Introduction
Materials and Methods
Fruit Materials and Treatments
Measurement of Marketable Fruit Rate
Evaluation of Postharvest Quality of Fruit
Evaluation of PPO Activity
Evaluation of POD Activity
Evaluation of MDA Content
Statistical Analysis
Results and Discussion
Effects of SNP on Marketable Fruit Rate
Effects of SNP on Quality Characteristics
Effects of SNP on PPO and POD Activities
Effects of SNP on MDA Content
Investigation of the Mechanism of Nitric Oxide Application in Postharvest Fruit
Conclusions
Introduction
Wax apple (Syzygium samarangense (Blume) Merr. & Perry), from the Myrtaceae family, has a bright color and superior flavor and is rich in nutrition, making it popular with consumers (Chen, 2020; Sun et al., 2020). Wax apple fruit is rich in protein, carbohydrates, vitamins, and minerals (Deng and Lin, 2010), and it can be consumed fresh or processed for juice, jam, and preserves (Zhou et al., 2011). Additionally, wax apple has medicinal benefits, including antibacterial activity, and can be used for cough relief, detoxification, and stomach distension reduction (Tina et al., 2011; Zhou et al., 2011). The fruits are gaining commercial importance with increasing recognition of their value to consumers (Chen et al., 2017). However, wax apple can only be stored at room temperature for a week, and the postharvest rot and disease restrict its consumption and distribution in the fruit market (Wu et al., 2018). The commodity value and shelf life of wax apple decline several days after harvest due to the shine and water loss (Ma et al., 2018) as well as microbial infections (Chen et al., 2020). To improve wax apple production, it is crucial to enhance its storage period and shelf life (Ma et al., 2018); however, few relevant studies have been conducted in China (Zhang et al., 2012a). Therefore, it is necessary to understand the physiological and biochemical changes that occur in the fruit after harvest and to devise an effective preservation method for retaining quality while suppressing maturity. In this study, we evaluated how SNP treatment changed the qualitative parameters and polyphenol oxidase, peroxidase, and malondialdehyde contents of postharvest wax apple.
Nitric oxide (NO) regulates physiological activity (Badiyan et al., 2004; Song et al., 2005), maturity, and senescence (Zhu et al., 2008) in plants as a ligand signaling transduction and a plant growth regulator. Additionally, NO has been suggested to delay the aging process of plant tissues and prolong storability by inhibiting ethylene synthesis (Jiang et al., 2010). Moreover, appropriate concentrations of NO delay the aging process of plants (Leshem and Haramaty, 1996). The respiration rate of postharvest wax apple fruit was significantly reduced and the fruit firmness was maintained after NO fumigation (Ye et al., 2012). In addition, NO treatment may decrease fruit softening by regulating enzyme activities related to the cell wall and lignin metabolism (Gao et al., 2016). The expression levels of related enzyme genes, including phenylalanine ammonia-lyase, peroxidase, and 4-coumarate:CoA ligase genes, have also been examined (Gao, 2016). The NO treatment of wax apple reduced the activities of peroxidase and phenylalanine ammonia-lyase (Hao et al., 2016) and polygalacturonase (PG) by affecting the expression level of the PG gene (Hao, 2017), thereby delaying postharvest fruit softening and senescence. NO reduces cinnamate-4-hydroxylase (C4H) activity by inhibiting the expression level of SsC4H (a C4H gene cloned from the wax apple fruit), thereby suppressing softening in wax apple (Huang et al., 2019, 2020).
SNP is a water-soluble sodium salt that can react with sulfhydryl groups in the cell to form cyanide and release NO (Ivankovich et al., 1978; Ma, 2018). SNP has been proven to function as an effective external donor of NO in sustaining both the appearance and inherent quality of wax apple after harvest (Liu et al., 2017). However, the use of SNP to extend the shelf life and maintain the postharvest quality of wax apple (‘Pink’) has not been documented. The purpose of this study is to explore the effects of SNP on postharvest wax apple (cv ‘Pink’) and to establish the appropriate concentration to preserve quality and suppress senescence.
Materials and Methods
Fruit Materials and Treatments
Wax apple (Syzygium samarangense, cv ‘Pink’) fruit were harvested from an orchard in Haikou, Hainan, China. Most fruit, especially tropical fruit, are harvested, stored, and processed at 80% maturity (Zhuang, 2001; Wu and Zhang, 2002; Qiu and Jiang, 2010; Kang et al., 2015), and this level of maturity is appropriate for wax apple to adapt to storage (Zhang et al., 2012a). The fruit were harvested in August, selected at 80% maturity, and had uniform shape, size, and color characteristics. The fruit were free of disease or mechanical injuries, and damage during transportation was minimized. In the preliminary test, wax apple fruit were exposed to various concentrations (0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, and 5.0 µmol·L-1) of sodium nitroprusside (SNP). According to Li et al. (2018), the physiological changes of fruit in the first few days of storage were tracked to determine the optimum concentration. As a consequence, the preliminary examined fruit were kept at 25°C for 4 days. In the final experiment, three concentrations of SNP (1, 2.5, and 5 µmol·L-1) were used, which may better depict the preservation effect of SNP on wax apple. The wax apples were randomly divided into different treatment groups. One group was soaked in distilled water as a control (group CK), and the other three were soaked in different concentrations of SNP (AR, Tianjin Beichen Fangzheng Reagent Factory, Tianjin, China) solution: group A (1 µmol·L-1), group B (2.5 µmol·L-1), and group C (5 µmol·L-1). According to Li (2011), Qi et al. (2019), and our previous study, the dipping time was 30 min. All samples were then naturally air-dried at an ambient temperature for 5 hours before being placed in an incubator (the temperature was 25°C, and the relative humidity was 90%). According to Zhang (2006), the shelf life of postharvest wax apple at 25°C is 6–7 days, after which the market value of the fruit is lost. Twelve fruits were randomly selected every day, and physiological indices were measured for 7 days.
Measurement of Marketable Fruit Rate
The marketable fruit rate was assessed to observe the mildew and shriveling of the epicarp (Qi and Xia, 2020; Wei et al., 2020). A marketable fruit refers to the fruit that is basically full and not wilting, and the rotten and moldy area accounts for less than 25% of the whole fruit. If the fruit begins to wilt or shrivel, or the rotten or moldy area is greater than 25%, it is considered unmarketable fruit. The total number of marketable fruits in each group was recorded every experimental day, and the marketable fruit rate was calculated as follows:
Evaluation of Postharvest Quality of Fruit
The soluble solids content (SSC) of fruit was measured using a refractometer (TOP TD-45, Zhejiang, China) at 25°C. According to Long and He (2002), the titratable acidity in wax apples was determined with NaOH (0.01 mol·L-1) and reported as a percentage. Pulp (3 g) was well ground in distilled water (50 mL), and the solution was boiled at 80°C for 30 min while shaking and cooled to room temperature. The prepared solution (10 mL) was pipetted into a clean conical flask and titrated immediately against standard sodium hydroxide using phenolphthalein indicator. The formula for measuring the titratable acidity (TA) of fruit was as follows, and the value was expressed as a percentage:
where V0 represents the volume of the filtrate used, V1 represents the volume of NaOH standard solution consumed, c represents the concentration of NaOH standard solution, m represents the mass of the pulp, and k represents the conversion factor of citric acid. The SSC and TA assays were repeated three times in each treatment group.
The Brix-acid ratio (BAR) of fruit was calculated based on the SSC/TA. The firmness of fruit pulp was measured using a fruit firmness tester (GY-1, Zhejiang Top Cloud-agri Technology Co., Ltd., China), and the diameter of the probe was 3.5 mm. An average of three points were chosen around the equatorial area of the fruit, and the experiment was repeated three times.
The anthocyanin contents of wax apple were estimated spectrophotometrically by Liu et al. (2003, 2007). One gram of peel tissue was cut into pieces and completely immersed in 0.1 mol·L-1 HCl solution (20 mL). The solution was placed in a thermostatic incubator (32°C) for 4 h and shaken several times during the extraction period. The mixture was then centrifuged at 13,000 × g for 5 min, and the absorbance of the supernatant was determined at 530 nm. The anthocyanin content was expressed as mg·kg-1, and three repetitions were implemented in each treatment group.
Evaluation of PPO Activity
Polyphenol oxidase (PPO) activity was measured in accordance with Jiang et al. (2002) with modifications. One gram of pulp from five fruits was well ground in 5 mL 10 mmol·L-1 phosphate buffer (pH 7.0) and centrifuged at 6,000 × g for 15 min. The supernatant (0.1 mL), 4 mL of phosphate buffer (10 mmol·L-1, pH 7.0), and 1 mL of 4-methylcatechol were reacted, and the change in absorbance at 420 nm was recorded. The determination values were defined as changes in enzyme units per minute per gram of fresh weight, and the measurement was repeated three times in each treatment group.
Evaluation of POD Activity
Peroxidase (POD) activity was assayed using the method described by Zou (2000) with minor modifications. One gram of flesh samples from five fruits was homogenized in 10 mmol·L-1 phosphate buffer (5 mL, pH 7.0), and the supernatant was collected after centrifugation (13,000 × g, 20 min, 4°C). The enzyme activity was determined in a 3-mL reaction mixture containing 10 mmol·L-1 phosphate buffer (2.91 mL), guaiacol (0.05 mL), H2O2 (0.02 mL), and extract (0.02 mL), with distilled water used as a control instead of the crude enzyme. The absorbance was estimated at 470 nm, and it was represented as the change in enzyme units per gram of fresh weight per minute. The POD activity assay was repeated three times in each group.
Evaluation of MDA Content
The malondialdehyde (MDA) content was measured according to Zou (2000) and Ding et al. (2015) with slight modifications. One gram of flesh (1 g) from five fruits was ground into a homogenate with 5% (w/v) trichloroacetic acid (5 mL) and then centrifuged at 12,000 × g for 10 min. The supernatant (2 mL) and trichloroacetic acid solution (2 mL) were mixed, and the solution was boiled at 95°C for 30 min. After cooling, the mixture was centrifuged at 12,000 × g for 10 min to measure absorbance at 600, 532, and 450 nm. The MDA content was estimated as mol·kg-1·FW using the formula 6.45 × (A532 − A600) − 0.56 × A450. The procedure was repeated three times in each treatment group.
Statistical Analysis
The experimental results are expressed as the average ± standard error (SE). The data obtained from each experiment were analyzed using the statistical program SAS version 9.4. One-way analysis of variance (ANOVA) was used to analyze the differences between treatments on each experimental day; two-way ANOVA was implemented to manifest the differences in variation trend between the four groups during storage. The Technique for Order Preference by Similarity to Ideal Solution (TOPSIS) was used to analyze the data and describe the measurement results. Differences between means at the 5% level were considered significant (p < 0.05).
Results and Discussion
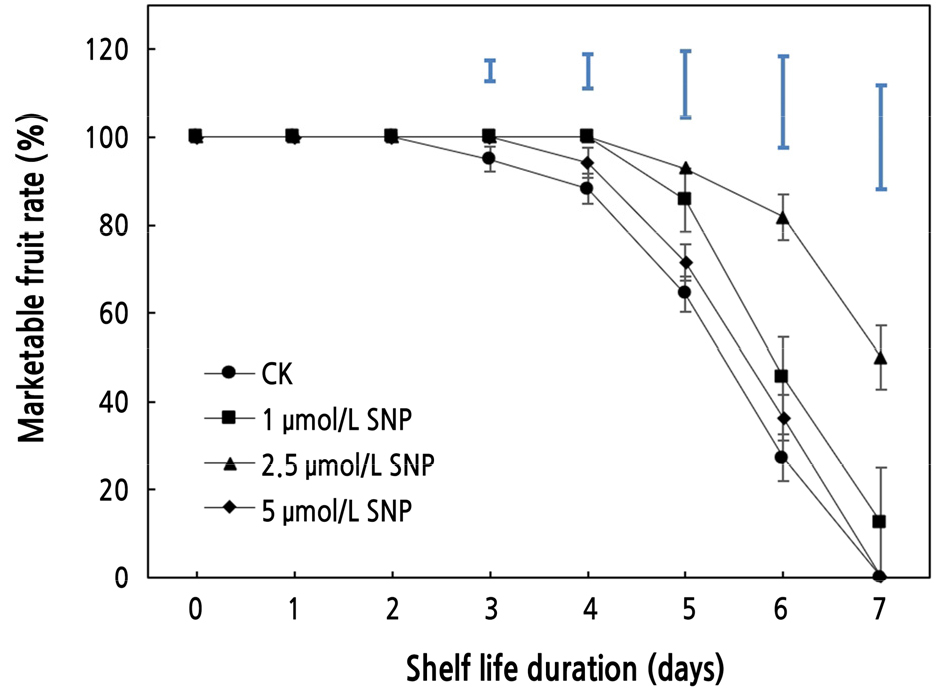
Effects of SNP on Marketable Fruit Rate
The flesh of the wax apple seems to be tender and sensitive to storage, and it is prone to decay and change color even though it is not processed after harvest (Zhang, 2006). As predicted, SNP had a significant effect (p < 0.05) on sustaining marketable fruit rate when compared to the control. Significant changes were not observed beyond the first 3 days, and the control fruit with an SNP concentration of 5 µmol·L-1 began to deteriorate on the fourth day (Fig. 1). The fruits soaking in SNP (1 and 2.5 µmol·L-1) maintained good marketable fruit rates over the first 4 days. On the fifth day, the marketable fruit rate of control (CK) declined to 64.29%, whereas that of fruit treated with SNP (2.5 µmol·L-1) persisted above 90%. On the sixth day, fruit treated with SNP (2.5 µmol·L-1) had a marketable fruit rate of 81.82% (three times that of the control), whereas the other concentration resulted in a marketable fruit rate of less than 50%. The SNP (2.5 µmol·L-1) value was observed to differ substantially (p < 0.05) across the other treatments, indicating that the fifth and sixth days were indispensable. Until the last day of storage, the marketable fruit rate at 2.5 µmol·L-1 SNP remained at ~50%. The marketable fruit rate represents the appearance of fruit and affects its market circulation and commercial value (Sivakumar and Korsten, 2010). Although due to the storability of fruit, a damage degree of less than 25% is typically acceptable for marketability, the rot and damage percentage of postharvest wax apple fruit on the market may not exceed 10%. At all intervals in late storage, the 2.5 µmol·L-1 SNP treatment retained the best marketable fruit rate, which was more than that of the control. Although treatments of 1, 2.5, and 5 µmol·L-1 showed an inhibitory effect on the reduction in marketable fruit rate, 2.5 µmol·L-1 was more efficient and beneficial, perhaps because the concentration of 1 µmol·L-1 was too low to sustain better preservation. Compared to 1 and 5 µmol·L-1 SNP, 2.5 µmol·L-1 SNP might have a more obvious effect on suppressing fruit rot and therefore could enhance storage resistance. This result implies that SNP, particularly at 2.5 µmol·L-1, can be utilized to reduce postharvest deterioration in wax apples, as it has a significant preservation effect.
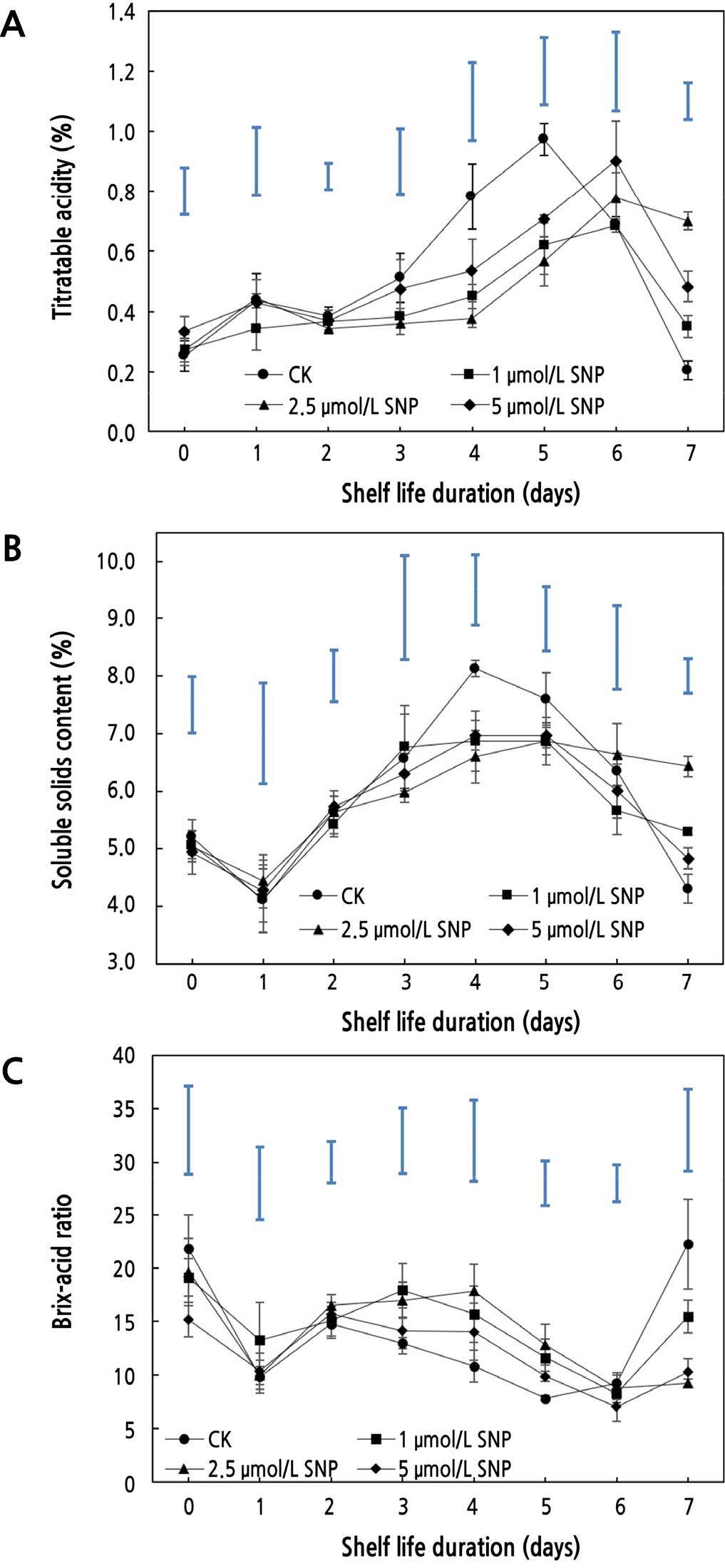
Effects of SNP on Quality Characteristics
Wax apple is a nonclimacteric fruit whose respiratory intensity does not change dramatically; thus, the organic matter content of the fruit may not change significantly during the early storage period. As shown in Fig. 2A and 2B, the titratable acidity (TA) and soluble solids content (SSC) in wax apples increased and subsequently decreased during the course of storage, which was consistent with the findings of Yang et al. (2016). The titratable acidity of the control (CK) peaked at 0.97% on the fifth day, whereas the other treatments peaked on the sixth day. The soluble solids content of the CK started to decline on the fifth day, but the reduction in the SNP treatment was delayed by a day (Fig. 2B). At the end of storage, the TA and SSC of all treatments decreased, with the control showing the clearest trend, indicating that SNP delayed the decline in flavor quality in wax apple. Organic matter in the fruit was continuously consumed over time, and the difference between treatments gradually widened. The titratable acidity and soluble solids contents in each treatment group were substantially different on the seventh day. The SNP concentration (2.5 µmol·L-1) of titratable acidity (0.70%) was more than three times that of control (0.20%), and its soluble solids content was almost 1.5 times that of control. It has previously been shown that a higher SSC may significantly retain fruit flavor quality throughout storage (Jiang et al., 2018), and fruit maturity and senescence after harvest will result in a decrease in flavor, which will influence taste (Sajid et al., 2019). Both the TA and SSC of the 2.5 µmol·L-1 SNP treatment remained higher at the end of storage, showing that SNP is a suitable preservative for retaining the flavor quality of postharvest wax apple. Moreover, 2.5 µmol·L-1 SNP might suppress the respiration of postharvest wax apple to inhibit the consumption of sugars and acids. Hence, the variations of SSC and TA in SNP (2.5 µmol·L-1) were relatively stable, and higher contents were maintained in the late storage period.
The Brix-acid ratio (BAR) fluctuated according to the changing trend, and the ratio was quite high on the third or fourth day (Fig. 2C). As one of the important indicators, the BAR contributes to the flavor and taste quality of fruit, and its fluctuation is also used to represent the rate of nutrient absorption (Wang et al., 2018). This experiment showed that the BAR of the control (CK) had the most obvious change during the storage period, which is speculated to be closely related to the substantial change in the TA content of the fruit in this group. On the seventh day, the BAR of the CK group grew by 2.29 times that of the preceding day. However, the fruit had started to mature and overripened by this day, and the flavor and aroma were much inferior, which was consistent with the finding of Montero et al. (2009). In the late storage period, the Brix-acid ratio of the 2.5 µmol·L-1 SNP treatment showed a slightly increasing trend, and by the seventh day, the Brix-acid ratio had only increased by 7.8%, indicating that SSC and TA with the SNP treatment were relatively stable, thus delaying ripening and senescence of the wax apple (Liang et al., 2015). SNP was beneficial in terms of maintaining the taste and flavor of the fruit as well as prolonging the shelf life, and 2.5 µmol·L-1 SNP had a better performance in terms of postharvest quality and senescence. Treatment with 2.5 µmol·L-1 SNP might suppress ripening and senescence by regulating the consumption and metabolism of sugars and acids so that a stable Brix-acid ratio could be maintained.
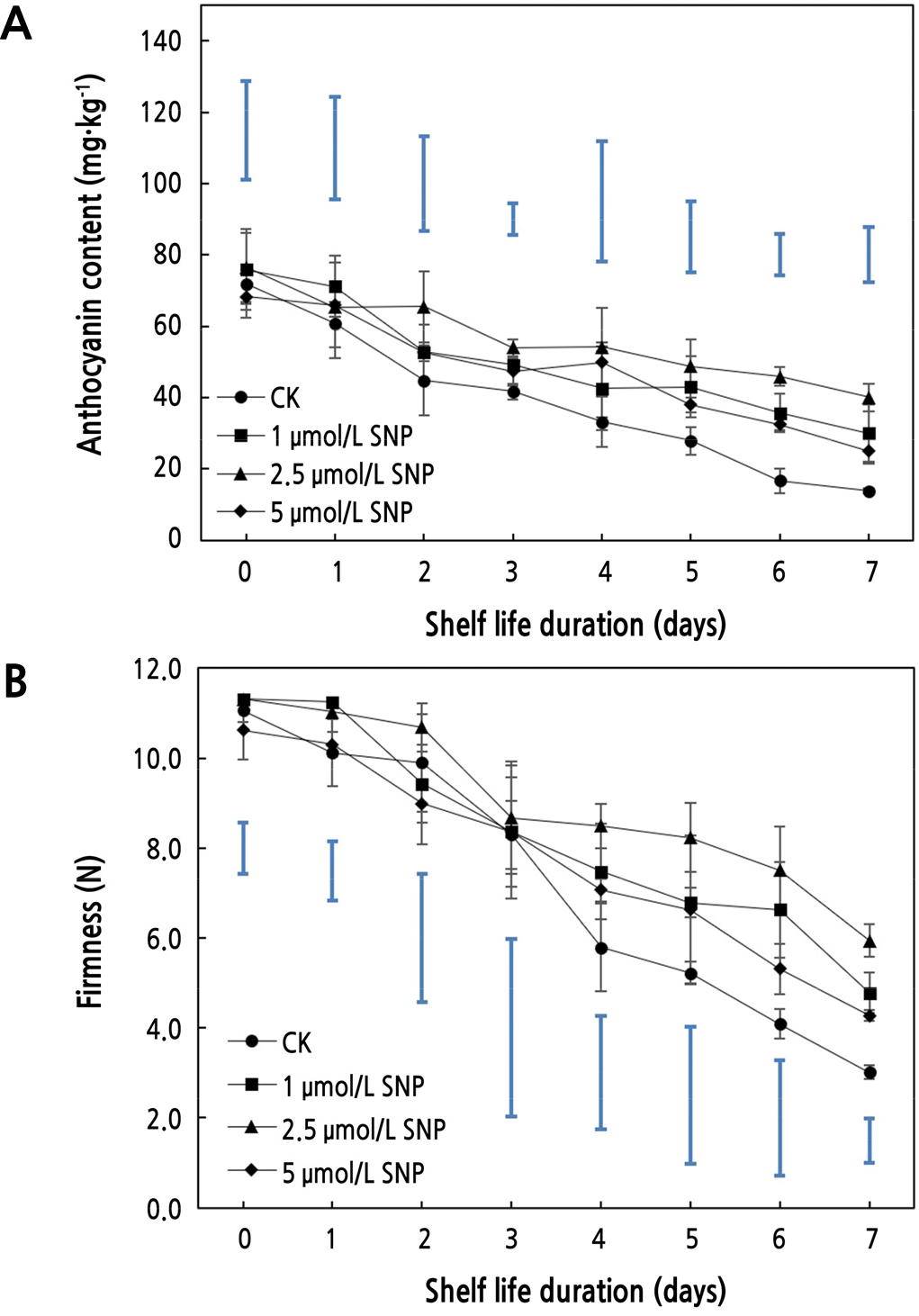
The anthocyanin content of fruit decreased over the test period (Fig. 3A); nevertheless, the content in response to treatment (2.5 µmol·L-1) always maintained the maximum level, which was comparable to Fan et al. (2017) and Lin et al. (2020b). During storage, the treatment concentrations of SNP (2.5 and 5 µmol·L-1) showed significant changes (p < 0.05) compared to the control. Overall, the anthocyanin content of the CK group began to drop significantly, while that of the fruit treated with SNP alleviated to some extent. On the fifth day, the anthocyanin content of the treatment (2.5 µmol·L-1) was considerably higher than that of the control (CK), reaching 2.75 times that of the control on the sixth day and 2.9 times on the seventh day. Anthocyanin influences the appearance and color of fruit (Qiao and Guo, 2019); furthermore, it is a natural and efficient free radical scavenger that may limit the development of free radicals by inhibiting lipid peroxidation and hindering the reaction of peroxy anions (Zhong et al., 2013). In the experiment, 2.5 µmol·L-1 SNP treatment significantly inhibited the degradation of anthocyanins, and a high anthocyanin content was maintained, which was consistent with Wang (2020) to some extent. SNP alleviated the breakdown of anthocyanins to maintain the color of the wax apple after harvest. It was speculated that 2.5 µmol·L-1 SNP might contribute significantly to inhibiting ethylene release in postharvest fruit compared to other concentrations, leading to enhanced anthocyanin content.
With the extension of storage time, the pulp tissue began to soften and the fruit firmness gradually decreased (Fig. 3B), closely resembling the results of Liu et al. (2017). SNP could have an important function (p < 0.05) in delaying the decrease in firmness of wax apples. The firmness of the fruit in the four treatment groups was reasonably comparable during the first 3 days of storage, and variations were observed on the fourth day, suggesting that the fourth day was the significant event in the firmness change of postharvest wax apple. The firmness of untreated fruit deteriorated substantially, and the effect of SNP on firmness maintenance was seen in the late storage period. On the seventh day, the firmness with treatment of 2.5 µmol·L-1 SNP was the highest and was significantly different from that of the control (1.97 times) (p < 0.05). As an important external indicator of fruit softening, firmness can reflect the shelf life and commercial properties of fruit to a certain extent. Softened fruit are more susceptible to microbial infection, resulting in nutritional breakdown and a further loss of firmness (Ye et al., 2012). In our study, the decline in firmness was significantly inhibited by SNP, especially at a 2.5 µmol·L-1 concentration, indicating that SNP could delay fruit softening and prolong shelf life. In addition, 2.5 µmol·L-1 SNP might play a significant role in delaying respiration and ethylene metabolism in postharvest wax apple fruit to suppress the decrease in firmness. Thus, SNP, a preservative with application potential, might be an important substitute for postharvest preservation of wax apple.
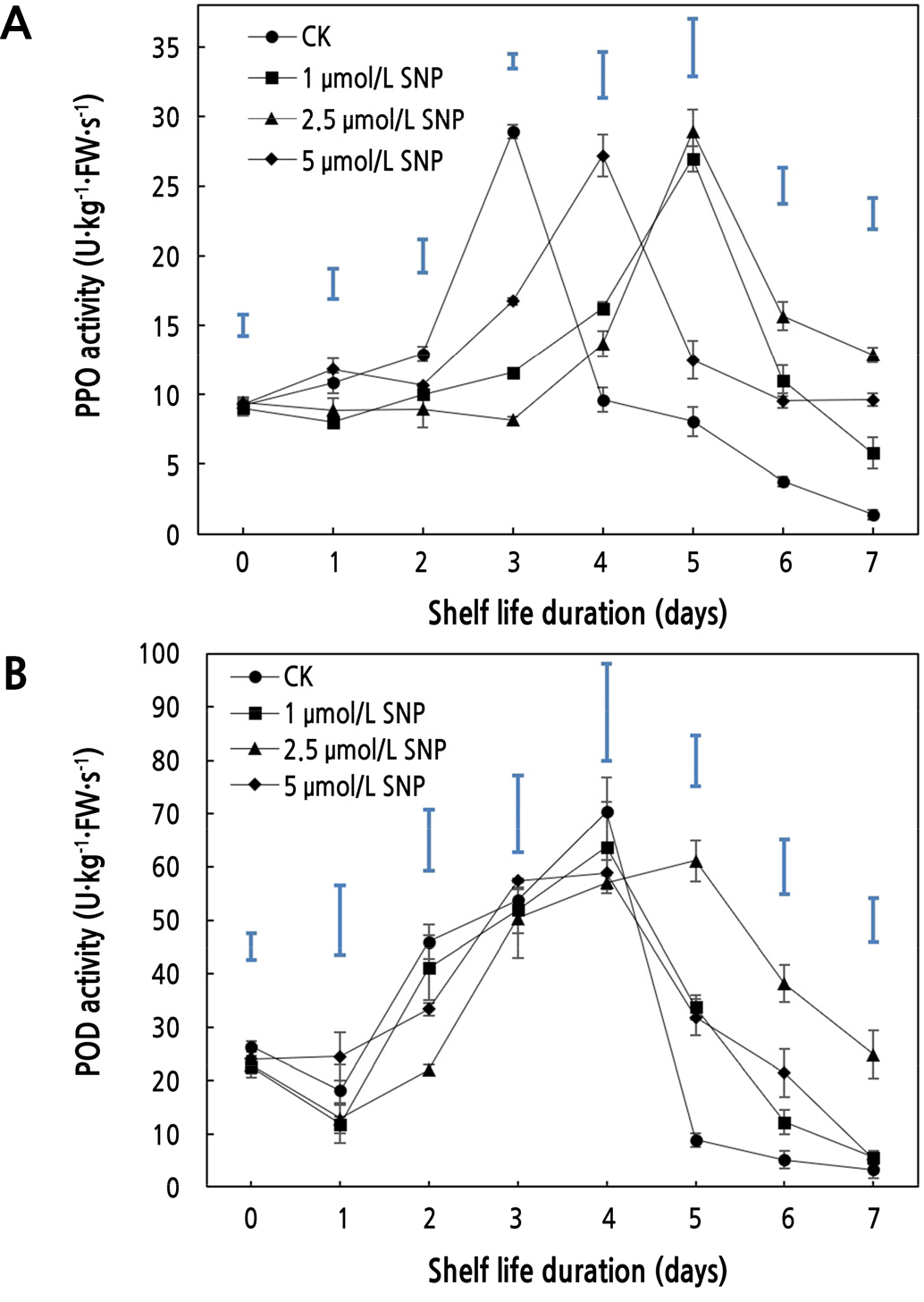
Effects of SNP on PPO and POD Activities
For all concentrations of SNP, the polyphenol oxidase (PPO) and peroxidase (POD) activities tended to increase initially and then decrease (Fig. 4A and 4B). Throughout the storage period, the changes in PPO and POD activities in response to 2.5 µmol·L-1 SNP showed significant differences (p < 0.05) from those in control (CK). The PPO activity of the CK group peaked on the third day, and the peak of fruit treated with SNP was delayed by 1 or 2 days. Similarly, maximal POD activity emerged on the fourth day in the control group, but 1 day later in the 2.5 µmol·L-1 SNP treatment group. Similar to the findings of Sun (2016), the delayed peaks may be related to the stress-induced and self-defense mechanism of plants. PPO and POD can catalyze the oxidation reaction in fruit and the conversion of phenolic compounds into quinone compounds, thereby causing browning and color change (Sajid et al., 2018). In the early storage period, the activities of the two enzymes were delayed by the SNP treatment, and the activities in group B were relatively lower, which was consistent with Zhang et al. (2018). The delayed enzyme activities might be related to the metabolic process of phenols, indicating that 2.5 µmol·L-1 SNP inhibited the accumulation of quinones, reduced oxidative damage in cells, and maintained the integrity of membrane lipids (Xiong, 2016), thereby reducing the appearance change and fruit damage of wax apple during the early storage period.
In addition, as terminal oxidases in plants, PPO and POD play an essential role in the postharvest senescence of fruit. As shown in Fig. 4A and 4B, the activities of the two enzymes were higher in fruit soaked in SNP, and a delay in the reduction in activities in group B was seen in the late storage period, which is comparable to Lin et al. (2020a). On the fifth day, the PPO and POD activity of the 2.5 µmol·L-1 SNP treatment were 3.59 and 6.88 times that of the control, respectively, indicating a significant difference (p < 0.05). The high activity of PPO can maintain the integrity of the membrane structure, therefore delaying fruit senescence (Li et al., 2019), and POD can suppress the accumulation of reactive oxygen species (ROS), thereby reducing oxidative damage (Zhang et al., 2014; Pandey et al., 2017; Guo et al., 2018). This result indicated that SNP might maintain high PPO and POD activities in the late storage period that lead to the delay of ripening and senescence of wax apple.
For the comprehensive analysis of the two enzymes, SNP delayed both enzyme activities in the early storage period, showing that quinone oxidation was suppressed to inhibit the browning and color change of wax apples; in the late storage period, the decrease in enzyme activities was suppressed to protect plant cells from oxidative damage and membrane damage so that the senescence of postharvest wax apple could be suppressed. During storage, 2.5 µmol·L-1 SNP had the most noticeable effect on wax apple ripening and senescence, indicating that this concentration seemed to delay PPO and POD activities in the early storage period more effectively and contribute significantly to delaying activity decrease in the late storage period. To some extent, 2.5 µmol·L-1 SNP delayed browning and color change in early storage and reduced cell damage in late storage, making it a more effective strategy for controlling postharvest senescence.
Effects of SNP on MDA Content
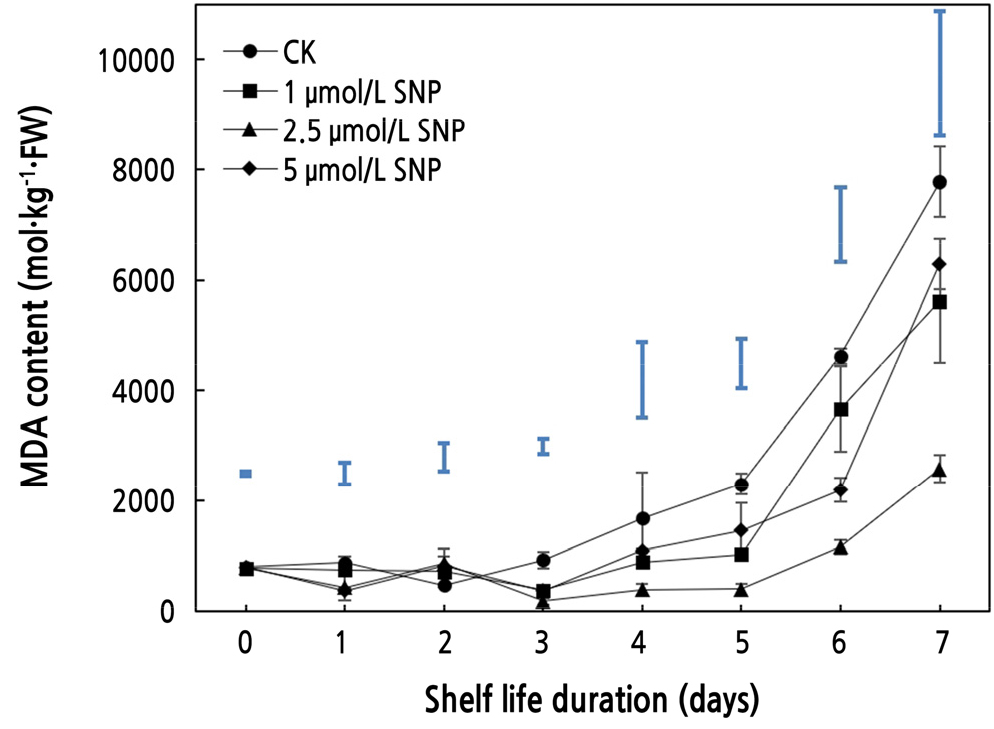
As shown in Fig. 5, the malondialdehyde (MDA) content of wax apples in the four groups (0, 1, 2.5, and 5 µmol·L-1) increased gradually, and the value of SNP (2.5 µmol·L-1) always maintained the lowest level, which was consistent with Shao et al. (2020). In the first 3 days, the MDA content did not change significantly, and differences between the treatment groups began to emerge on the fourth day (Fig. 5). Overall, the content of the control (CK) group increased dramatically, while that of the other groups was suppressed to a certain extent. On the sixth day, the level of the control was 3.99 times greater than that of SNP (2.5 µmol·L-1), which indicated a significant difference (p < 0.05), and it was 3.01 times greater on the last day. MDA, as one of the prominent components of membrane lipid peroxidation, is an indicator of biomembrane system degradation (Zhou et al., 2015; Zhang et al., 2020). Oxidative stress can damage cell membranes and disrupt the membrane lipid structure, leading to an increase in MDA content in fruit (Sun et al., 2011). Therefore, SNP treatment, especially at 2.5 µmol·L-1, could inhibit the accumulation of MDA, defend the cell membrane structure from damage, and hinder the lipid peroxidation process (Masoud et al., 2019) to postpone postharvest senescence and extend the shelf life of wax apple.
Finally, TOPSIS performed a complete review of all indicators for the last 4 days of data. As shown in Table 1, the CK group scored fourth in the last 4 days, while the SNP group (2.5 µmol·L-1) scored first. These data confirm that SNP may delay the maturity and senescence of wax apples after harvest and that 2.5 µmol·L-1 SNP has a superior preservation effect.
Table 1.
TOPSIS comprehensive evaluation of different treatments in the last four days
| Day | Treatment | D+ | D- | D++ D- | C | Order |
| Day 4 | CK | 0.8655 | 0.3931 | 1.2586 | 0.3123 | 4 |
| 1 µmol·L-1 | 0.6596 | 0.3212 | 0.9808 | 0.3275 | 3 | |
| 2.5 µmol·L-1 | 0.5449 | 0.7220 | 1.2669 | 0.5699 | 1 | |
| 5 µmol·L-1 | 0.6190 | 0.5552 | 1.1742 | 0.4728 | 2 | |
| Day 5 | CK | 1.1821 | 0.2773 | 1.4594 | 0.19 | 4 |
| 1 µmol·L-1 | 0.7098 | 0.6416 | 1.3514 | 0.4748 | 2 | |
| 2.5 µmol·L-1 | 0.2773 | 1.1821 | 1.4594 | 0.81 | 1 | |
| 5 µmol·L-1 | 0.8930 | 0.3821 | 1.2751 | 0.2997 | 3 | |
| Day 6 | CK | 1.3279 | 0.0539 | 1.3818 | 0.039 | 4 |
| 1 µmol·L-1 | 0.9328 | 0.5359 | 1.4687 | 0.3649 | 3 | |
| 2.5 µmol·L-1 | 0.0782 | 1.3245 | 1.4027 | 0.9443 | 1 | |
| 5 µmol·L-1 | 0.7963 | 0.5833 | 1.3796 | 0.4228 | 2 | |
| Day 7 | CK | 1.7307 | 0 | 1.7307 | 0 | 4 |
| 1 µmol·L-1 | 1.2778 | 0.5436 | 1.8214 | 0.2985 | 3 | |
| 2.5 µmol·L-1 | 0 | 1.7307 | 1.7307 | 1 | 1 | |
| 5 µmol·L-1 | 1.3967 | 0.6267 | 2.0234 | 0.3097 | 2 |
The Euclidean distance closeness between the control and concentration of treatments and the ideal solutions and negative ideal solutions were listed. D+ indicates positive ideal solution distance; D- indicates negative ideal solution distance; C indicates relative approach degree; Order represents the effect order of four treatments in each experimental day in TOPSIS comprehensive evaluation.
Investigation of the Mechanism of Nitric Oxide Application in Postharvest Fruit
NO regulates numerous physiological and biochemical reactions in plants, and its active chemical properties (both oxidizing and reducing) have a variety of effects on preservation (Zhang et al., 2012b). Many studies have shown that NO can significantly alter the activities of PPO and POD, therefore delaying fruit ripening and extending shelf life in terms of phenolic compound metabolism and reactive oxygen metabolism (Wang et al., 2015; Hafiz et al., 2017). In addition, NO, which is closely related to ethylene biosynthesis, is also the key factor regulating the ripening and senescence of horticultural plants. Exogenous NO could improve the NO levels of fruit and vegetable tissue and inhibit ethylene production through its cofactor of oxidative inactivation inhibition of ACC oxidase (ACO) activity (Leshem and Haramaty, 1996; Xie et al., 2015). Therefore, the variation in fruit when strong respiration is suppressed and the maturation and senescence of fruit tissues are delayed.
Conclusions
SNP has the potential to be applied to wax apples after harvest as an efficient and practical treatment to maintain quality and delay maturity during storage. In general, SNP played a significant role in preserving postharvest physiological properties in our study. Soaking wax apples in SNP maintained appreciable flavor qualities, such as TA and SSC, and suppressed the decay, softening, and anthocyanin degradation. Moreover, SNP delayed the activities of PPO and POD and sustained the low MDA content to reduce cell damage and delay fruit aging. It appears that the beneficial or detrimental effects of NO may depend on concentration and sensitivity of genotypes. High concentrations of NO may cause toxicity mainly due to its reaction with superoxide anion. By contrast, application of NO in low concentrations is useful for increasing the postharvest life of wax apples and other horticultural products. Therefore, our results suggest that the application of SNP, especially at 2.5 µmol·L-1, is a promising strategy for postharvest wax apple management and distribution.