the L0×NI, L0×I, and L90×NI treatments, respectively. The b* value (from blue to yellow), representing blueness, was also lower in the L90×I group by 62.29, 62.06, and 64.18% compared to the L0×NI, L0×I, and L90×NI groups, respectively (Table 1).
Table 1.
Effects of nocturnal supplemental lighting and different irrigation regimes during plant growth on the color of ‘Charming Black’ rose stems after 7 days of vase display
| Treatmentsz | Color characteristics | ||||
| L* | a* | b* | C* | h° | |
| Standardy | 15.58 | 47.57 | 9.93 | 48.6 | 11.79 |
| L0×NI | 20.23 a | 54.79 a | 11.35 a | 55.95 a | 11.70 a |
| L0×I | 19.05 a | 55.11 a | 11.28 a | 56.25 a | 11.57 a |
| L90×NI | 18.03 a | 51.42 a | 11.95 a | 52.79 a | 13.08 a |
| L90×I | 14.42 b | 28.72 b** | 4.28 b* | 29.04 b** | 8.48 b |
L* scale: light vs. dark, where a low number (0–50) indicates dark and a high number (51–100) indicates light. a* scale: red vs. green, where a positive number indicates red and a negative number indicates green. b* scale: yellow vs. blue, where a positive number indicates yellow and a negative number indicates blue. C* scale: chroma, represents the hypotenuse of a right triangle created by joining points (0, 0), (a*, b*), and (a*, 0). h° scale: determined from the angle between the hypotenuse and 0° on the a* axis. L0×NI : Normal daylength, not irrigated; L0×I : Normal daylength, irrigated; L90×NI : Extended daylength, not irrigated; L90×I : Extended daylength, irrigated.
The vacuolar pH was measured in flower petals four times during the vase period. The vacuolar pH of the roses at harvest was around 4.0. As the petals aged, the vacuolar pH increased. Supplemental lighting lead to a significant difference in vacuolar pH from that of the short-day treatments during the vase life. The roses cut from plants grown under shorter days showed a dramatic increase in the vacuolar pH during the first 4 days after harvest, while roses from longer day plantshad pH values that remained relatively constant during the initial 4 days. However, the L90×I treatment did result in a significant increase in vacuolar pH after 7 days, whereas the L90×NI group showed only a moderate increase. At the end of the vase life, the pH value for the L90×NI group was increased by 3.5% while that of the L90×I group was increased by 4% compared with the initial values, indicating that the roses cut from plants grown under supplemental lighting and with irrigation remained less acidic (Fig. 9).
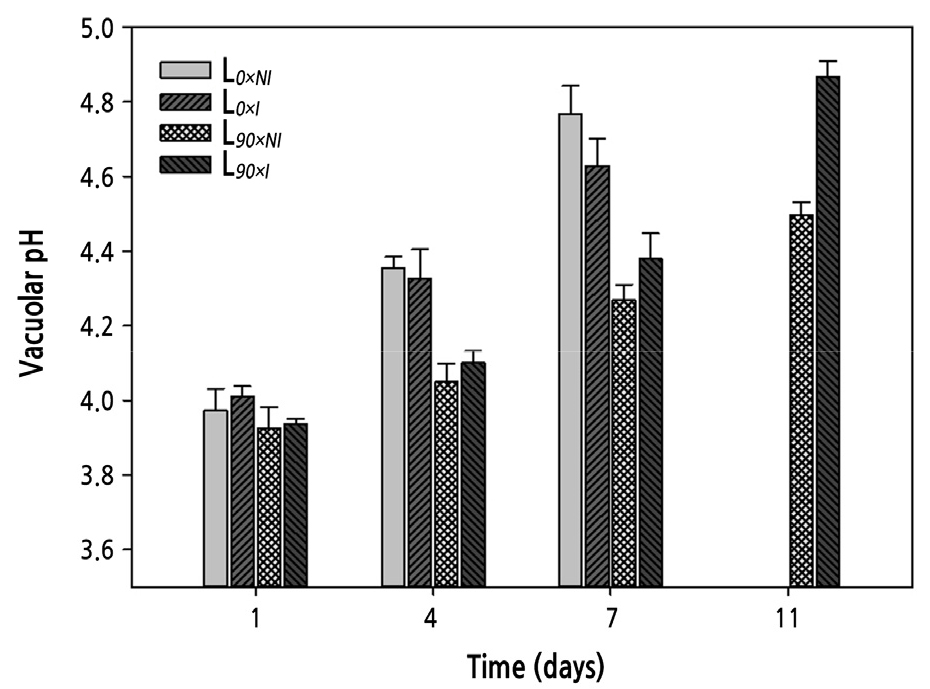
Fig. 9.
Effects of nocturnal supplemental lighting and different irrigation regimes during plant growth on vacuolar pH of petals from ‘Charming Black’ during vase display. L0×NI : Normal daylength, not irrigated; L0×I : Normal daylength, irrigated; L90×NI : Extended daylength, not irrigated; L90×I : Extended daylength, irrigated. Vertical bars indicate mean standard errors (n = 3).
Discussion
The vase life of cut roses is an important factor that directly affects the commodity value. Therefore, it is important to investigate how to delay senescence and prolong the vase life of cut flowers (Zhao et al., 2018). In the present study using the rose cultivar ‘Charming Black’, day-lengthening supplemental lighting treatments significantly extended the vase life of cut roses by 2–4 days as compared to flowers grown without supplemental lighting. This finding contrasts results showing that supplemental lighting either had no effect or decreased the vase life of cut roses (Zieslin and Mor, 1990) and results showing that continuous light has an adverse effect on the vase life of cut roses (Mortensen and Gislerød, 1999). Alternatively, increasing the PPFD during the growing period increases the vase life in roses (Fjeld et al., 1994). An increase in the preharvest PPFD level increased the vase life in the cut flower Eustoma (Islam et al., 2003). Thus, the effect of supplemental lighting on the vase life of cut flowers seems variable, possibly due to the influence of other factors, perhaps high air humility, light source/spectra, or irrigation.
Senescence in cut roses is accompanied by numerous physiological and biochemical changes. Soluble protein content is related to flower senescence, with enhanced content directly impacting the ornamental quality and vase life of cut Chrysanthemum morifolium flowers (Fan et al., 2015). In our cut roses, the soluble protein content increased initially and then decreased during the early postharvest period (loose pointed bud stage), after which protein decomposition reduced the content of soluble protein during the late postharvest period. Supplemental lighting significantly increased the soluble protein content in cut roses compared with that of the plants grown in shorter days, with the unirrigated plants showing the highest soluble protein content on the 5th day postharvest.
According to our previous study, the rose plants under supplemental lighting showed a better growth response; the plants grew faster and the percentage of blind shoots decreased (Shi and Kim, 2014). Our results show that optimal light conditions during the growth period might ensure high carbohydrate levels in the plants at harvest and may promote membrane stability, thereby prolonging the vase life.
Water shortage is a major factor affecting vase life, as water stress may lead to premature senescence, bent neck, and wilting (Särkkä, 2004). Cut roses treated with supplemental lighting showed higher water uptake and relative fresh weight. Slootweg and Meeteren (1991) found that the postharvest transpiration rate of cut roses was much higher when the roses had been grown under a 20-h photoperiod compared with those grown under the natural daylength. When the daylength increased from 16 to 24 h, the dry weight of cut roses increased. In this study using ‘Charming Black’ roses, supplemental lighting without irrigation improved vase life and the longevity of postharvest quality. Adding irrigation decreased the vase life and quality at supreme bloom, with loosening and blackening petals appearing 10 days after harvest. A cut rose with a long vase life must maintain water uptake at the stem base, the ability to transport water through the stem and its organs, and the ability to retain water in the flower (Halevy, 1972; Zieslin, 1989). In some conditions, the amount of water uptake does not compensate for water loss in roses (Mortensen and Gislerød, 1999). Therefore, the vase life of cut roses is dependent on the balance between water uptake and transpiration loss (Van Doorn, 1997, 2001). The major postharvest problem is caused by a negative water balance (Mortensen, 2001; Mortensen and Fjeld, 1998; Torre and Fjeld, 2001). Our experiment showed that treatment with supplemental light and irrigation (L90×I) reduced the uptake of water from the vase and increased water loss through transpiration, resulting in a shorter vase life. The moderation of water loss may be related to xylem vessel development. Vessels placed at lower humidity during culture showed better control over water loss (Ghashghaie et al., 1992; Short et al., 1987; Ziv et al., 1987). The higher water loss rate under the L90×I treatment suggests that plentiful irrigation may be partly responsible for the negative water balance (water loss > water uptake), as a result of stomatal malfunctioning (Mortensen et al., 2007; Pettersen et al., 2007; Rezaei et al., 2006). Supplemental lighting and irrigation increased the rate of water loss from the leaves in the cut roses. Stomatal responsiveness to desiccation is affected when leaves develop at high humidity (Mortensen and Fjeld, 1998; Giday et al., 2013).
Growth under supplemental lighting and irrigation (L90×I) increased water loss not only in leaves but also in the flower petals, resulting in increased numbers of senescing petals, membrane permeability changes, hydrolysis of cell components (Halevy and Mayak, 1979), and blackening/blueing of the petals. The discoloration of red roses has often been observed under water deficit, implying that soluble carbohydrates may not be available for respiration, and that amino acids and proteins are then depleted. Research has also shown that discoloration or bluing was generated by a small increase in pH (Asen et al., 1971). Significant increases in pH under the L90×I condition might speed the bluing/blackening and senescence of the petal. It can be concluded that constant irrigation and high water content of the rockwool media during the growth period of the rose plants attenuates stomatal responsiveness, resulting in excessive water loss in roses after harvest, which then causes a considerable reduction in vase life.
The present study indicates that growth of rose plants under supplemental lighting but without irrigation will significantly delay senescence and extend the vase life of cut roses. This regime has the potential of increasing the vase life through promoting desirable morphological and physiological characteristics at harvest time, such as low water loss and high internal sucrose contents. These characteristics are achievable through the use of specific growth methods. Future experiments can determine the optimal water content of the rockwool media and the best irrigation regime to use with supplemental lighting when cultivating cut roses for durable postharvest quality.