Introduction
Materials and Methods
Plant Material
Effect of Temperature Variation on Growth and Flowering
Treatment with Silver Ion under Low Temperature (20/15°C)
ACC Content
ACC Oxidase Activity
Ethylene Production
Experimental Design and Statistical Analyses
Results
Effects of Temperature Variation on Growth Rate
Effects of Temperature Variation on Flower Bud Formation
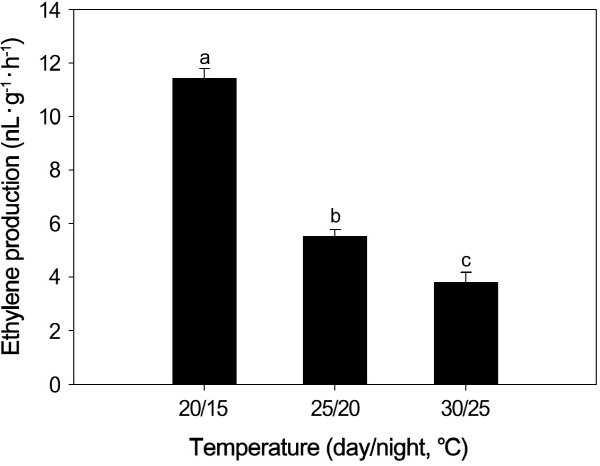
Effects of Temperature Variations on Ethylene Production
Effects of Silver Ion on Growth and Flowering
Effects of Silver Ion on ACC Content and ACC Oxidase under Low Temperature (20/15°C)
Effects on Ethylene Production under Low Temperature (20/15°C).
Discussion
Introduction
Passion fruit (Passiflora spp.) are popular in many sub-tropical and tropical countries. The results of previous studies have shown that the flowering of passion fruit is enhanced by appropriate regulation of environmental factors, such as photoperiod (Menzel and Simpson, 1988; Nave et al., 2010), temperature (Menzel et al., 1987), water content (Menzel et al., 1986; Staveley and Wolstenholme, 1990), and the application of plant growth regulators including aminoethoxyvinylglycine (AVG) and silver thiosulfate (STS) (Barbosa et al., 2001; Reis et al., 2003).
Seasonal temperature variations are usually less than 3-4°C in tropical areas (diurnal temperature variation is usually 8-10°C). Simon and Karnatz (1983) showed that day/night temperatures of 25-30/20°C increased shoot growth and flowering, but reduced yields in purple passion fruit cuttings. Menzel et al. (1987) showed that the vegetative growth of purple × golden hybrids ‘Purple Gold’, ‘E-23’, and ‘Lacey’ was greater at 20/15°C, 25/20°C, and 30/25°C compared to those at 15/10°C under low solar radiation levels of 9.8 MJ m-2 day-1 (234 cal・cm-2・day-1)(in winter in Queensland, Australia). For ‘E-23’ and ‘Purple Gold’, the increased vegetative growth at 25/20°C and 30/25°C compared to those at 20/15°C was associated with reduced flowering. The growth of shoot was greater at 33/28°C compared with 23/18°C and 28/23°C (day/night temperature) in the purple passion fruit (Utsunomiya, 1992).
Among the 400 species of Passiflora, there are 50-60 edible species of passion fruit but only half of them are grown solely for their fruit, and among those that have been commercially cultivated are P. edulis Sim., P. edulis f. flavicarpa Deg., and their cross-breeding, i.e. ‘Tai-nung No.1’, P. edulis × P. edulis f. flavicarpa. Passion fruit ‘Tai-nung No.1’ tolerates a wide range of climatic conditions from sea-level to altitudes of up to 600 meters, and is the most popular passion fruit in Taiwan. Most Passiflora species, including passion fruit ‘Tai-nung No.1’ are not cold-tolerant for outdoor cultivation and require protected cultivation in cool temperature zones (Chang and Cheng, 1992). Many subtropical and tropical plants are chilling-sensitive; when stressed by low temperature, the tissues of such plants respond by producing ethylene (Max et al., 2011; Orihuel- Iranzo et al., 2010; Tacken et al., 2010). Several studies have demonstrated that the leaves of the Passiflora species stored at 0°C can release ethylene (Chen and Patterson, 1985), and the more sensitive species produced larger amounts of ethylene than those more tolerant to chilling (MacRae et al., 1986). Therefore, ethylene produced by plant tissue during cold winter conditions, is presumably an important inhibitor of growth and flowering in some Passiflora plants.
This study evaluated the effects of different day/night temperature regimes on growth and flower formation of Passiflora Tai-nung No.1 (Passiflora edulis × P. edulis f. flavicarpa) and also the effects of AgNO3 and STS on growth and flowering of young, potted Passiflora ‘Tai-nung No.1’ plants grown at the low temperature regime (20/15°C).
Materials and Methods
Plant Material
Experiment 1. Passion fruit plants (Tai-nung No.1, Passiflora edulis × P. edulis f. flavicarpa) were obtained in June from a commercial passion fruit nursery and planted in 18-cm diameter pots containing 2.9 L of a 2:2:1 (v/v) mix of vermiculite, perlite, and peat moss, and then were grown in a phytotron (25/20°C day/night temperature) under natural light, at 60-80% RH. On 5 August, the plants had an average height of 50.2 cm and an average of 7.4 nodes when the various treatments started. The average PPFD (photosynthetic photon flux density) at noon was 400 μmol・m-2・s-1. Plants were watered (to soil moisture content greater than 80%) when the surface of the medium became slightly dry. For slow-release fertilization, 3.0 g of Osmocote ® (14:14:14 N:P:K; Scotts Company, Marysville, OH, USA) was applied to each pot at monthly intervals.
Experiment 2. Passiflora Tai-nung No.1 grafted seedlings were purchased from a nursery in Puli, Taiwan. These plants were transplanted into 2.9 L plastic pots containing 1:1:1 (v/v) mix of vermiculite, perlite, and peat moss, then grown in an open area of the Experimental Farm at 20-28°C and an average noontime PPFD of 397.5 μmol・m-2・s-1. For slow- release fertilization, 3.0 g of Osmocote ® was applied to each pot at monthly intervals. After treatment, plants with average height of 52.4 cm and nodes of 7.9 were transferred to a phytotron at 20/15°C (day/night temperature), and average noon PPFD was 150 μmol・m-2・s-1.
Effect of Temperature Variation on Growth and Flowering
Plants were grown in the phytotrons at 30/25°C, 25/20°C or 20/15°C (day/night temperature), and exposed to the day temperature for 12 h (6 a.m. to 6 p.m.). Each treatment was applied to four potted plants. Each vine was treated weekly with Peter’s solution (20:20:20 N:P:K; Scotts Company, Marysville, OH, USA). The growth rate, plastochron (the interval of time between the appearances of successive leaf primordia at the shoot apex), days to first flower formation and the number of flower buds were recorded weekly (Experiment 1).
Treatment with Silver Ion under Low Temperature (20/15°C)
Plants were sprayed with AgNO3 or STS both at 0.5 or 1.0 mM, or distilled water (control) and then subjected to low temperature conditions (20/15°C) for one month. STS and AgNO3 stock solutions were prepared as follows: 0.1 M STS stock solution was prepared by dissolving 1.58 g of STS in 100 mL of water, whereas 0.1 M AgNO3 stock solution was prepared by dissolving 1.7 g of AgNO3 in 100 mL of water. The stock solutions were stored in the dark until needed to prepare the STS. Each treatment was applied to five potted plants. Growth rate, plastochron and internode length were determined, and the days to the first flower bud formation, the node number to initial flower bud were recorded (Experiment 2).
ACC Content
Detached passion fruit leaves after AgNO3 or STS treat-ments were used. ACC (1-aminocyclopropene-1-carboxylate) content was assayed using the method described by Lizada and Yang (1979). For each sample 0.5 g fresh weight (FW) of leaves was washed in a test tube with 5 mL of 80% (v/v) ethanol for subsequent extraction of leaves in a hot water bath at 70°C for 20 min, followed by centrifugation. This procedure was repeated twice, and the mixture of the two extracts was further concentrated under vacuum until it became anhydrous. Distilled water was then added to a final volume of 1.0 mL. All samples were then stored in test tubes at 0°C to be analyzed within 2 weeks.
To perform the analyses, two 0.5 mL aliquots of each sample were mixed with 0.1 mL 50 mM HgCl2. Then 0.1 mL 100 mM ACC was added to one of these sub-samples. Nothing was added to the other. The final volumes were brought to 1.8 mL by adding distilled water and the mixtures were placed in test tubes, which were sealed with sleeve stoppers. While the tubes were submerged in crushed ice, 100 μL of a 2:1 (v/v) mixture of iced 5% (v/v) NaOCl and saturated NaOH was added to each test tube. The contents were vortex mixed for 5 sec., then each tube was placed in crushed ice for 2.5 min. They were then vibrated for 1 sec. and 1 mL of the gas was extracted for analysis using a GC-14A gas chromatograph (Shimazdu, Japan) fitted with a flame ionization detector. The carrier gas was nitrogen at a flow rate of 0.42 mL・s-1 and the standard gas (Hsinan Inc., Taipei, Taiwan) was 1.0 μL・L-1 ethylene.
ACC Oxidase Activity
Detached passion fruit leaves after AgNO3 or STS treatments were used. The ACC oxidase detection method was from a previously described method (Mekhedov and Kende, 1996; Ververdis and John, 1991). A 1.0 mL gas sample was injected into a GC-14A gas chromatograph (Shimazdu, Japan) fitted with a flame ionization detector.
Ethylene Production
Detached passion fruit leaf after temperatures (30/25°C, 25/20°C and 20/15°C) or AgNO3 or STS treatments were placed in a 40 mL sealed plastic jar at 20-28°C for 2 h under cool-white fluorescent lamps providing 15-16 μmol・m-2・s-1. A 1.0 mL gas sample was injected into a Shimadzu GC-14A gas chromatograph with a flame ionization detector. The carrier gas was nitrogen and the standard gas was 1 mg・L-1 ethylene.
Experimental Design and Statistical Analyses
The experiments were arranged in a completely randomized design. The data were analyzed using ANOVA for comparing means of treatment variables at p < 0.05. CoStat 6.2 (CoHort Software, Monterey, CA, USA) was used for the analysis.
Results
Effects of Temperature Variation on Growth Rate
Shoot growth at 30/25°C was faster than either 25/20°C or 20/15°C, where the plants produced more leaves with shorter plastochrons and enhanced internode elongation (Table 1). With respect to the plastochron, leaf formation was slowest in the 20/15°C treatment (4.5 days), indicating that vegetative growth of the shoot increases with increasing temperatures (Table 1).
Effects of Temperature Variation on Flower Bud Formation
The mean days to the first flower bud and the total number of flower buds per plant did not significantly differ between 25/20°C and 30/25°C treatments (Table 2). However, plants grown at 30/25°C had a higher rate of aborted flower buds than those grown at 25/20°C. No flower formation was observed on the plants at 20/15°C (Table 2).
Effects of Temperature Variations on Ethylene Production
Ethylene production in the leaves of passion fruit was significantly higher when plants were grown at the relatively lower temperature (20/15°C), compared to 25/20° and 30/25° temperatures (Fig. 1).
Effects of Silver Ion on Growth and Flowering
Shoot growth of passion fruit ‘Tai-nung No.1’ was signifi-cantly inhibited by both AgNO3 and STS treatments at 20/15°C. The plants treated with AgNO3 or STS had lower growth rates and higher plastochron values than controls (Table 3). Those treated with 1.0 mM AgNO3 had the highest plastochron values followed by those treated with 1.0 mM STS, indicating that ethylene action inhibitors delayed leaf formation. However, AgNO3 and STS enhanced flower formation in passion fruit compared to the untreated control where no flowers were formed (Table 4). Internode length were not influenced (Table 3). All plants treated with AgNO3 or STS formed their first flower buds approximately 2 weeks after treatment.
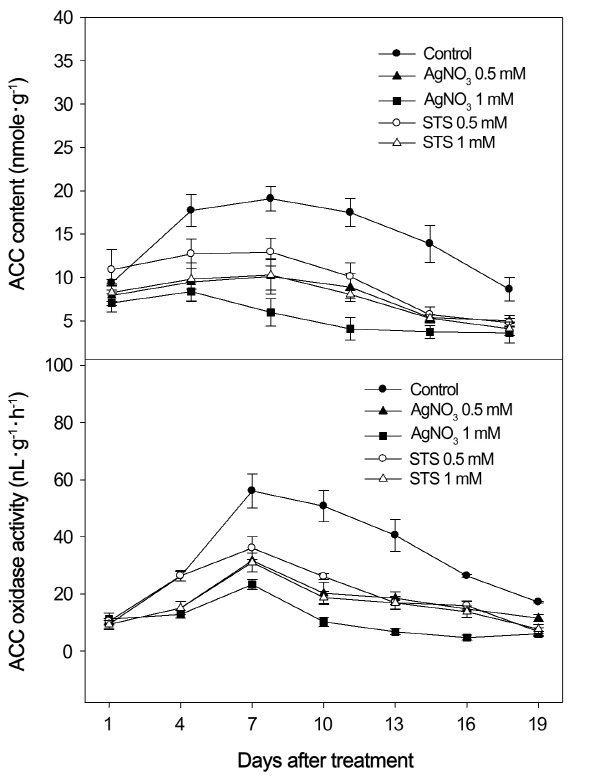
Effects of Silver Ion on ACC Content and ACC Oxidase under Low Temperature (20/15°C)
The ACC content and ACC oxidase activity in the leaves of passion fruit plants exposed to the 20/15°C temperature condition were significantly decreased by both AgNO3 and STS treatments throughout 19 days of treatments (Fig. 2).
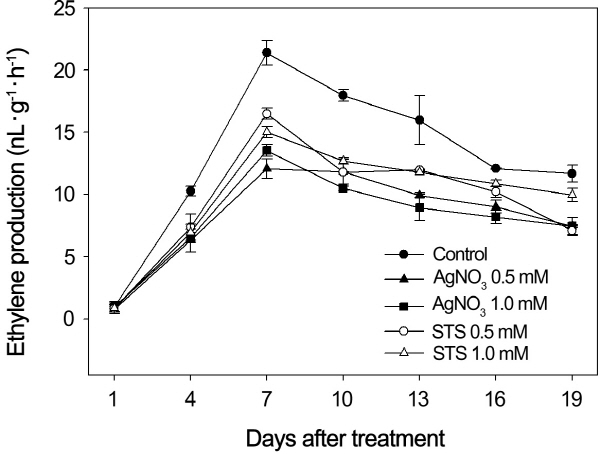
Effects on Ethylene Production under Low Temperature (20/15°C).
Low temperature treatment at 20/15°C obviously increased ethylene production during the first 7 days (Fig. 3). AgNO3 and STS treatments significantly retarded ethylene production.
Discussion
This study revealed that temperature significantly affects vegetative growth and flowering of passion fruit. High temperatures accelerate vegetative growth of passion fruit. These observations are consistent with those of Meinke and Karnatz (1990), Menzel et al. (1986, 1987), Simon and Karnatz (1983), and Utsunomiya (1992). Utsunomiya (1992) studied the growth and flowering response of the cool-loving purple passion fruit at 23/18°C, 28/23°C, and 33/28°C, and found that the lower temperature treatment (23/18°C) promoted flowering and fruit set, while the higher temperature treatments (28/23°C or 33/28°C) resulted in faster fruit development and better fruit quality. In this study, we investigated the growth and flowering response of the heat-tolerant ‘Tai-nung No. 1’ at 20/15°C, 25/20°C, and 30/25°C. We found that low temperature (20/15°C) inhibited flowering in ‘Tai-nung No. 1’ passion fruit, while higher temperatures (25/20°C and 30/25°C) promoted flowering. We also found that application of ethylene response inhibitor (AgNO3 or STS) under low temperature induced flowering. Thus, the results of this study are significantly different from that found by Utsunomiya (1992). This significant difference in flowering is most probably due to different climatic preferences between these two different types of passion fruit, one cool-loving and the other heat-tolerant.
In this study, plants grown at 25/20°C exhibited the earliest flower bud formation than those grown at higher (30/25°C) or lower (20/15°C) temperature regimes, and produced their first flower buds on lower nodes and had lower abortion rate of flower buds. The same effects were noted in tomatoes (Calvert, 1959; Hussey, 1963a; Ohyama et al., 2005). According to previous studies, flower formation in passion fruit was affected by a critical range (20-30°C) of temperatures (Chang and Cheng, 1992). Beyond this range, no flower formation is induced (Chang and Cheng, 1992). This confirms the results of previous study demonstrating the inhibition of the growth and flowering of the passion fruit species during winter in Taiwan (Chang and Cheng, 1992).
An interesting question is why plants grown at higher temperatures form their initial flower buds on relatively higher nodes and have higher abortion rates of flower buds (Table 2). Hussey (1963a, 1963b) proposed that developing leaves and apical meristems of Lycopersicon esculentum compete vigorously for assimilates. In the case of tomato, high temperatures are beneficial for proliferation of leaf primordia, while low temperatures or the removal of young leaves may cause early transformation of apical meristems to inflorescence (Hussey, 1963a, 1963b; Sawhney, 1983). Thus we propose that passion fruit plants grown at high temperatures exhibit more vigorous vegetative growth by expending more nutrition on the vegetative growth of stems and leaves, which reduces nutrition available for flower formation. Therefore, more leaves are needed to produce more assimilates to support the formation and development of flower buds. Also plant hormone is related to floral induction. More vigorous growth produces more GA and/or auxin out of more young tissues, which delays floral initiation (Zhu and Davies, 1997). Additionally, treating tomato plants with gibberellic acid (GA) reduced flower bud formation and caused them to produce their first flower buds on higher nodes (Abdul and Harris, 1978; Sasaki et al., 2005). At high temperature, young leaves of tomato contained relatively higher GA content leading to enhanced plant growth, while flower formation and development were suppressed (Abdul and Harris, 1978; Beppu et al., 2001; Leonard and Kinet, 1982). The same effects were observed in grape (Ziv et al., 1981). Additionally, we found that treating passion fruit with paclobutrazol (gibberellin biosynthesis inhibitor) suppressed branch growth, caused the plants to produce their first flower buds on lower nodes, and promoted flower bud formation (data not shown). These observations may be associated with increases of GA in passion fruit grown at high temperatures. Based on the nutrient diversion hypothesis proposed by Sachs (1977), GA-induced vegetative growth caused the competition for nutrients between the formation of flower buds and the growth of branches and leaves; hence, enhanced growth of vegetative tissues will suppress the formation and development of flower buds.
Light intensity is another important factor affecting flower formation. In Experiment 1, the passion fruit plants were transferred to different temperature under high light intensity (400 μmol・m-2・s-1) in the summer, and flower formation occurred more quickly at 30/25°C than at 25/20°C (data not shown). The number of flower buds did not significantly differ between 30/25°C and 25/20°C treatments. Additionally, no flower formation occurred among passion fruit plants transferred to three different temperature regimens (30/25°C, 25/20°C or 20/15°C) under low light intensity (100-150 μmol・m-2・s-1) (data not shown). With low light intensity below a critical range, flowering of passion fruit was not induced at any temperature. Therefore, light intensity may moderate the impact of temperature on flowering of passion fruit. The same effects were observed in tomato (Calvert, 1959; Hussey, 1963a; Ohyama et al., 2005). In tomato, inflorescence occurred much earlier at higher temperatures (25°C) than at lower temperatures (15°C) under 318 μmol・m-2・s-1, but occurred more slowly at 159 μmol・m-2・s-1 (Hussey, 1963a). At low light intensity (150 μmol・m-2・s-1), floral development of tomato was enhanced as average air tem-perature decreased (Ohyama et al., 2005). High temperature apparently delayed flower formation and development under very low light intensity (50 μmol・m-2・s-1)(Calvert, 1959).
Ethylene is a plant hormone that regulates many aspects of plant growth and development ranging from seed ger-mination to organ senescence (Sisler and Yang, 1984). Ethylene promotes or inhibits vegetative growth (Jackson et al., 1981; Wheeler et al., 1996), promotes or inhibits the initiation of flowering (Elfving et al., 2004; Saltveit, 1999), and plays an important role in fruit maturity (Ritenour et al., 1999). Ethylene is also responsible for the transfer from the vegetative to the flowering stage in bougainvillea (Liu and Chang, 2011b). These indicate that ethylene is bi-directionally involved in regulating the flowering control of some plants. In Experiments 1 and 2, plants exhibited no flower formation at low temperature (20/15°C). The production of ethylene in the leaves of passion fruit ‘Tai-nung No.1’ at low temperature (20/15°C) was significantly higher than that at other temperatures (25/20°C and 30/25°C). All plants treated with AgNO3 or STS formed their first flower buds starting approximately 2 weeks after treatment, even in the 20/15°C conditions. These data indicate that silver ion, an inhibitor of ethylene action, promotes flower formation in passion fruit at low temperatures. Additionally, ACC content, ACC oxidase activity and ethylene production in the leaves of passion fruit ‘Tai-nung No.1’ at low temperature (20/15°C) were significantly inhibited by those chemical treatments. These results suggest that suppressed vegetative growth led to greater availability of nutrients for reproductive growth of plants under the low temperature condition. It seems that ethylene may play a positive role in the vegetative growth of passion fruit under the low temperature condition. The chemical treatment could enable transfer of plant nutrients to flower bud formation via reduction in ACC content, ACC oxidase activity and ethylene production.
Passion fruit leaves, stems or flowers produced similar amounts of ethylene by the end of the experiment (data not shown). Therefore, ethylene production in the passion fruit leaves is a good indicator for the entire shoot. The same effects were noted in bougainvillea (Liu and Chang, 2011a).
Although the number of flower buds in the STS-treated plants was comparable to that in AgNO3-treated plants, the leaves of STS-treated plants exhibited less sign of chemical toxicity than those of AgNO3-treated plants (data not shown). This indicated that the anionic complex STS, which is mobile and less phytotoxic in plant transport system (Veen, 1983, 1987), has less chemical toxicity than AgNO3 on the leaves of Passiflora spp. Silver, applied in the form of thiosulfate, is a very effective inhibitor of ethylene responses, but this heavy metal cannot be used on food and feed, and has been objected by environmentalists. Recently, a non-toxic antagonist of ethylene action, 1-methylcyclopropene (1-MCP), was discovered and has since been widely used (Blankenship and Dole, 2003). Therefore, the use of AgNO3 or STS can be substituted with 1-MCP for future applications to avoid silver ion toxicity.
In conclusion, temperature has significant effects on the growth and flowering of Passiflora. Ethylene production is easily induced by low temperature in Passiflora plants, which are especially chilling-sensitive plants (Chen and Patterson, 1985). Our study suggests that ethylene is an important inhibitor of flowering in Passiflora spp. Using ethylene action inhibitors offers the possibility of inducing flower formation under low temperature conditions in Passiflora plants.