Introduction
Materials and Methods
Plant materials
Growth conditions
Measurement of intumescence
Measurement of growth characteristics
Statistical analysis
Results and Discussion
Intumescence development of grafted seedlings during cultivation
Susceptibility of pepper and tomato cultivars to intumescence
Stress level of pepper and tomato-grafted seedlings with and without intumescence (Fv/Fm)
Growth of pepper- and tomato-grafted seedlings by cultivars
Seedling quality changes for different pepper and tomato cultivars
Conclusion
Introduction
Intumescence is a physiological disorder characterized by the abnormal enlargement of epidermal or palisade cells on the surfaces of leaves and stems in plants, resulting in tumor-like tissue (Wetzstein and Frett, 1984; Morrow and Tibbits, 1988). Previously, the terms intumescence, edema, oedema, excrescence, neoplasm, gall, genetic tumors, and leaf lesions were used interchangeably to refer to disorders with similar symptoms (Pinkard et al., 2006). As a result, the factors reported to cause intumescence are diverse, including the plant water status, temperature, hormones, plant genetics, any pest injuries, fungal infections, mechanical and chemical injuries, nutritional status, and the quality of the light, making it difficult to match a specific factor to a clear cause (Pinkard et al., 2006).
According to Eguchi et al. (2016), intumescence is not an injury caused by diseases or pests, such as fungi, microorganisms, or insects; rather, it is a physiological disorder resulting from an inappropriate growing environment, including a lack of light in the UV-B wavelength range (280–315 nm), monochromatic red light (600–700 nm), over humidification, and the plant moisture status in plant factories with artificial lighting (PFAL) systems (Eisa and Dobrenz, 1971; Heidi and Runkle, 2015; Misu et al., 2018). Intumescence reduces the aesthetic value of ornamental plants and, in severe cases, can inhibit photosynthesis due to leaf abscission (Lang et al., 1983b; Rud et al., 2009; Williams et al., 2016). These instances of intumescence mainly occur in Solanaceae crops such as pepper (Capsicum annuum L.), tomato (Solanum lycopersicum L.), potato (Solanum tuberosum L.), and eggplant (Solanum melongena L.), where susceptibility is highly variable among cultivars within the same species (Eisa and Dobrenz, 1971; Lang et al., 1983a; Petitte and Ormrod, 1986; Massa et al., 2008; Ozawa et al., 2018). Nonetheless, the exact causes and mechanisms are poorly understood.
Recent work has shown that intumescence injuries occur in nurseries that produce plug-grafted Solanaceae seedlings. Grafting improves plant growth by increasing disease resistance, increasing tolerance to adverse environmental conditions, and enhancing water uptake. Hence, it is gaining popularity in Asian countries, such as Korea, Japan, and Taiwan, and globally (RDA, 2020a). The process of producing vegetable plug-grafted seedlings consists of four stages: 1) scion and rootstock nursery growth, 2) grafting, 3) healing (the connection of new vasculature at the rootstock and scion site) and acclimatization, and 4) nursery growth after acclimatization until shipping (Maeda, 2004). During the healing stage, intumescence occurs at the scions in the LED healing chamber. Severe intumescence injury impairs photosynthesis in grafted seedlings and induces leaf abscission and downward curling (Lang et al., 1983a; Misu et al., 2018). Intumescence injuries persist on older leaf surfaces of shipped seedlings and reduce the product value of plug seedlings.
Grafted pepper and tomato seedlings account for more than half of the purchases under protected cultivation (67.3% and 50.9%, respectively). Pepper- and tomato-grafted seedlings are ranked as the second- and fifth-largest exports to Japan, respectively (An et al., 2018). Intumescence injuries to grafted pepper and tomato seedlings can lead to domestic and export claims for nursery operators when the seedlings are sold. Nevertheless, research on methods capable of preventing intumescence has focused mainly on tomatoes as opposed to peppers. The tomato intumescence-susceptible rootstock cultivars ‘B-blocking,’ ‘Beaufort,’ and ‘Maxifort’ have been used for intumescence research in PFAL. However, studies of intumescence injuries and intumescence-susceptible cultivars in plug-grafted seedling production systems are limited. Therefore, this study was conducted to investigate the development of intumescence in grafted chili pepper seedlings (crunchy, nokkwang, sweet, cheongyang, and kkwari peppers) and tomatoes (ordinary and jujube-shaped cherry tomatoes) predominantly cultivated in plug-seedling nurseries.
Materials and Methods
Plant materials
Pepper (Capsicum annuum L.) rootstock ‘Thankyoudaemok’ (Nongwoo Bio Co. Ltd., Suwon, Korea) and tomato (Solanum lycopersicum L.) rootstock ‘B-blocking’ (Takii Seed Co. Ltd., Gangnam, Korea) were sown in 50-cell trays filled with a commercial growing medium (Plantworld, Nongwoo Bio Co. Ltd.) on August 17, 2022. The following day, pepper and tomato scions were each sown in 105-cell trays. The characteristics of the pepper and tomato scions used in the experimental cultivars are summarized in Tables 1 and 2, respectively.
Table 1.
Cultivars and characteristics of the pepper scion cultivars used in experiments
Table 2.
Cultivars and characteristics of the tomato scion cultivars used in experiments
Growth conditions
The experiments were conducted in an LED healing chamber at the “ED” plug seedling nursery in Gyeongsangnamdo, Korea. The scions were grafted 28 days after sowing, and the grafted seedlings were immediately placed in the LED healing chamber for 4 d. The light quality inside the healing chamber was measured using a light wavelength meter (ILT950; International Light Technologies Inc., Peabody, MA, USA) with a red/blue ratio of 6:4. The photoperiod was set to 18/6 h (light/dark). The light intensity levels were 31 ± 5 and 26 ± 5 µmol·m-2·s-1 photosynthetic photon flux density (PPFD) at the height of the growing point for pepper and tomato, respectively. Temperature and relative humidity were set to 25 ± 1°C and 99 ± 1%, respectively. Data were collected using a data logger (88162 AZ EB, AZ Instrument Corp., Taichung, Taiwan).
Measurement of intumescence
The incidences of intumescence in plants (IMP) and leaves (IML) were calculated using the following equations:
IMP (%) = (number of intumescence plants/total number of plants) × 100
IML (%) = (number of intumescence leaves/number of total leaves) × 100
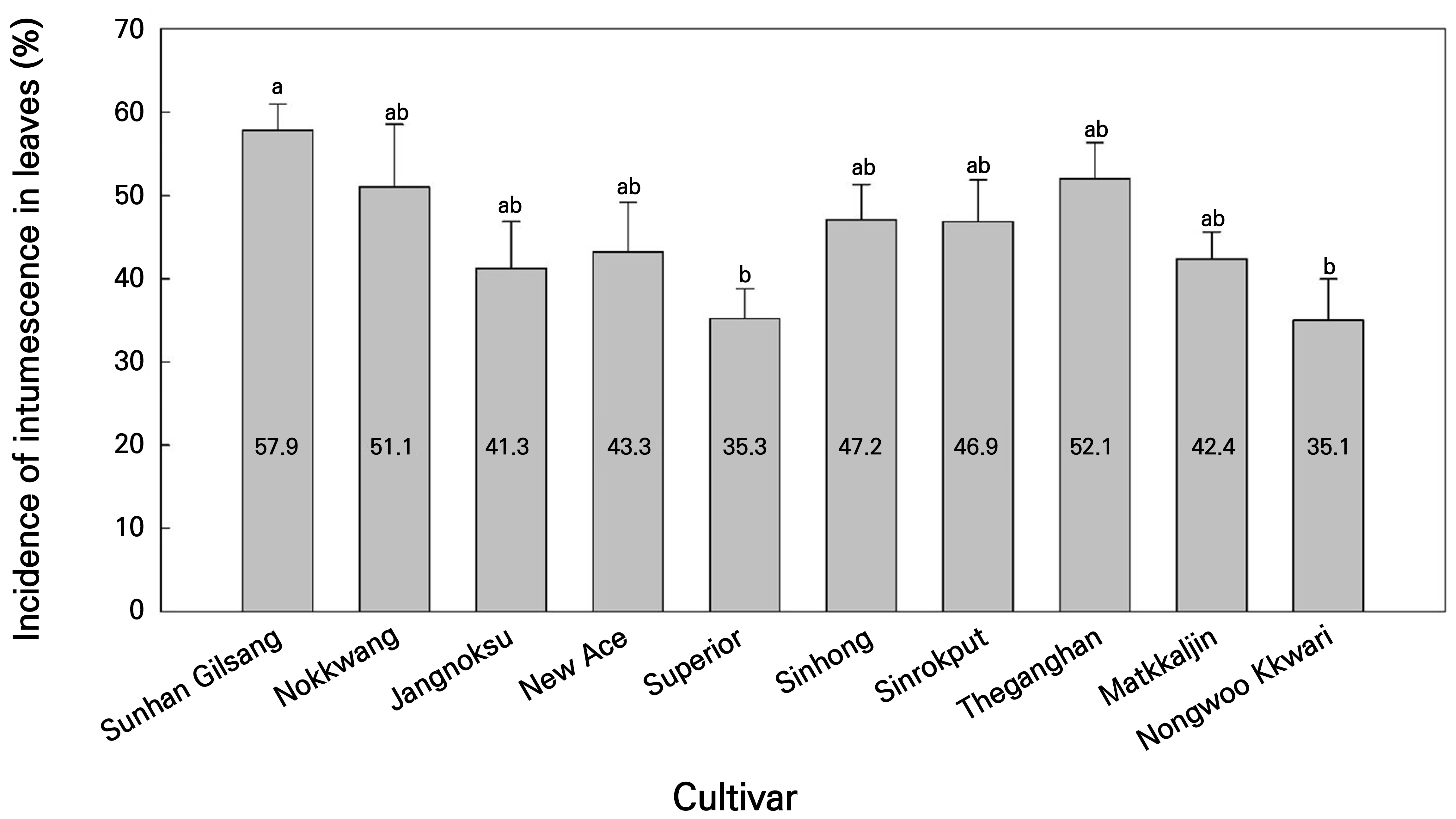
Upon any discovery of abnormal outgrowth, such as a blister, on even one leaf (Figs. 1A and 2A), the plants were defined as intumescence plants. IMP in the pepper- and tomato-grafted seedlings in the LED healing chamber was monitored for 1–4 d after grafting. Even for the same IMP, the severity of the intumescence injury, as judged, could vary depending on whether there were more or fewer intumescence leaves. Therefore, for peppers, which had high IMP in most cultivars (Fig. 3), IML was additionally measured to understand intumescence susceptibility in more detail. Before shipping, the incidence of intumescence leaves per plant was calculated as IML. The stress levels (Fv/Fm) of the plants were measured using a portable chlorophyll fluorometer (FP 100, PSI spol. s r.o., Drasov, Czech Republic). Before shipping, the measurement site was the middle section of the third or fourth leaf from the cotyledon of the grafted seedlings. Leaves with and without intumescence were separated by cultivar and analyzed in a greenhouse.
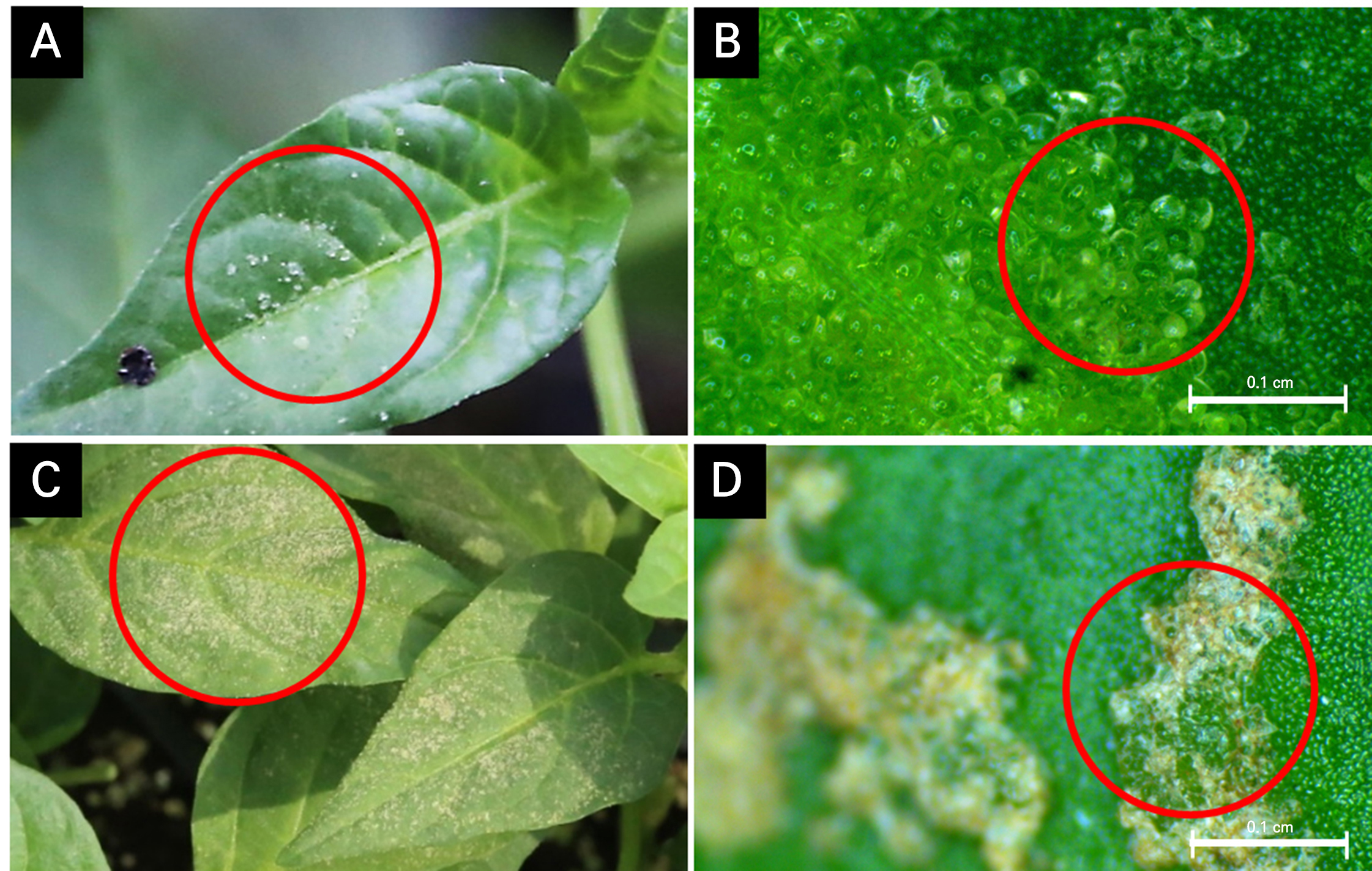
Fig. 1.
Intumescence development on the leaf surfaces of grafted pepper seedlings during cultivation. Early stage with translucent blisters on the adaxial side of pepper leaves in the LED healing chamber (A), as shown in the light microscope image (B); in later stages, after the healing and acclimatization, the blisters turn brown (C), as shown in the light microscope image (D). Red circles highlight different intumescence development outcomes.
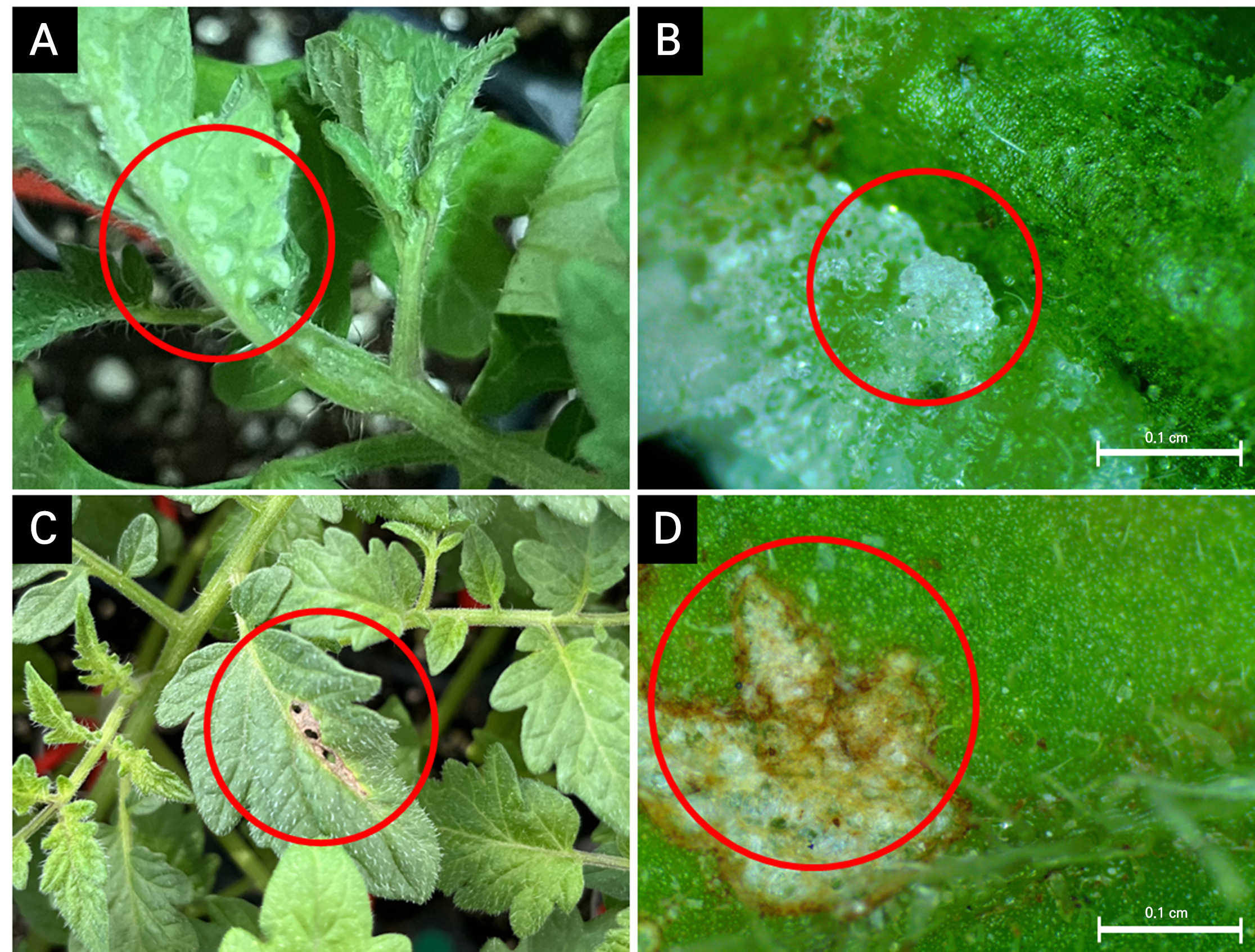
Fig. 2.
Intumescence development on the leaf surface of grafted tomato seedlings during cultivation. Early stage with blistering on the abaxial side of tomato leaves in the LED healing chamber (A), as shown in the light microscope image (B); in later stages, after the healing and acclimatization, the blister turned brown and necrosis developed (C), as shown in the light microscope image (D). Red circles highlight different intumescence development outcomes.
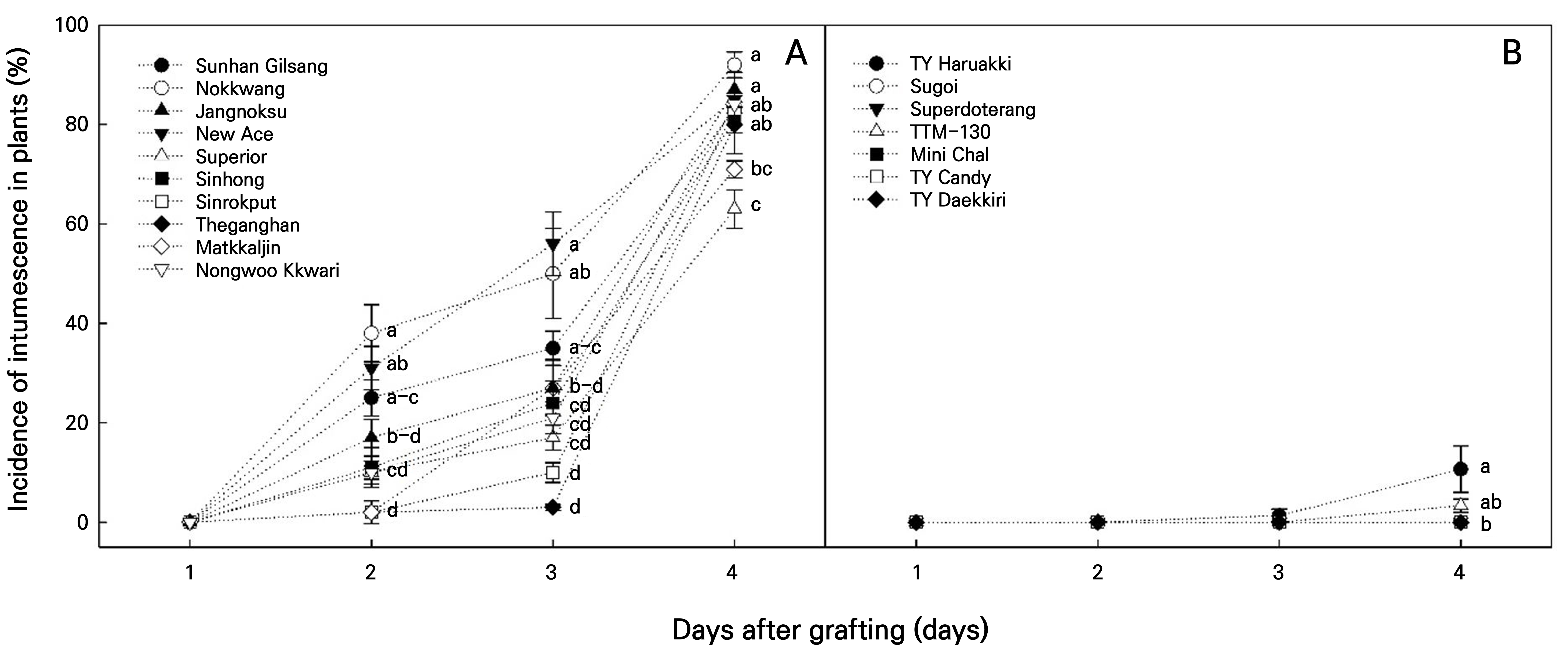
Fig. 3.
Incidence of intumescence in plants of different grafted pepper (A) and tomato (B) seedlings in the LED healing chamber. Vertical bars represent the standard deviation of the mean (n = 150). Different letters above the bars indicate significant differences according to Tukey’s test at p < 0.05.
Measurement of growth characteristics
Growth characteristics were measured before shipping on September 28, 2022. In each case, the seedling remained at the nursery from September 19 to 28, 2022 (13 d after grafting). The plant height, leaf length, leaf width, number of leaves, stem diameter, soil plant analysis development (SPAD) value, leaf area, and shoot and root fresh and dry weights were measured. Plant height was measured from the soil line to the growing point. The stem diameter was measured using a Vernier caliper (CD-20CPX, Mitutoyo Co. Ltd., Kawasaki, Japan), and the SPAD values were determined using a chlorophyll meter (SPAD-502; Konica Minolta Inc., Tokyo, Japan), with three fully expanded leaves measured and the average value then used. Leaf area was measured using a leaf area meter (LI-3000, LICOR Inc., Lincoln, NE, USA), and fresh and dry weights were measured using an electronic balance (EW220-3NM, Kern and Sohn GmbH., Balingen, Germany). Dry weight was measured after drying the plant in an oven (Venticell-222, MMM Medcenter Einrichtungen GmbH, Planegg, Germany) for 72 h at 70°C. The leaf area ratio (LAR) and compactness of the pepper and tomato seedlings were examined at three stages: before grafting on September 15, 2022, after healing on September 19, 2022, and on before shipping on September 28, 2022. This was done using analysis standards for research in agricultural science and technology (RDA, 2012). The equations are as follows:
LAR (cm2·g-1) = leaf area (cm2) / dry weight of shoot (g)
Compactness (mg·cm-1) = dry weight of shoot (mg) / plant height (cm)
Statistical analysis
The experiment utilized a block design with 50 plants per treatment in triplicates, and twelve uniform seedlings per treatment were collected for the growth measurements. The statistical analysis method used was the analysis of variance (ANOVA), which was done with SAS (SAS 9.4, SAS Institute Inc., Cary, NC, USA), and comparisons between the means were done using Tukey’s test. Differences were considered statistically significant at p < 0.05. Graphs were displayed using SigmaPlot software (SigmaPlot 14.5; Systat Software Inc., San Jose, CA, USA).
Results and Discussion
Intumescence development of grafted seedlings during cultivation
Figs. 1 and 2 show the development of intumescence in the pepper- and tomato-grafted seedlings during cultivation. In the peppers, small blisters were found on the adaxial leaf surfaces during healing in the LED healing chamber (Fig. 1A and 1B). After healing and acclimatization, the small blisters on the leaves turned brown and became more prominent (Fig. 1C and 1D). In contrast, the blistering on the tomatoes appearing on the abaxial leaf surface was larger than that on the peppers (Fig. 2A and 2B). After healing and acclimatization, the large blisters became brown and necrotic, leaving noticeable, large wounds on the adaxial surface (Fig. 2C and 2D). The incidence of intumescence in plants (IMP) continued to increase during healing in the LED healing chamber for both the pepper and tomato plants (Fig. 3). However when exposed to natural light after healing, the intumescence no longer expanded, and the tips of the blisters turned brown and remained on the leaves of the grafted seedlings. Intumescence is a physiological disorder characterized by the abnormal enlargement of epidermal or palisade cells on the surfaces of leaves and stems in plants, resulting in tumor-like tissue (Wetzstein and Frett, 1984; Morrow and Tibbits, 1988). Intumescence injury can occur on the adaxial surface, abaxial surface, or on both leaf surfaces depending on the plant species (Williams et al., 2016). Spencer et al. (2021) observed a case of severe intumescence injury on the adaxial leaf surface of pepper, and Suzuki et al. (2020) identified enlarged epidermal cells on the abaxial leaf surfaces of tomatoes using light microscopy. The intumescence development process in the pepper- and tomato-grafted seedlings here aligns with earlier findings for ornamental sweet potato (Ipomoea batatas L.) and tomato leaves correspondingly reported by Craver et al. (2014) and Williams et al. (2016). For example, the tips of blisters exhibited discoloration, turning black in ornamental sweet potatoes and brown and necrotic in both ornamental sweet potato and tomato leaves (Craver et al., 2014; Williams et al., 2016). Under specific conditions, such as exposure to natural light, intumescence no longer expanded and the plants exhibited senescence, accompanied by discoloration of the blisters. Craver et al. (2014) identified the development of blisters on tomato and ornamental sweet potato as ‘intumescence.’ The lesions found in these species resulted in cellular abnormalities, predominantly affecting the epidermis and leading to cell and tissue senescence. Therefore, the symptoms observed here in the LED healing chamber of the plug-seedling nursery were indicative of intumescence.
Susceptibility of pepper and tomato cultivars to intumescence
The IMP (%) of the peppers was higher than that of the tomatoes (Fig. 3). For the peppers, intumescence was not observed until 1 d after grafting (Fig. 3A). However, from day 2, all cultivars showed an IMP of more than 2%, especially ‘Nokkwang’ (38.7%), ‘New Ace’ (31.3%), and ‘Sunhan Gilsang’ (25.0%) which showed a significantly higher IMP that were followed by a rapid increase in each case (Fig. 3A). Conversely, the ‘Matkkaljin’ (2.3%), ‘Sinrokput’ (2.0%), and ‘Theganghan’ (2.0%) peppers showed the lowest IMP (Fig. 3A). At 4 d, the end of the healing period, all cultivars had an IMP of over 62.7%, with ‘Nokkwang’ (92.0%) having the highest and ‘Superior’ (62.7%) having the lowest IMP. For the tomatoes, intumescence was initially observed 3 d after grafting in 1.3% of ‘TY Haruaki’ (Fig. 3B). On the 4 d, only two cultivars, ‘TY Haruaki’ (10.7%) and ‘TTM-130’ (3.3%), had intumescence injuries. However, none of the cherry tomato cultivars exhibited intumescence. Intumescence was observed in all pepper cultivars after healing, unlike in tomatoes, where only two cultivars exhibited intumescence. To identify cultivars that were highly susceptible to pepper intumescence, the IML (%) in pepper seedlings was calculated (Fig. 4). The crunchy pepper ‘Sunhan Gilsang’ had a significantly higher IML of 57.9%, while the sweet pepper ‘Superior’ and the kkwari pepper ‘Nongwoo Kkwari’ had significantly lower IML of 35.3% and 35.1%, respectively (Fig. 4). Based on these results, two pepper cultivars that were highly susceptible to intumescence were identified. One was the crunchy pepper ‘Sunhan Gilsang,’ which showed a high IMP (25.0%) 2 d after grafting and the highest IML (57.9%; Figs. 3A and 4), and the other was the nokkwang pepper ‘Nokkwang,’ which showed the highest IMP (92.0%) 4 d after grafting (Fig. 3). Conversely, among the cultivars used in the experiment, a moderately resistant cultivar was the sweet pepper ‘Superior,’ which showed the lowest IMP (63.0%) 4 d after grafting and a significantly lower IML (35.3%; Figs. 3A and 4). For the tomatoes, only two cultivars, ‘TY Haruaki’ (10.7%) and ‘TTM-130’ (3.3%), exhibited intumescence (Fig. 3B) and were assumed to be susceptible. The IMP outcomes for both the peppers and tomatoes increased as healing progressed (Fig. 3). During the first 1–2 d after the grafting of the vegetable seedlings, water absorption in the scion and rootstock was hindered due to the cutting between them. To promote cell division and to connect the vascular bundle between the scion and the rootstock, the temperature was maintained at 25–30°C and the relative humidity was kept in a saturated state (RDA, 2020a, 2020b). Intumescence occurred in pepper- and tomato-grafted seedlings after 2 and 3 d, respectively (Fig. 3). It has been reported that pepper graft-take occurs two days after grafting (Jang et al., 2009). According to Jeffree and Yeoman (1983) and Turquois and Malone (1996), tomato grafting occurs between three and five days. Therefore, it can be assumed that the intumescence of the grafted seedlings in the LED healing chamber is related to the ‘vascular bundle connection’ or ‘water uptake’ between the scion and rootstock. A rapid increase in the relative humidity or soil moisture content may be accompanied by cell wall enlargement and rupture owing to the increased pressure in the xylem (Miyama and Yasui, 2021). The scion was exposed to sudden high humidity and LED light for grafting. In addition, the connection between the rootstock and scion can cause a rapid change in the water transpiration pressure in the scion, leading to cell enlargement after 1–2 d in the cut state. Pepper plants are susceptible to water stress (Smittle et al., 1994). Therefore, intumescence injuries are expected to occur more frequently in grafted pepper seedlings during plug-seedling production.
Stress level of pepper and tomato-grafted seedlings with and without intumescence (Fv/Fm)
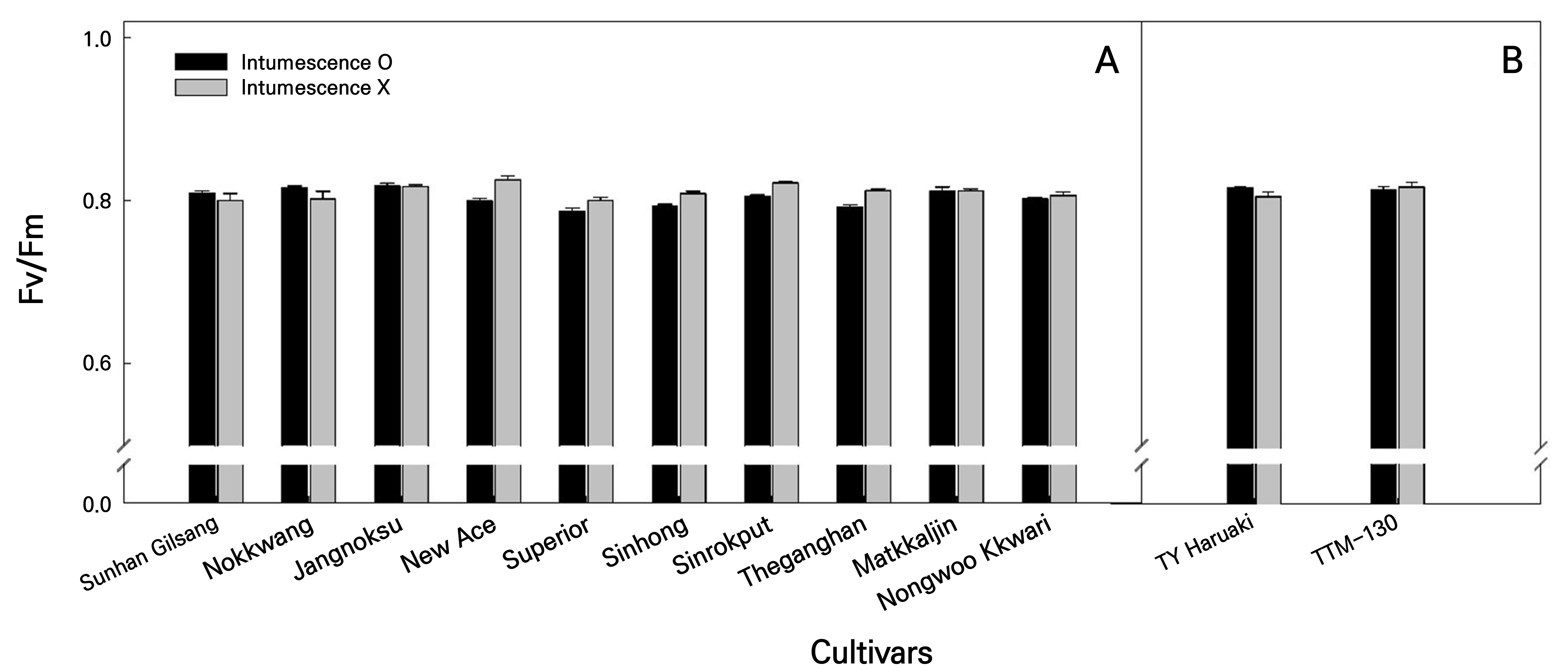
Intumescence injuries from the LED healing chamber persisted in the older leaves of the pepper- and tomato-grafted seedlings. However, the Fv/Fm ratio of pepper seedlings was between 0.78 and 0.84 regardless of the presence of intumescence (Fig. 5). In tomatoes, there was no significant (p > 0.05) difference in the Fv/Fm ratio of the leaves in the two cultivars ‘TY Haruaki’ and ‘TTM-130.’ The chlorophyll fluorescence index has been used as a means of the non-destructive assessment of the photosynthetic capacity of plants (Moradi and Ismail, 2007; Hazrati et al., 2016) or as an indicator of the stress physiology of a crop (Krause and Weis, 1991; Willits and Peet, 1999). The Fv/Fm of healthy leaves is approximately 0.83 (Johnson et al., 1993; Maxwell and Johnson, 2000), and according to Kycko et al. (2018), when leaves are exposed to biotic or abiotic types of stress, their Fv/Fm rates decrease. However, the remaining intumescence injury on the older leaves of the grafted seedlings before shipping appeared to have no considerable effect on plant stress levels.
Growth of pepper- and tomato-grafted seedlings by cultivars
The plant heights of the pepper-grafted seedlings were between 11 and 14 cm before shipping, regardless of the cultivar, and there were 6–10 leaves in the seedlings (Table 3). The crunchy pepper ‘Sunhan Gilsang’ had a thin stem diameter (3.1 mm) and a low SPAD (29.0). In contrast, the nokkwang pepper ‘Nokkwang’ had a significantly higher SPAD (38.1). The sweet pepper ‘Superior’ had the largest leaf area (66.4 cm2) and the heaviest fresh (2.8 g) and dry weight (0.3 g) of the shoots. However, the cheongyang pepper ‘Sinhong’ had the thinnest diameter (2.9 mm) and the lightest fresh (2.1 g) and dry weight (0.18 g) of the shoots, and ‘Theganghan’ had the smallest leaf area (46.1 cm2). The plant heights of the tomato-grafted seedlings were 7.3–10.0 cm, and there were four to five leaves regardless of the cultivar (Table 4). Jujube-shaped cherry tomato cultivars tended to have more leaves than ordinary tomato cultivars. The ordinary tomato cultivars ‘TY Haruaki’ and ‘TTM-130’ tended to have lower SPAD values of 33.2 and 34.9, a larger leaf area of 67.0 cm2 and 72.0 cm2, and higher fresh weights of 3.7 g and 3.4 g, respectively. Conversely, the jujube-shaped cherry tomato cultivars ‘TY Candy,’ ‘TY Daekkiri,’ and ‘Mini Chal’ exhibited relatively more leaves, higher SPAD values, a narrower leaf area, and lighter fresh and dry weights of shoots than the ordinary tomato cultivars. The growth rate of cherry tomatoes is higher than that of ordinary tomatoes after the development of three to four true leaves (RDA, 2020a). ‘Superior,’ a moderately intumescence-resistant pepper cultivar, is a sweet pepper characterized by a thick stem diameter, high fresh and dry weights, and a large leaf area. It is not spicy compared to other peppers (Tables 2 and 4). However, the highly susceptible intumescence cultivar ‘Sunhan Gilsang’ is a crunchy pepper with a thinner stem diameter and very mild spiciness. In tomatoes, the intumescence-susceptible cultivars ‘TY Haruaki’ and ‘TTM-130’ are both ordinary tomato cultivars that are characterized by a low number of leaves, a low SPAD value, a large leaf area, and heavy shoot fresh and dry weights compared to jujube-shaped cherry tomato cultivars that produce large tomato fruits (Tables 3 and 5). Both peppers and tomatoes are native to South America and are cultivated in areas with high levels of UV radiation (Cho et al., 2000; Seo and Sung, 2006; de Vries et al., 2016; Jang et al., 2023). Wild tomato species (Solanum habrochaites) are observed in South America at high altitudes where UV radiation is abundant (de Vries et al., 2016). UV-B inhibits cellular hypertrophy (Weston et al., 2000); therefore, cultivation in a drastically lower UV environment than the native environment results in intumescence injuries due to cellular hypertrophy (Eguchi et al., 2016). In contrast, jujube-shaped cherry tomatoes are cultivated with domestically developed cultivars in Korea, covering approximately 70% of the cultivated area (Kim and Kim, 2016); they did not exhibit intumescence in this study. However, the association between the growth characteristics of these cultivars and their susceptibility to intumescence requires further investigation.
Table 3.
Growth characteristics of the different grafted pepper seedlings used in experiments after 13 d of grafting (n = 12)
| Cultivar |
Plant height (cm) |
Number of leaves |
Stem diameter (mm) | SPAD |
Leaf area (cm2/plant) | Shoot | |
|
Fresh weight (g/plant) |
Dry weight (g/plant) | ||||||
| Sunhan Gilsang | 11.4 cdz | 7.4 c-e | 3.1 bc | 29.0 d | 52.2 b-d | 2.2 cd | 0.20 bc |
| Nokkwang | 12.2 bc | 7.9 b-d | 3.2 ab | 38.1 a | 57.2 a-c | 2.3 b-d | 0.25 a |
| Jangnoksu | 12.6 a-c | 7.8 b-d | 3.3 ab | 38.5 a | 53.2 bc | 2.3 b-d | 0.24 ab |
| New Ace | 10.8 d | 6.4 e | 3.2 ab | 30.8 cd | 60.6 ab | 2.5 a-c | 0.22 a-c |
| Superior | 11.6 cd | 7.2 de | 3.2 ab | 34.4 bc | 66.4 a | 2.8 a | 0.3 a |
| Sinhong | 12.3 a-c | 8.0 b-d | 2.9 c | 32.3 b-d | 50.2 cd | 2.1 d | 0.18 c |
| Sinrokput | 13.1 ab | 8.3 a-d | 3.3 ab | 32.4 b-d | 53.8 a-c | 2.4 b-d | 0.23 a-c |
| Theganghan | 12.2 bc | 8.5 a-c | 3.1 a-c | 33.8 a-c | 46.1 d | 2.1 cd | 0.21 a-c |
| Matkkaljin | 12.9 ab | 8.8 ab | 3.4 a | 35.5 ab | 61.2 ab | 2.7 ab | 0.25 a |
| Nongwoo Kkwari | 13.6 a | 9.6 a | 3.2 ab | 34.1 a-c | 60.3 ab | 2.6 ab | 0.24 ab |
Table 4.
Growth characteristics of the different grafted tomato seedlings used in experiments after 13 d of grafting (n = 12)
| Cultivar |
Plant height (cm) |
Number of leaves |
Stem diameter (mm) | SPAD |
Leaf area (cm2/plant) | Shoot | |
|
Fresh weight (g/plant) |
Dry weight (g/plant) | ||||||
| TY Haruakki | 8.3 abz | 4.3 ab | 4.0 a | 33.2 c | 67.0 ab | 3.7 a | 0.31 ab |
| Sugoi | 8.1 ab | 4.2 b | 3.8 ab | 35.2 a-c | 61.4 ab | 3.3 ab | 0.32 a |
| Super Dotaerang | 7.3 b | 4.2 b | 3.5 b | 36.0 a-c | 56.4 ab | 2.9 bc | 0.27 a-c |
| TTM-130 | 9.2 ab | 4.3 ab | 3.9 ab | 34.9 bc | 72.0 a | 3.4 ab | 0.27 a-c |
| TY Candy | 8.3 ab | 4.7 ab | 3.9 ab | 39.0 a | 50.0 b | 2.7 bc | 0.23 bc |
| TY Daekkiri | 10.0 a | 4.5 ab | 4.1 a | 38.4 ab | 49.1 b | 2.4 c | 0.22 c |
| Mini Chal | 9.2 ab | 5.0 a | 3.8 ab | 36.9 a-c | 55.2 ab | 2.8 bc | 0.29 a-c |
Seedling quality changes for different pepper and tomato cultivars
Fig. 6A shows the changes in the LAR outcomes of the pepper seedlings. The susceptible cultivars ‘Sunhan Gilsang,’ ‘Nokkwang,’ and the moderately resistant cultivar ‘Superior’ showed no significant differences (p > 0.05) in LAR before grafting. However, the LAR of ‘Sunhan Gilsang’ decreased substantially after healing. Before shipping, the LAR of ‘Sunhan Gilsang’ increased rapidly, indicating a thin leaf thickness similar to those of the other cultivars. LAR is calculated as the leaf area divided by the dry weight of the shoots, indicative of the leaf thickness, with lower values indicating thicker leaves (Jo et al., 2021). During the healing process, there can be interruptions in the transport of nutrients and water and disruptions in photosynthesis between the scion and rootstock. Scions are susceptible to water stress, which is attributed to a decrease in internal water due to transpiration until the establishment of the xylem connection (Jang et al., 2014). In contrast to other cultivars, ‘Sunhan Gilsang’ exhibits high sensitivity to intumescence (Fig. 4) and a reduced leaf area under conditions of high relative humidity and water stress (Del Amor and Marcelis, 2004). The reduced leaf area likely contributed to the decreased LAR of ‘Sunhan Gilsang’ after grafting. Yang et al. (2024) reported that leaves become thicker when exposed to UV-B, and these thicker leaves can retain more moisture and are advantageous for drought due to grafting. On the contrary, the ‘Sunhan Gilsang’ is judged to be sensitive to moisture stress due to its thin leaf thickness. Fig. 6B shows the changes in LAR for the tomato cultivars. No significant differences (p > 0.05) in LAR were observed before grafting or after healing. However, the LAR of ‘TTM-130’ was significantly higher in grafted seedlings before shipping. Although intumescence occurred in the tomato-grafted seedlings after healing, no significant difference was observed in the LAR outcome. Fig. 6C shows no significant difference in the compactness of pepper seedlings before grafting. However, on September 19 and 28 2022, ‘Superior’ exhibited the highest degree of compactness, whereas ‘Sinhong’ exhibited the lowest. Compactness, calculated as the dry weight of the shoot divided by the plant height, indicates tissue fidelity, with higher values indicating high-quality seedlings that had experienced less stress after transplantation (Lee et al., 2016). Uniform pepper seedlings with no significant differences in compactness were used before grafting. Before grafting, ‘Sunhan Gilsang’ showed the lowest degree of compactness. No significant difference existed between ‘Sinhong’ and ‘Sunhan Gilsang’ before grafting. The varying levels of the compactness of grafted seedlings likely represent different characteristics of each cultivar. For tomato seedlings, there was no difference in compactness between the cultivars before grafting or after healing (Fig. 6D). However, the grafted seedlings tended to show lower compactness in the cases of the jujube-shaped cherry tomato cultivars ‘TY Daekkiri’ and ‘TY Candy.’ However, ‘TY Daekkiri’ and ‘TY Candy’ are cultivars in which intumescence was not observed. Therefore, we can conclude that the intumescence sensitivity of tomato-grafted seedlings did not directly affect seedling quality.
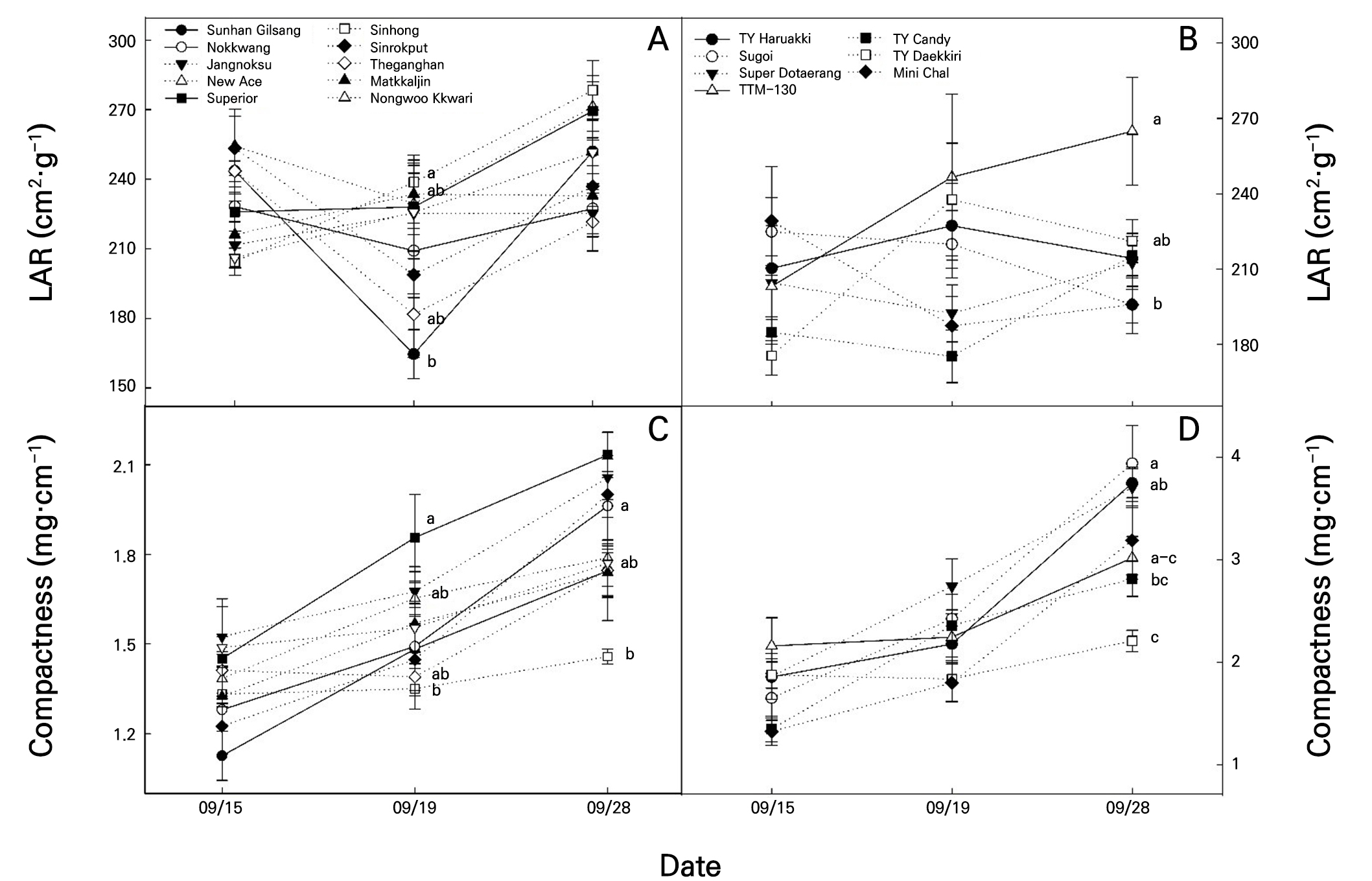
Fig. 6.
LAR (A) and compactness (C) of pepper seedlings, and LAR (B) and compactness (D) of tomato seedlings before grafting, after healing, and before shipping. LAR, leaf area ratio. Vertical bars represent the standard deviation of the mean (n = 12). Different letters above the bars indicate significant differences according to Tukey’s test at p < 0.05.
Conclusion
Nurseries that produce grafted plug seedlings have found severe intumescence injuries in the scion. This study was conducted to identify scion cultivars susceptible to intumescence while grafting pepper and tomato seedlings. Ten pepper and seven tomato cultivars were selected for an examination of their susceptibility to intumescence. Intumescence was observed in all pepper cultivars and in only two tomato cultivars. The results here indicate that the ‘Sunhan Gilsang’ crunchy pepper and the ‘Nokkwang’ nokkwang pepper are highly susceptible cultivars, whereas the ‘Superior’ sweet pepper exhibits moderate resistance. For tomatoes, only ‘TY Haruakki’ and ‘TTM-130’ exhibited intumescence in the experiments conducted here. However, further studies are required to establish an association between growth and intumescence or any significant impairment due to intumescence injury.