Introduction
Materials and Methods
Myrtaceae extracts preparation
Identification of Alternaria species by sequence analysis
Preliminary phytochemical screening of leaf extracts from four Myrtaceae plants
Phytotoxicity of four Myrtaceae extracts on lettuce grown in hydroponics
In vitro antifungal activity of Myrtaceae extracts on the growth of Alternaria sp.
GC/MS and LC/MC-TOF analyses of the Myrtaceae extracts
Evaluation of EEMC and EECV as an inducer on development of Alternaria leaf spot and the induction of defense enzymes in lettuce grown in a hydroponic system
Results and Discussion
Identification of Alternaria species by sequence analysis
Phytochemical screening of four Myrtaceae leaf extracts
Phytotoxicity of four Myrtaceae extracts on lettuce grown in hydroponics
In vitro antifungal activity of Myrtaceae extracts against growth of Alternaria sp.
Phytochemical profile of the Myrtaceae extracts by GC/MS and LC/MC-TOF
Evaluation of EEMC and EECV as an inducer on development of Alternaria leaf spot and induction of defense enzymes in lettuce grown in a hydroponic system
Conclusion
Introduction
For leafy vegetable production, synthetic fungicides have been widely employed to combat foliar diseases, including Alternaria leaf spot. However, the use of chemical fungicides is increasingly restricted due to concerns about food safety, particularly within organic farming practices (Horsfield et al. 2010; Wang et al. 2016; Yang et al. 2019; Wu et al. 2023). Hence, a transfer from synthetic to natural safe fungicide is needed. Plant extracts are of great significance in this regard. Scientific studies have been carried out worldwide on the antifungal activities of plant extracts against the fungal pathogens of plant diseases, which have resulted in the development of promising sources of natural fungicides and botanical fungicides offering a potential solution to agricultural challenges (Jantasorn et al. 2016; Dethoup et al. 2018; Dethoup et al. 2019; Sukdee 2023). In general, plant extracts have secondary metabolites (e.g., phenolics, phenolic acids, terpenoids, essential oils, alkaloids, tannins, flavonoids, coumarins, lectins and polypeptide) which represent biologically active substances (Gurjar et al. 2012; Pinto et al. 2018). Several researchers have uncovered the potential of medicinal plants to control plant pathogens, both by direct fungal toxic actions and due to the ability to stimulate the accumulation of molecules with elicitor features capable of inducing defense responses (Bonaldo et al. 2004; Celoto et al. 2008; Bulhões et al. 2012; Pinto et al. 2018). These resistance mechanisms may include the accumulation of phenolic compounds, phytoalexins and pathogenesis-related proteins such as β-1,3-glucanase, chitinase, peroxidase, phenylalanine ammonia lyase and polyphenol oxidase (Barros et al. 2010). Plant extract enhancers of the defense response in Ponkan mandarin seedlings against Alternaria alternata f. spp. citri infection were reported by Pinto et al. (2018). In 2012, Gurjar et al. reviewed the efficacy of plant extracts in plant disease management, finding that thousands of phytochemicals with inhibitory effects on all types of microorganisms in vitro should be subjected to in vivo testing to evaluate how well they control the incidence of disease in crops. The latest studies of natural alternatives for chemical synthetic pesticides, including the application of neem extract to manage postharvest losses due to postharvest diseases (e.g., Aspergillus flavus, A. niger and Botrytis cinerea) of fresh produce in tropical and subtropical fruits, were reviewed by Tzortzakis and Proestos (2024). In Thailand, the popularity of botanical fungicides is also increasing. Quite a number of phytochemical extracts have been evaluated during the search for plant-based antifungal agents for plant disease management, some recently reported studies include those on indigenous plants, such as Hydnocarpus anthelminthicus, Crateva magna, Caesalpinia sappan (Jantasorn et al. 2016), Acorus calamus (Dethoup et al. 2019) and black pepper (Kunasakdakul and Suwitchayanon 2012).
Myrtaceae plants, specifically Callistemon viminalis, Melaleuca cajuputi, Syzygium jambos, and Syzygium malaccense, are naturally found in Australia, southeast Asian countries, including Thailand, Malaysia and Indonesia (Bharat and Praveen 2016; Salem et al. 2017; Patel et al. 2019; Isah et al. 2023). Various plant species from the Myrtaceae family have long been widely used for medicinal purposes given their antimicrobial, anti-inflammatory and antioxidant activities (Imatomi et al. 2013; Al-Abd et al. 2015; Rita et al. 2017; Salem et al. 2017; Puig et al. 2018; Patel et al. 2019; Vasconcelos et al. 2022). The phytochemical extract of C. viminalis demonstrated strong to moderate antibacterial effects against certain plant bacterial pathogens (El-Hefny et al. 2017) as well as in vitro antifungal effects (Somnuek et al. 2020; Somnuek et al. 2021). Essential oil extracted from M. cajuputi has been widely used worldwide for many purposes. Most of the compounds found in this plant possess aromatic, antibacterial and insecticide properties (Sharif et al. 2019). Keereedach et al. (2020) reported that Thai cajuput oil can be used to create new potential combination therapies to combat the antifungal resistance of Candida albicans. In addition, several studies have found the excellent antimicrobial effects of M. cajuputi extracts against bacteria, viruses, protozoa and fungal species due to the presence of specific phytoconstituents (Isah et al. 2023). S. jambos extract showed antimicrobial activity on the growth of both gram-positive and gram-negative bacteria (Mohanty and Cock 2010), while only gram-negative bacteria were susceptible to the extract of S. malaccense (Bouzada et al. 2009). Although multiple studies have analyzed chemical compounds and the antibacterial activities of extracts from different Myrtaceae family plants (Al-Abd et al. 2015; Salem et al. 2017; Isah et al. 2023), few studies have evaluated the potential of these extracts in in vivo testing to control the incidence of fungal plant diseases.
Therefore, the objective of our research was to investigate the possibility of employing ethanolic extracts from Myrtaceae plants as an alternative approach for managing Alternaria leaf spot in lettuce. For this purpose, an experimental series was carried out to determine the following: (1) the phytotoxicity of Myrtaceae plant extracts on the cultivated crop, (2) the direct effects of Myrtaceae extracts on mycelial growth and spore germination of a fungal pathogen of lettuce leaf spot, (3) the phytochemical profile of Myrtaceae plant extracts according to liquid chromatography (LC) / mass spectrometry (MS) and gas chromatography (GC) / MS, (4) the potential of Myrtaceae plant extracts as an inducer for defense-related enzymes for controlling Alternaria leaf spot in lettuce grown in hydroponics and (5) the correlation between disease severity and defense enzymes.
Materials and Methods
Myrtaceae extracts preparation
Four Myrtaceae plants were studied here. Callistemon viminalis (CV), Syzygium jambos (SJ),and Syzygium malaccense (SM) were collected from an area in Bangkok province, while Melaleuca cajuputi (MC) was collected from Chumphon province in Thailand.
Crude extracts using 95% ethanol
Fresh leaves of the Myrtaceae plants were cleaned with tap water and dried in open air. The leaf samples were then completely dried using a hot air oven (Memmert) at 50°C. The dried leaves were ground and extracted using the Soxhlet extraction technique with 95% ethanol, after which the ethanol solvent was evaporated with a rotary evaporator (Buchi, Rotavapor R300) at 50°C. The sticky extracts were kept at 4°C for further study.
Crude extracts using 50% ethanol
The method used to extract the Callistemon viminalis leaves was identical to that used with the 95% ethanolic crude extracts but with 50% ethanol instead as a solvent.This extract as well was kept at 4°C for further study.
Identification of Alternaria species by sequence analysis
The Alternaria isolate in this experiment obtained from our previous research (Somnuek et al. 2020) was morphologically identified and already proven with regard to its pathogenicity. Here, the identification of Alternaria species was confirmed by molecular identification. The genomic DNA of the tested fungus was extracted and the extracted DNA was used as the template for amplification with the ITS1 primer (5’ TCCGTAGGTGAACCTGCGG 3’) and ITS4 primer (5’ TCCTCCGCTTATTGATATGC ’3) of the internal transcribed spacer (ITS) of rDNA regions via polymerase chain reaction (PCR), with the process conducted according to the work of Mohammadi and Bahramikia (2019). The purified PCR products were sequenced by Bionics Co., Ltd. of Korea. Sequence similarity analyses were conducted using the Basic Local Alignment Search Tool (BLAST) in the GenBank NCBI database.
Preliminary phytochemical screening of leaf extracts from four Myrtaceae plants
Phytochemical compounds such as phenols, flavonoids, tannins, alkaloids, and terpenoids showed antifungal properties on plant pathogenic fungi, and they acted in a synergistic manner (Tiwari et al. 2011; Gurjar et al. 2012). Therefore, this part of the experiment was undertaken to screen the targeted phytochemical compounds from the ethanolic crude extracts of Callistemon viminalis (95% EECV), Melaleuca cajuputi (95% EEMC), Syzygium jambos (95% EESJ),and Syzygium malaccense (95% EESM). Phytocompounds were detected according to a slightly modified version of the methods of Harborne (1998), Iqbal et al. (2015) and Dubale et al. (2023). The qualitative results were assessed by means of colorimetric reactions and were presented here as positive (+) for the presence of and negative (–) for the absence of phytochemicals.
Phytotoxicity of four Myrtaceae extracts on lettuce grown in hydroponics
Plant extracts, an important source of bioactive compounds, have been suggested as a viable, environmentally friendly option for plant disease control. However, plant extracts may also have negative impacts on cultivated crops. Therefore, a preliminary test was conducted here to find the extract with the least negative effect on lettuce grown in a hydroponic system. The experiment utilized a 4 × 4 × 2 factorial in a completely randomized design (CRD) with 3 replications (3 plants per replicate). Factor A was the type of plant extract (EECV, EEMC, EESM, and EEMJ), Factor B was the concentration of the extract used (0, 5,000, 25,000 and 50,000 ppm), and Factor C was the number of applications (one time and two times). The tested lettuce plants were grown in a deep water culture (DWC) system, which was constructed from a plastic box (25 × 34 × 15 cm) and filled with a nutrient solution (EC = 1.6–1.8 mS/cm, pH = 5.8–6.2) (Benoit and Ceustermans 1995). Subsequently, 2 ml of each prepared extract was sprayed on 15-day-old lettuce seedlings, and this step was repeatedly sprayed on 30-day-old lettuce seedling group. Phytotoxicity was evaluated according to the incidence of necrosis and discoloration at 1 to 3 days after the foliar spray. Moreover, the lettuce growth parameter of leaf greenness of the lettuce under test was monitored weekly using a chlorophyll meter (SPAD-502, Konica Minolta Sensing, Inc., Japan) after foliar spraying, whereas the fresh weights of the plants were recorded at the end of the cultivation period.
In vitro antifungal activity of Myrtaceae extracts on the growth of Alternaria sp.
Effect of four Myrtaceae extracts on mycelial growth
The effect of the ethanolic extracts from the four Myrtaceae plants on the mycelium growth of Alternariabrassicicola was investigated using 4 × 4 factorials in a completely randomized design (CRD). Factor A indicated the 4 Myrtaceae plants, and Factor B represented the 4 concentrations of the extracts used. The crude extracts were tested with regard to the mycelial growth of A. brassicicola by a poisoned food technique. A fungus was cultured on potato dextrose agar (PDA; Sisco Research Laboratories Pvt. Ltd., India) at room temperature (~25°C). Mycelial disks, 0.5 cm in diameter, were cut from the margins of the colonies and inoculated onto PDA mixed with each extract. The sizes of A. brassicicola colonies in the control plate and the plate containing the extract were determined at 3, 5, and 7 days after inoculation. Mycelial growth inhibition was calculated using the following formula: Mycelial growth inhibition (%) = (DC–DT)/DC × 100
where DC = diameter of the Alternaria colony on the control plate
DT = diameter of the Alternaria colony on the plate containing the extract
Effect of potentially selected Myrtaceae extracts on spore germination
Two plant extracts (95% EEMC and 95% EECV) showing high-potential antifungal activity in the mycelium growth test described above were selected and tested further for their effect on A. brassicicola spore germination. The experiment was carried out by CRD. One mL of spore suspension (approximately 105 spores/mL) was inoculated into 1 mL of PDB in test tubes with 5 extract concentrations (1,000, 5,000, 10,000, 15,000 and 50,000 ppm). Sterilized water and mancozeb (500 ppm) were used as inoculated and chemical controls, respectively. Then, spore germination of the tested fungus was observed under a light microscope at 24, 48, and 72 h.
GC/MS and LC/MC-TOF analyses of the Myrtaceae extracts
GC/MS and LC/MS have often been used to analyze phytochemical compounds in plant extracts. The two methods are more alike than different. The only key difference between the systems is that GC/MS uses a gas mobile phase while LC/MS uses a liquid mobile phase. Therefore, GC/MS appears to be more applicable to more volatile and gas samples.
GC/MS analyses of the 95% EEMC and 95% EECV were carried out according to a slightly modified method based on Al-Abd et al. (2015) and Hassan et al. (2022). A gas chromatograph-mass spectrometer (7890 B GC–5977A MSD, Agilent Technologies, Inc., USA) was employed for these analyses. A HP-5 Ultra Inert column (30 m × 250 µm × 0.25 µm, Agilent Technologies) with helium (He, flow rate = 40 cm/s) as the carrier gas was used. The oven was initially programmed to run at a temperature of 60°C (hold time 2 min), a rate of 7°C/min to 150°C (hold time 1 min), a rate of 2°C/min to 230°C (hold time 1 min), and a rate of 15°C/min to 300°C (hold time 15 min). The injection volume of 1 µL operated in the split mode with a ratio of 10:1. The MS transfer line and ion source temperatures were maintained at 230°C and 150°C, respectively, and the mass spectra detector voltage was set at 70 eV. The scan range was from 35 to 600 m/z. Peaks and chemical constituents were identified by a MassHunter Workstation Software Quantitative Analysis Version B.09.00 Unknown Analysis.
The LC/MS analyses of the 95% EEMC and 95% EECV were conducted according to a slightly modified method based on Al-Abd et al. (2015). The system for analyzing the samples consisted of a high-performance liquid chromatograph- mass spectrometer-quadrupole time-of-flight [ExonLCTM AD Series (LC) and X500R QTOF system (QTOF),-SCIEX] with dual electrospray ionization (ESI). The LC separation steps were performed using a 2.1 mm (i.d.) narrow-bore SB-C18 (length 150 mm, particle size 3.5 mM) analytical column. The LC parameters used were: 25°C for the autosampler temperature, 1 µL for the injection volume, 25°C for the column temperature, and 0.4 mL/min for the flow rate. A gradient system consisting of 0.1% formic acid in water as solvent A and 0.1% formic acid in acetonitrile as solvent B was used. The mass spectra data were acquired using an ESI capillary voltage of (+) 4000 V in the positive ion mode with the fragmentor set to 125 V. Other conditions were set to 45 psi for the liquid nebulizer, a flow rate of 10 L/min with the drying gas, and vaporizer temperatures maintained at 300°C for the nitrogen drying gas. Moreover, the ionization interface was operated in positive mode. The data were collected and analyzed using SCIEX OS 2.1.0.
Evaluation of EEMC and EECV as an inducer on development of Alternaria leaf spot and the induction of defense enzymes in lettuce grown in a hydroponic system
In addition to a direct effect evaluation, we also focused on an indirect effect of the extracts as inducers on Alternaria leaf spot disease severity as well as on the activity of defense enzymes. Two experiments were separately conducted, one to assess the effect of 95% EEMC (15,000 and 50,000 ppm) and the other to test 95% EECV (15,000 and 50,000 ppm). Moreover, 50% EECV, given its potential to stimulate lettuce growth as determined in our previous research (Somnuek et al. 2020), was also included in the 95% EECV experiment for further evaluation as an inducer on defense enzyme activity.
The EEMC effect
Preparation and experimental design
In order to make certain of the indirect effect of EEMC as an inducer, a hydroponic experiment with lettuce was carried out. First, lettuce seeds were germinated in a plastic tray on a moist sponge with nutrient solution (EC = 1, pH = 5.8–6.2) for 14 days. The seedlings were then transferred to grow in DWC with nutrient solution (EC = 1.6–1.8 mS/cm, pH= 5.8–6.2). The 95% EEMC at 15,000 and 50,000 ppm were prepared and foliar sprayed on the above lettuce seedlings (1 mL/plant). The treated seedlings were inoculated with a 100 µL of testedspore suspension (approximately 106 spores/mL) a day after spraying with the plant extract. CRD with 3 replications (3 plants per replicate) was used in this experiment, as follows:
T1 = Healthy control, T2 = Inoculated control, T3 = Chemical control (mancozeb 500 ppm)
T4 = 95% EEMC (15,000 ppm), T5 = 95% EEMC (50,000 ppm)
Determination of disease severity
The treated leaves were observed at 1, 3, 5 and 7 days after inoculation (DAI), and a disease index was scored using a scale of 0 to 4 for the lesion size: 0 = no infection, 1 = 1 to 5 mm, 2 = 5 to 10 mm, 3 = 10 to 15 mm, and 4 ≥ 15 mm. Disease severity (DS) of the tested lettuce leaves was analyzed using the following equations:
% DS = [∑ (number of infected leaves × disease index) / number of total leaves × the highest disease index] × 100.
In addition, the plant growth parameters, such as the greenness value (by chlorophyll meter; SPAD-502, Konica Minolta Sensing, Inc., Japan) and fresh weight were checked at 1, 3, 5, 7 days after spraying and at harvest.
Induction of plant defense enzymes due to the effect of the inducer
During pathogenesis, plant defense enzymes were also determined. The aforementioned treated lettuce leaves were collected at 1, 3, 5, and 7 days after foliar spraying. Three treated leaves were sampled from each replication of the treatment and were ground using liquid nitrogen. Then, 1 g of the tested leaf sample in each case was homogenized with 2 mL of 0.1 M sodium phosphate buffer (pH 7.0). The homogenate was centrifuged for 20 min at 10,000 rpm in a cooling centrifuge at 4°C. Subsequently, peroxidase, chitinase, and β-1,3-glucanase were detected according to a modified version of the method introduced by Verburg and Huynh (1991), Boller and Mauch (1988), Gupta et al. (2013) and Selvaraj and Ambalavanan (2013).
β-1,3-glucanase activity
The homogenate of 62.5 µL was mixed with laminarin (4% w/v in 0.05 M sodium acetate buffer, pH 5.0) of 62.5 µL for 10 min at 40°C. The reaction was then stopped by adding 375 µL of DNS and heating this solution for 5 min in a boiling water bath. The absorbance was detected by a spectrophotometer at 500 nm. The enzyme activity was expressed as µmol glucose/g of fresh leaves.
Chitinase activity
The homogenate of 0.4 ml was mixed with colloidal chitin (0.1% w/v in 0.05 M sodium acetate buffer, pH 5.0) at a ratio of 1:1 and incubated at 37°C for 2 hrs with the product of N-acetyl glucosamine (GlcNAc). Then, the chitinase activity was detected with a spectrophotometer at 585 nm. The enzyme activity was expressed as µmol GlcNAc/g of fresh leaves.
Peroxidase (PO) activity
The enzyme supernatant of 100 µl was taken along with 0.05 M pyrogallol. To initiate the enzyme reaction, 1% H2O2 in an amount of 0.5 ml was added. The change in the absorbance was recorded by a spectrophotometer at 420 nm at 30 sec intervals for 3 min from zero seconds of incubation at room temperature. The result was expressed as the change in unit/g of fresh leaves.
The EECV effect
In this experiment, the preparation and experimental design, including the determination of disease severity and the plant defense enzyme activity, were carried out in the same manner as in the 95% EEMC experiment. Treatments in the experiment were as follows:
T1 = Healthy control, T2 = Inoculated control, T3 = Chemical control (mancozeb 500 ppm)
T4 = 50% EECV (15,000 ppm), T5 = 50% EECV (50,000 ppm), T6 = 95% EECV (15,000 ppm),
T7 = 95% EECV (50,000 ppm)
Correlation of disease severity and plant defense enzymes
In order to understand the relationship between the severity of leaf spot disease and the induced plant defense enzymes, as well as to confirm that these defense enzymes have a role in leaf spot management in lettuce, the correlation coefficients (r) between disease severity and the defense enzymes of β-1,3-glucanase, chitinase and peroxidase were estimated by standard statistical calculations. Simple regression equations (Y= a + bx) were also developed for all variables.
Results and Discussion
Identification of Alternaria species by sequence analysis
Alternaria species (Somnuek et al. 2020) was confirmed by ITS identification. The nucleotide sequences in the tested isolate were approximately 568 bp in size. The nucleotide sequence of the tested Alternaria isolate Alt-LL1 (OR226735) allowed an identification of Alternariabrassicicola. Our findings were in agreement with those of many researchers who reported that A. brassicicola was the causal agent of leaf spot disease in a number of vegetable crops, including lettuce (Pattanamahakul and Strange 1999; Dethoup et al. 2018; O’Neill 2019; Blagojević et al. 2020).
Phytochemical screening of four Myrtaceae leaf extracts
Our study confirmed the presence of qualitative phytochemical compounds, specifically phenol, flavonoids, and tannins, in all tested extracts (Table 1). High levels of phenols and flavonoids were especially detected in the 95% EEMC. Terpenoids were found only in the 95% EEMC and 95% EECV while alkaloids were not found in any of the extracts. Our findings were in accordance with those of many researchers who reported that the targeted phytochemical compounds, i.e., phenol, flavonoids, tannins, alkaloids and terpenoids, were found in several Myrtaceae plants, such as the fruit extract of M. cajuputi (Isnaini et al. 2023), C. viminalis extract (Salem et al. 2017), an extract from the leaves and bark of S. jambos (Wamba et al. 2018), the leaf extract of S. malaccense (Patel et al. 2019) and an extract from Psidium cattleianum leaves(Faleiro et al. 2016). These targeted phytocompounds were detected in other plant extracts as well (Tiwari et al. 2011; Gurjar et al. 2012).
Table 1.
Phytochemical compounds of 95% ethanolic crude extracts from the leaves of 4 Myrtaceae plants
| Phytochemical compound | Plant extract | |||
| EEMC | EECV | EESJ | EESM | |
| Phenols | ++z | + | + | + |
| Flavonoids | ++ | + | + | + |
| Tannins | + | + | + | + |
| Alkaloids | – | – | – | – |
| Terpenoids | + | + | – | – |
Phytotoxicity of four Myrtaceae extracts on lettuce grown in hydroponics
The phytotoxic effect of the four Myrtaceae extracts on lettuce was assessed by the foliar spray technique. Of the 4 Myrtaceae extracts evaluated here, none of the treatments showed phytotoxicity (such as browning and discoloration) on lettuce at 15 and 30 days (Table 2). Regarding the plant growth parameters, the SPAD values did not differ significantly (p < 0.05) from control and the other treatments, while the fresh weights were dependent on the plant extracts and corresponding concentrations but not on the application times. The 95% EECV at all concentrations significantly (p < 0.05) stimulated the fresh weight of lettuce (65.5–70.2 g/plant) compared to those treated with other plant extracts (39–43.5 g/plant). In addition, an increase in the extract concentration appeared to stimulate lettuce growth more strongly (Table 2 and Fig. 1). This result also agreed with our previous research (Somnuek et al. 2020), which reported that 3 ethanolic extracts from C. viminalis (50%, 70% and 95% EECV) at 5,000–50,000 ppm showed no phytotoxic effect and still increased seed germination and the growth of lettuce. More notably, the water extract of this plant showing no phytotoxicity could still promote the growth of rice (Bali et al. 2017). Apart from the 95% EECV, the research on the phytotoxic effect of the other 3 tested plant extracts was rather limited, most likely due to the fact that these plants were Thai native plants. Furthermore, no phytotoxicity of extracts from other plants within Myrtaceae, such as Myrcia tomentosa (Imatomi et al. 2013), Myrciaria dubia (Kunth) McVaugh (Rita et al. 2017), Eucalyptus globulus (Puig et al. 2018), clove, tea tree, jaboticaba and guava (Carmello and Cardoso 2018; Teixeira et al. 2018), was also noted together with the stimulating effects on growth parameters (e.g., germination percentage, germination speed index, leaf number, root length, and yield) in lettuce. In contrast, phytotoxic effects of Myrcia vittoriana (Myrtaceae) in a plant model (lettuce and onion) have recently been reported by Vasconcelos et al. (2022). Because the 4 Myrtaceae plant extracts have been proven so far from this experiment to have no negative effects on lettuce, their antifungal activities against Alternaria sp. in vitro and against leaf spot of lettuce grown in a hydroponic system should be investigated further.
Table 2.
Phytotoxicity test of 95% ethanolic crude extracts from 4 Myrtaceae plants on lettuce grown in a hydroponic system
|
Factor A (Plant extract) |
Factor B (Concentration) |
Factor C (Application time)z |
Phytotoxicityy (3 DAT) | SPAD value |
Fresh weight (g/plant) |
| EECV | 0 ppm | One time | – | 31.6 ax | 40.9 c |
| Two times | – | 30.2 a | 42.5 c | ||
| 5,000 ppm | One time | – | 31.4 a | 65.5 b | |
| Two times | – | 31.5 a | 67.3 ab | ||
| 25,000 ppm | One time | – | 30.8 a | 68.9 ab | |
| Two times | – | 32.4 a | 67.4 ab | ||
| 50,000 ppm | One time | – | 32.5 a | 68.3 ab | |
| Two times | – | 30.7 a | 70.2 a | ||
| EEMC | 0 ppm | One time | – | 33.4 a | 40.9 c |
| Two times | – | 32.2 a | 42.5 c | ||
| 5,000 ppm | One time | – | 33.2 a | 39.0 c | |
| Two times | – | 31.6 a | 43.5 c | ||
| 25,000 ppm | One time | – | 30.6 a | 40.1 c | |
| Two times | – | 32.2 a | 41.1 c | ||
| 50,000 ppm | One time | – | 31.3 a | 40.6 c | |
| Two times | – | 31.9 a | 40.1 c | ||
| EESJ | 0 ppm | One time | – | 32.0 a | 40.9 c |
| Two times | – | 30.6 a | 42.5 c | ||
| 5,000 ppm | One time | – | 33.8 a | 40.4 c | |
| Two times | – | 33.2 a | 39.6 c | ||
| 25,000 ppm | One time | – | 32.0 a | 41.1 c | |
| Two times | – | 32.0 a | 39.4 c | ||
| 50,000 ppm | One time | – | 32.0 a | 40.2 c | |
| Two times | – | 30.8 a | 41.2 c | ||
| EESM | 0 ppm | One time | – | 32.0 a | 40.9 c |
| Two times | – | 32.8 a | 42.5 c | ||
| 5,000 ppm | One time | – | 32.0 a | 39.0 c | |
| Two times | – | 30.5 a | 39.2 c | ||
| 25,000 ppm | One time | – | 32.0 a | 40.2 c | |
| Two times | – | 30.2 a | 40.0 c | ||
| 50,000 ppm | One time | – | 32.3 a | 40.6 c | |
| Two times | – | 32.5 a | 40.4 c | ||
| C.V. (%) | 4.56 | 7.59 | |||
| A | ns | ÚÚ | |||
| B | ns | ÚÚ | |||
| C | ns | ns | |||
| A × B | ns | ÚÚ | |||
| A × C | ns | ns | |||
| B × C | ns | ns | |||
| A × B × C | ns | ns |
In vitro antifungal activity of Myrtaceae extracts against growth of Alternaria sp.
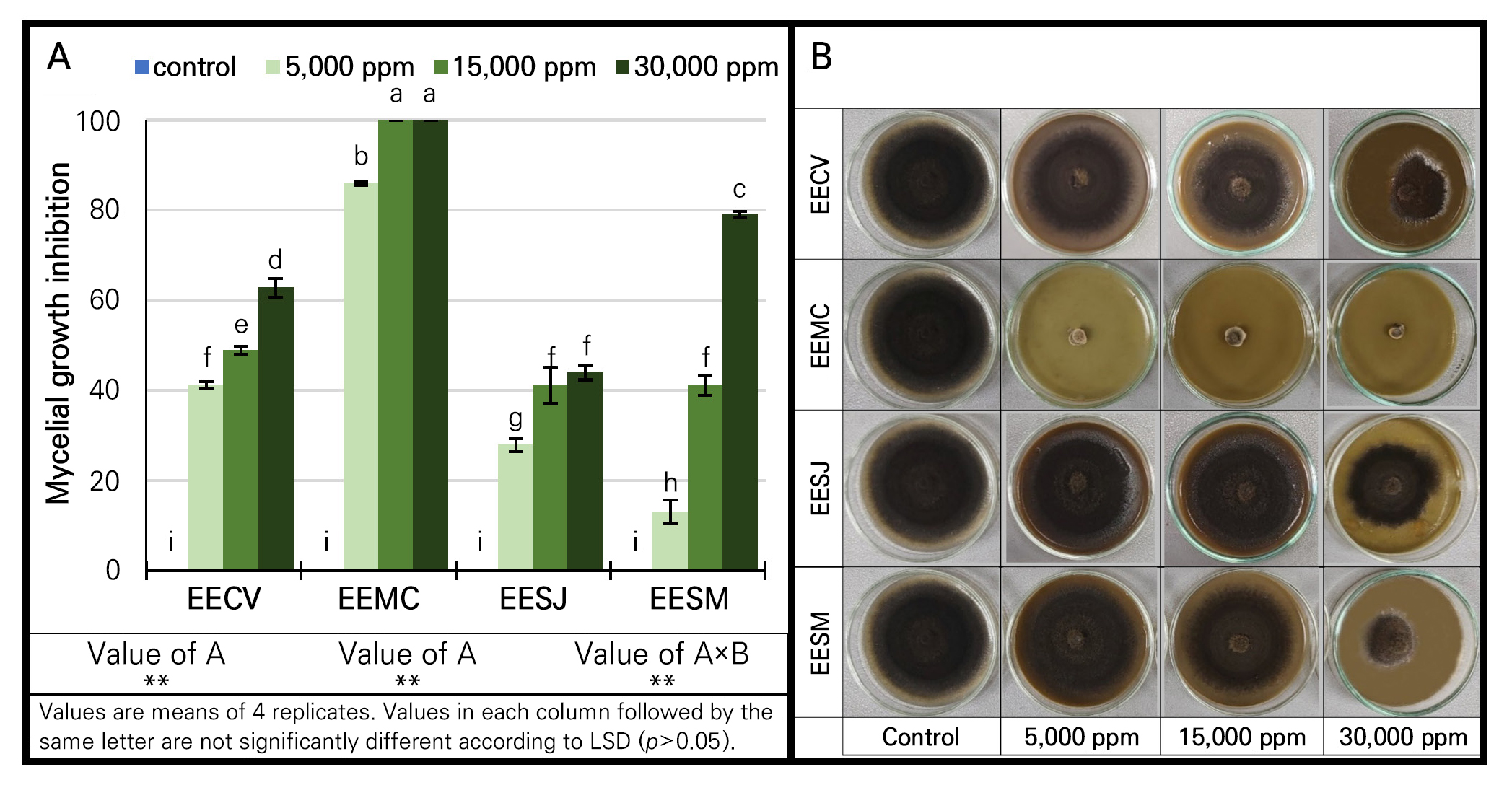
Effect of four Myrtaceae extracts on mycelial growth
The antifungal activities of crude ethanolic extracts from the four Myrtaceae plants, i.e., Callistemon viminalis (95% EECV), Melaleuca cajuputi (95% EEMC), Syzygium jambos (95% EESJ) and Syzygium malaccense (95% EESM) at different concentrations were significantly (p < 0.05) noted against A. brassicicola mycelial growth. Moreover, their effect was dependent on the concentration of the extract and on the Myrtaceae plant species (Fig. 2). The 95% EEMC at all tested concentrations (5,000, 15,000 and 50,000 ppm) statistically (p < 0.05) showed the highest inhibitory effect (85–100%), while 95% EECV was the next most effective extract, with activity in the range of 40–63%. The 95% EESM and 95% EESJ presented inhibitory effects in the corresponding ranges of 15–80% and 30–40% (Fig. 2). The antifungal activity found in this experiment is attributable to the presence of phytochemical compounds such as phenolics, flavonoids, tannins and terpenoids. These phytocompounds were reported to have good antifungal effects on plant pathogens (Al-Abd et al. 2015; Bharat and Praveen 2016; Salem et al. 2017; Patel et al. 2019). The mode of action of the phytochemicals includes membrane disruption, enzyme inactivation, protein binding and toxicity (Tiwari et al. 2011; Gurjar et al. 2012). Moreover, the significant in vitro antifungal activities of 4 extracts were in good agreement with the aforementioned phytochemical screening test, which showed the highest phytochemical substance levels in the 95% EEMC and 95% EECV. This study was in line with those of other researchers who found that M. cajuputi (essential oil) had strong inhibitory effects on Alternaria spp. (Pawar and Thaker 2007; Siddique et al. 2018), Aspergillus spp. (Thanaboripat et al. 2007; Thanaboripat 2011; Bharat and Praveen 2016; Thanaboripat et al. 2016; Siddique et al. 2018), Fusarium spp. (Pawar and Thaker 2007; Siddique et al. 2018) and Penicillium digitatum (Siddique et al. 2018). Apart from having antifungal activity, the methanolic extract of M. cajuputi also exhibited antimicrobial activity against certain bacteria, such as Bacillus spp., Enterococcus faecalis (Ukit et al. 2019), S. aureus, Vibrio cholerae, Shigella dysenteriae, and Staphylococcus epidermidis (Al-Abd et al. 2015; Ukit et al. 2019). Of the 4 extracts tested here, the 95% EECV result confirmed the inhibitory effect, as noted in our previous research (Somnuek et al. 2020), against Alternaria sp. For 95% EESJ, our result agreed with other studies testing seed and leaf extracts. The seed extract was effective at controlling certain fungi, e.g., Candida albicans, Microsporum canis, and M. gypseum (Sakander et al. 2015), while the leaf extract inhibited the bacteria, namely Salmonella typhi (Murugan et al. 2011), Aeromonas hydrophila, Alcaligenes faecalis, Bacillus cereus and Staphylococcus aureus (Mohanty and Cock 2010). In the 95% EESM case, S. malaccense leaf extracts showed inhibition activity against Pseudomonas aeruginosa, Salmonella typhi, and Shigella sonnei (Bouzada et al. 2009).
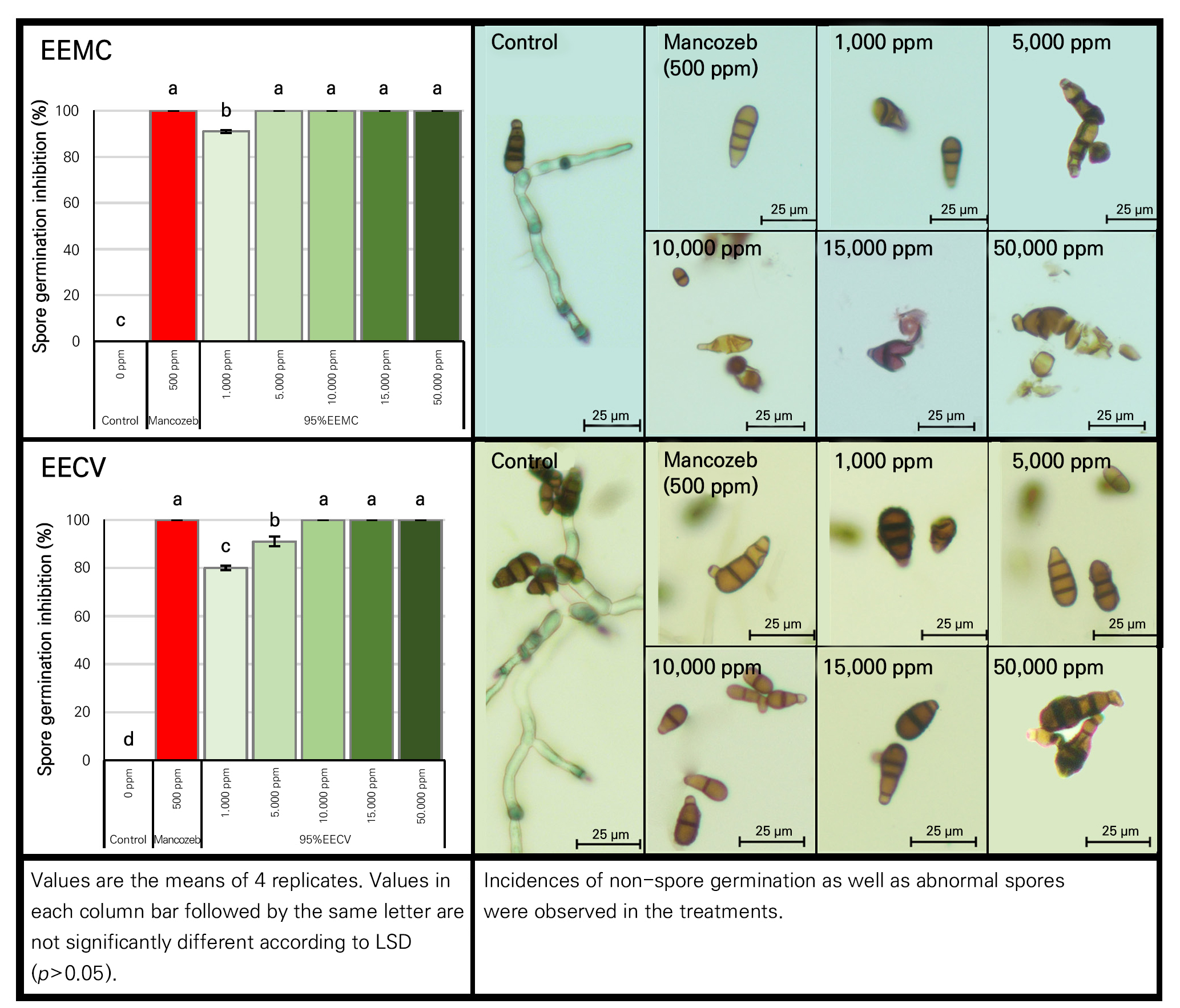
Effect of potentially selected Myrtaceae extracts on spore germination
Potentially selected Myrtaceae extracts tested here, i.e., 95% EEMC and 95% EECV, at different concentrations (1,000, 5,000, 10,000, 15,000 and 50,000 ppm) were further evaluated for their antifungal activities on spore germination of A. brassicicola. Both 95% EEMC and 95% EECV at all tested concentrations indicated a significant (p < 0.05) inhibitory effect on spore germination (Fig. 3). Complete inhibition was noted upon the application of 95% EEMC at 5,000–50,000 ppm (Fig. 3), while abnormal spores were detected at all tested concentrations. At 72 hrs, abnormalities of A. brassicicola spores were detected, taking the forms of collapsed spores, degraded cell walls, and ungerminated spores (Fig. 3). For 95% EECV, complete inhibition of spore germination was initially noted at 10,000 ppm and up. However, abnormal spores were only found at the highest extract concentration (50,000 ppm). Overall, we found that the higher concentration of plant extract induced the more potent inhibition of the growth of the pathogen in terms of mycelial growth and spore germination. Subsequently, the corresponding phytochemical profile responsible for this potent activity was further clarified in the following experiment, as described below.
Phytochemical profile of the Myrtaceae extracts by GC/MS and LC/MC-TOF
The 95% EEMC and 95% EECV were selected from the four Myrtaceae extracts due to their potential plant-growth stimulating effect and antifungal activities from the experiment described above. Subsequently, both 95% EEMC and 95% EECV were analyzed for non-targeted phytochemical compounds by GC/MS QTOF and LC/MS QTOF techniques. An overview of all annotated compounds of 95% EEMC and 95% EECV was given in Tables 3 and 4. These compounds were summarized along with their retention time, molecular formula, m/z, peak area and known bioactivity from the literature.
Table 3.
Phytochemical compounds from the 95% ethanolic extract of Thai M. cajuputi (95% EEMC) analyzed by GC/MS QTOF and LC/MS QTOF
| Compound name |
RT (min) |
Molecular formula | m/z |
Peak area (%) | Known bioactivity from literature | |
| By GC/MS QTOF | ||||||
| Terpenoids | ||||||
| 1 | Copaene | 16.74 | C15H24 | 204 | 0.20 | antimicrobial (Scur et al. 2016) |
| 2 | β-Elemene | 17.12 | C15H24 | 204 | 0.64 | anticancer (Xie et al. 2020) |
| 3 | Caryophyllene | 17.87 | C15H24 | 204 | 0.41 | antimicrobial (Selestino Neta et al. 2017) |
| 4 | α-Muurolene | 19.35 | C15H24 | 204 | 0.33 | antimicrobial (Marinas et al. 2021) |
| 5 | β-Selinene | 19.59 | C15H24 | 204 | 0.95 | antifungal (Ding et al. 2017) |
| 6 | γ-Selinene | 19.82 | C15H24 | 204 | 0.39 | antifungal (Foudah et al. 2021) |
| 7 | Elemol | 21.26 | C15H26O | 222 | 0.35 | antimicrobial (Noriega et al. 2020) |
| 8 | Spathulenol | 22.22 | C15H24O | 220 | 0.45 | antimicrobial (Fu et al. 2022) |
| 9 | Caryophyllene epoxide | 22.44 | C15H24O | 220 | 2.53 | antimicrobial (Selestino Neta et al. 2017) |
| 10 | Muurola-4,10(14)-dien-1β-ol | 23.78 | C15H24O | 220 | 1.54 | unknown |
| 11 | Caryophylla-4(12),8(13)-dien-5α-ol | 24.00 | C15H24O | 220 | 1.71 | antimicrobial (Selestino Neta et al. 2017) |
| 12 | 10,10-Dimethyl-2,6-dimethylenebicyclo[7.2.0]undecan-5β-ol | 24.12 | C15H24O | 220 | 3.89 | unknown |
| 13 | β-Eudesmol | 24.39 | C15H26O | 222 | 0.27 | antimicrobial (Noriega et al. 2020) |
| 14 | Selin-6-en-4α-ol | 24.61 | C15H26O | 222 | 2.12 | antibacterial (Cordeiro et al. 2020) |
| 15 | Aromadendrene epoxide | 24.75 | C15H24O | 220 | 2.45 | antimicrobial (Mulyaningsih et al. 2011) |
| 16 | 8-Methoxycedrane | 25.27 | C17H28O2 | 264 | 0.74 | unknown |
| 17 | (1R,7S,E)-7-Isopropyl-4,10-dimethylenecyclodec-5-enol | 25.44 | C15H24O | 220 | 1.17 | unknown |
| 18 | Pluchidiol | 26.97 | C13H20O2 | 208 | 0.21 | antimicrobial (Karimi et al. 2019) |
| 19 | Proximadiol | 29.55 | C15H28O2 | 222 | 0.25 | unknown |
| 20 | α-Eudesmol | 30.29 | C15H26O | 222 | 1.40 | antimicrobial (Noriega et al. 2020) |
| 21 | Squamulosone | 30.90 | C15H22O | 218 | 0.78 | unknown |
| 22 | 6,10,14-Trimethyl-2-pentadecanone | 31.46 | C18H36O | 268 | 0.43 | antibacterial (Naidoo et al. 2014) |
| 23 | Corymbolone | 31.55 | C15H24O2 | 236 | 0.40 | antifungal (Hussein et al. 2016) |
| 24 | 4,4,8-Trimethyltricyclo[6.3.1.0(1,5)]dodecane-2,9-diol | 31.67 | C15H26O2 | 238 | 0.54 | unknown |
| 25 | Estra-1,3,5(10)-trien-17β-ol | 32.82 | C18H24O | 256 | 0.91 | anticancer (Khan et al. 2022) |
| 26 | Phytol | 38.60 | C20H40O | 296 | 19.14 |
antimicrobial (Saha and Bandyopadhyay 2020; Yusoff et al. 2020; Petpheng et al. 2023) |
| 27 | γ-Sitosterol | 41.19 | C29H50O | 414 | 0.54 | antibacterial (Subramaniam et al. 2014) |
| 28 | 28-Norolean-17-en-3-ol | 42.70 | C29H48O | 412 | 0.57 | antiviral (Darshani et al. 2022) |
| Terpenoids | 45.31 | |||||
| Phenolics | ||||||
| 29 | Ethyl α-d-glucopyranoside | 23.67 | C8H16O6 | 208 | 1.66 | antioxidant (Dai et al. 2022) |
| 30 | 2-Hydroxy-5-methoxybenzaldehyde,TMS derivative | 25.82 | C11H16O3Si | 224 | 0.26 | antibacterial (Durgadevi et al. 2019) |
| Phenolics | 1.92 | |||||
| Flavonoids | ||||||
| 31 | Pinostrobin | 32.53 | C11H10O4 | 206 | 0.32 | antibacterial (Marliyana et al. 2018) |
| Flavonoids | 0.32 | |||||
| Naphthalenes & Aromatics | ||||||
| 32 | 1,2,3,4-Tetrahydronaphthalene-1,2-diol,5,6-dimethoxy- | 23.15 | C12H16O4 | 164 | 8.45 | unknown |
| 33 | 9-Butyl-9H-fluoren-9-ol | 36.55 | C17H18O | 238 | 0.40 | unknown |
| Naphthalenes & Aromatics | 8.85 | |||||
| Other compounds | ||||||
| 34 | 2,5-Dimethoxythiophenol | 15.71 | C8H10O2S | 170 | 0.26 | unknown |
| 35 | Butyric acid, 2,3-epoxy-, ethyl ester | 21.47 | C11H16O | 164 | 0.23 | unknown |
| 36 | 2',3',4' Trimethoxyacetophenone | 26.46 | C11H14O4 | 210 | 7.16 | antibacterial (Freitas et al. 2020) |
| 37 |
2,5,5,8a-Tetramethyl-4-methylene-6,7,8,8a-tetrahydro-4H,5H- chromen-4a-yl hydroperoxide | 28.99 | C14H22O3 | 238 | 1.23 | unknown |
| 38 | Octahydro-1-(2-octyldecyl)- pentalene | 30.51 | C26H50 | 362 | 3.63 | unknown |
| 39 | 10-Methylanthracene-9-carboxaldehyde | 33.23 | C16H12O | 220 | 11.63 | unknown |
| 40 | 2-Isopropyl-10-methylphenanthrene | 37.35 | C18H18 | 234 | 5.32 | unknown |
| 41 | 5-Methyl-6,7,8,9-tetrahydroisothiazolo[5,4-C]isoquinolin-1(2H)-one | 37.79 | C11H12N2OS | 220 | 12.53 | unknown |
| Other compounds | 41.99 | |||||
| Total | 98.39 | |||||
| By LC/MS QTOF | ||||||
| Terpenoids | ||||||
| 1 | Ingenol | 15.36 | C20H28O5 | 348 | 9.17 | anti-HIV (Fujiwara et al. 1996) |
| Terpenoids | 9.17 | |||||
| Phenolics | ||||||
| 2 | Acetaminophen | 11.99 | C8H9NO2 | 151 | 9.93 | antifungal (Srikanth et al. 2005) |
| 3 | 6-Gingerol | 12.51 | C17H26O4 | 294 | 2.75 | antifungal (Xi et al. 2022) |
| 4 | 3,5-Di-tert-butyl-2-hydroxybenzaldehyde | 27.74 | C15H22O2 | 234 | 6.81 | antimicrobial (Zhao et al. 2020) |
| Phenolics | 19.49 | |||||
| Flavonoids & Naphthalene | ||||||
| 5 | Cyanidin-3-O-sophoroside | 0.58 | C27H31O16+ | 611 | 1.34 | antibacterial (Tan et al. 2019) |
| 6 | 6,2'-Dimethylflavone | 15.72 | C17H14O4 | 282 | 4.21 | antifungal (Mangoyi et al. 2015) |
| Flavonoids & Naphthalene | 5.55 | |||||
| Steroids & Benzofurans | ||||||
| 7 | 5β-Androstane-3β,17β-diol | 12.54 | C19H32O2 | 292 | 3.84 | unknown |
| 8 | Dichlorofluorescein | 14.80 | C20H10Cl2O5 | 401 | 3.95 | unknown |
| Steroids & Benzofurans | 7.79 | |||||
| Ethylene glycol | ||||||
| 9 | Nonaethylene glycol | 0.58 | C18H38O10 | 415 | 10.28 | antimicrobial (Shukla et al. 2012) |
| 10 | Decaethylene glycol | 0.58 | C20H42O11 | 459 | 10.42 | antimicrobial (Shukla et al. 2012) |
| 11 | Pentaethylene glycol | 12.51 | C10H22O6 | 238 | 5.55 | antimicrobial (Shukla et al. 2012) |
| Ethylene glycol | 26.25 | |||||
| Vitamins | ||||||
| 12 | R-lipoic acid | 0.46 | C8H14O2S2 | 207 | 6.63 | PGPMz (Elkelish et al. 2021) |
| Vitamins | 6.63 | |||||
| Fatty acid & Glucosides | ||||||
| 13 | 2,3-Dinor-11β-prostaglandin F2α | 12.56 | C18H30O5 | 326 | 9.45 | unknown |
| 14 | Harpagide | 14.4 | C15H24O10 | 364 | 3.46 | unknown |
| Fatty acid & Glucosides | 12.91 | |||||
| Other compounds | ||||||
| 15 | 1-(1',3'-Benzodioxol-5'-yl)-2-butanamine | 0.42 | C11H15NO2 | 193 | 1.03 | unknown |
| 16 | Salicylic acid, valerate | 0.82 | C12H14O4 | 222 | 3.70 | ISRy (War et al. 2011; Sangpueak et al. 2021) |
| 17 | 2-Acetyl-5-(tetrahydroxybutyl)imidazole | 12.47 | C9H14N2O5 | 230 | 1.24 | unknown |
| 18 | Leukotriene B3 | 12.54 | C20H34O4 | 338 | 3.34 | antibacterial (Serban et al. 2018) |
| Other compounds | 9.31 | |||||
| Total | 97.1 | |||||
Table 4.
Phytochemical compounds from the 95% ethanolic extract of Thai C. viminalis (95% EECV) analyzed by GC/MS QTOF and LC/MS QTOF
| Compound name |
RT (min) |
Molecular formula | m/z |
Peak area (%) | Known bioactivity from literature | |
| By GC/MS QTOF | ||||||
| Terpenoids | ||||||
| 1 | α-Phellandrene | 8.96 | C10H16 | 136 | 2.04 | antimicrobial (İşcan et al. 2012) |
| 2 | o-Cymene | 9.39 | C10H14 | 134 | 0.84 | antimicrobial (Tadtong et al. 2016) |
| 3 | 1,8-Cineole | 9.52 | C10H18O | 154 | 14.0 |
antimicrobial (Tadtong et al. 2016; Şimşek and Duman 2017; Kim et al. 2018) |
| 4 | α-Terpinene | 10.77 | C10H16 | 136 | 0.19 | antifungal (Marei and Abdelgaleil 2018) |
| 5 | Terpinen-4-ol | 12.64 | C10H18O | 154 | 0.36 |
antifungal (Marei and Abdelgaleil 2018; Kamiya et al. 2024) |
| 6 | L-α-Terpineol | 12.89 | C10H18O | 154 | 1.42 | antifungal (Marei and Abdelgaleil 2018) |
| 7 | 2-Acetoxy-1,8-cineole | 15.24 | C12H20O3 | 212 | 0.26 | antimicrobial (Tadtong et al. 2016) |
| 8 | Caryophyllene | 17.86 | C15H24 | 204 | 0.35 | antimicrobial (Selestino Neta et al. 2017) |
| 9 | γ-Selinene | 19.81 | C15H24 | 204 | 0.20 | antifungal (Foudah et al. 2021) |
| 10 | Spathulenol | 22.21 | C15H24O | 220 | 0.76 | antimicrobial (Fu et al. 2022) |
| 11 | Isoleptospermone | 23.65 | C15H22O4 | 266 | 1.01 | unknown |
| 12 | Pluchidiol | 29.46 | C13H20O2 | 208 | 0.57 | unknown |
| 13 | α-Phellandrene, dimer | 29.82 | C20H32 | 272 | 0.76 | antimicrobial (İşcan et al. 2012) |
| 14 | Phytyl acetate | 31.42 | C22H42O2 | 338 | 0.49 | antifungal (Foudah et al. 2021) |
| 15 | 4,4,8-Trimethyltricyclo[6.3.1.0(1,5)]dodecane-2,9-diol | 32.82 | C15H26O2 | 238 | 0.33 | unknown |
| 16 | Phytol | 42.67 | C20H40O | 296 | 0.53 |
antimicrobial (Saha and Bandyopadhyay 2020; Yusoff et al. 2020; Petpheng et al. 2023) |
| 17 | Vitamin E | 65.48 | C29H50O2 | 430 | 0.56 | PGPMz (Muñoz and Munné-Bosch 2019) |
| 18 | γ-Sitosterol | 68.15 | C29H50O | 414 | 1.91 | antibacterial (Subramaniam et al. 2014) |
| 19 | Lanosterol | 68.76 | C30H50O | 426 | 0.52 | unknown |
| 20 | 28-Norolean-17-en-3-ol | 69.25 | C29H48O | 412 | 1.02 | antibacterial (Kim et al. 2015) |
| 21 | α-Amyrin | 69.56 | C30H50O | 426 | 0.40 | antibacterial (Chung et al. 2011) |
| 22 | γ-Sitostenone | 70.52 | C29H48O | 412 | 0.64 | antibacterial (Subramaniam et al. 2014) |
| 23 | Betulinaldehyde | 73.64 | C30H48O2 | 440 | 0.88 | antibacterial (Chung et al. 2011) |
| Terpenoids | 30.04 | |||||
| Phenolics | ||||||
| 24 | 2'-Hydroxy-5'-methoxyacetophenone, 3-methylbutylether | 20.33 | C14H20O3 | 236 | 1.70 | unknown |
| 25 | 2,6-Di-tert-butyl-4-hydroxy-4-methylcyclohexa-2,5-dien-1-one | 23.24 | C15H24O2 | 236 | 1.77 | unknown |
| Phenolics | 3.47 | |||||
| Flavonoids | ||||||
| 26 | Hesperetine | 58.40 | C17H14O7 | 330 | 0.39 | antimicrobial (Carevic et al. 2022) |
| 27 | Prosogerin E | 60.00 | C18H16O7 | 332 | 2.06 | antibacterial (Shamsudin et al. 2022) |
| 28 | Guaiacin | 60.63 | C20H24O4 | 328 | 0.32 | unknown |
| 29 | 8-hydroxysalvigenin | 64.71 | C18H16O5 | 312 | 0.71 | antimicrobial (Alreshidi et al. 2020) |
| 30 | 4',7-Di-O-methylnaringenin | 65.01 | C19H18O5 | 326 | 1.79 | antibacterial (Kozłowska et al. 2017) |
| Flavonoids | 5.27 | |||||
| Aromatic & Fatty acids | ||||||
| 31 | Isolongifolene | 19.59 | C15H24 | 204 | 0.48 | unknown |
| 32 | 1,2,3,4,5,6,7,8-Octahydro-2-naphthol, 4-methylene-2,5,5-trimethyl- | 24.79 | C14H22O | 206 | 0.25 | unknown |
| 33 | 2,4,6-Octatriene, 3,4-dimethyl- | 9.23 | C10H16 | 136 | 0.26 | unknown |
| 34 | Hexadecanoic acid, ethyl ester | 37.81 | C18H36O2 | 284 | 0.30 | unknown |
| 35 | Ethyl oleate | 44.93 | C20H38O2 | 310 | 0.20 | unknown |
| Aromatic & Fatty acids | 1.49 | |||||
| Quinones & Steroids | ||||||
| 36 | 5-Hydroxy-2,2,6,6-tetramethyl-4-cyclohexene-1,3-dione | 14.26 | C10H14O3 | 182 | 1.04 | unknown |
| 37 | Androst-1-en-3-one, 17-(acetyloxy)-4,5-epoxy-,(4β,5β,17β)- | 50.33 | C21H28O4 | 344 | 2.14 | unknown |
| Quinones & Steroids | 3.18 | |||||
| Other compounds | ||||||
| 38 | 6-acetyl-2,2,4,4-tetramethylcyclohexane-1,3,5-trione | 16.37 | C12H16O4 | 224 | 1.59 | unknown |
| 39 | 2-Cyclopropene-1-carboxylic acid, 2-(1,1-dimethyl-5-oxohexyl)-, methyl ester | 18.68 | C13H20O3 | 224 | 0.31 | unknown |
| 40 | 2-Hydroxy-5-methoxybenzaldehyde, TMS derivative | 19.16 | C11H16O3Si | 224 | 0.32 | unknown |
| 41 | 2,2,4-Trimethyl-4-trimethylsilylethynylcyclopentane-1,3-dione | 21.01 | C13H20O2Si | 236 | 25.0 | unknown |
| 42 | 1-Ethyl-2,2,4a,7,7-pentamethyl-1,2,3,4,4a,5,6,7-octahydro[1,8]naphthyridine | 22.96 | C15H28N2 | 236 | 0.72 | unknown |
| 43 | 4a,6a-Dimethyl-2-oxo-1a,2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11-tetradecahydrocyclopenta[7,8]phenanthro[1,10ab]oxiren-7-yl acetate pk2 | 51.49 | C21H28O4 | 344 | 7.63 | unknown |
| 44 | 2-Amino-3-cyano-4-methyl-4,6-dipyridin-4-ylcyclohexa-1,5-dien-1,3-dicarboxylic acid, diethyl ester | 61.33 | C24H24N4O4 | 432 | 0.32 | unknown |
| 45 | 2,6,10,14,18,22-Tetracosahexaene,2,6,10,15,19,23-hexamethyl- | 62.28 | C30H50 | 410 | 0.76 | unknown |
| Other compounds | 36.65 | |||||
| Total | 80.1 | |||||
| By LC/MS QTOF | ||||||
| Terpenoids | ||||||
| 1 | 11-Keto-β-boswellic acid | 16.22 | C30H46O4 | 471 | 6.96 | antimicrobial (Jaroš et al. 2022) |
| Terpenoids | 6.96 | |||||
| Phenolics | ||||||
| 2 | Metaproterenol | 13.34 | C11H17NO3 | 212 | 5.33 | unknown |
| Phenolics | 5.33 | |||||
| Flavonoids | ||||||
| 3 | Leiocarposide | 12.63 | C27H34O16 | 653 | 2.41 | antimicrobial (Toiu et al. 2019) |
| 4 | Peonidin cation | 15.24 | C16H13O6+ | 301 | 1.03 | antifungal (Chen et al. 2023) |
| Flavonoids | 3.44 | |||||
| Coumarins, Alkaloid & Steroids | ||||||
| 5 | 7-Methoxycoumarin | 0.80 | C10H8O3 | 177 | 2.11 | antifungal (Chen et al. 2023) |
| 6 | Quinupramine | 12.12 | C21H24N2 | 305 | 1.04 | antimicrobial (Caldara and Marmiroli 2018) |
| 7 | 5β-Pregnan-3α,17,20β21-tetrol-11-one | 12.20 | C21H34O5 | 366 | 1.39 | unknown |
| 8 | γ-Muricholic acid | 15.28 | C24H35D5O5 | 391 | 1.70 | antibacterial (Watanabe et al. 2017) |
| 9 | 5β-Androstane-3α,17β-diol | 12.07 | C19H32O2 | 275 | 1.33 | unknown |
| Coumarins, Alkaloid & Steroids | 7.57 | |||||
| Ethylene glycol | ||||||
| 10 | Nonaethylene glycol | 0.81 | C18H38O10 | 432 | 5.00 | antimicrobial (Shukla et al. 2012) |
| 11 | Decaethylene glycol | 0.81 | C20H42O11 | 459 | 6.10 | antimicrobial (Shukla et al. 2012) |
| Ethylene glycol | 11.1 | |||||
| Vitamins | ||||||
| 12 | 1,25-Dihydroxy vitamin D2 | 16.23 | C27H44O3 | 411 | 2.07 | PGPM (Buchala and Pythoud 1988) |
| Vitamins | 2.07 | |||||
| Fatty acid | ||||||
| 13 | 18-Carboxy dinor leukotriene B4 | 12.12 | C18H26O6 | 321 | 3.53 | unknown |
| 14 | 16,16-Dimethylprostaglandin E2 | 12.23 | C22H36O5 | 363 | 1.20 | unknown |
| 15 | Tafluprost (free acid) | 12.25 | C22H28F2O5 | 411 | 8.59 | unknown |
| Fatty acid | 13.32 | |||||
| Other compounds | ||||||
| 16 | Etidocaine | 12.05 | C17H28N2O | 277 | 2.05 | unknown |
| 17 | N-Desethylamodiaquine | 12.12 | C18H18ClN3O | 328 | 2.05 | unknown |
| 18 | Nigerose | 12.19 | C12H22O11 | 365 | 5.49 | PGPM (Ichimura et al. 2022) |
| 19 | Unoprostone isopropyl ester | 12.33 | C25H44O5 | 407 | 1.23 | unknown |
| 20 | Glu-Ile | 13.49 | C11H20N2O5 | 261 | 5.21 | PGPM (Qiu et al. 2020) |
| 21 | Cinnamic acid | 15.24 | C9H8O2 | 149 | 2.12 | antifungal (Guo et al. 2020) |
| 22 | 3β-Hydroxy-5-cholenoic acid | 15.49 | C24H38O3 | 357 | 7.98 | unknown |
| 23 | Octamethylcyclotetrasiloxane | 15.54 | C8H24O4Si4 | 297 | 1.14 | unknown |
| Other compounds | 27.27 | |||||
| Total | 77.06 | |||||
As shown in Table 3, the analysis of the 95% EEMC by GC/MS revealed 41 components comprising 98.39% of the total extract composition, represented by 45.31% of terpenoids, 1.95% of phenolics, 0.32% of flavonoids, 8.85% of naphthalene and aromatics as well as 41.99% of other compounds. Of terpenoids containing 28 phytochemicals, phytol (14%) was the predominant component, followed by 10,10-dimethyl-2,6-dimethylenebicyclo[7.2.0]undecan-5β-ol (3.89%), caryophyllene epoxide (2.53%), aromadendrene epoxide (2.45%) and selin-6-en-4α-ol (2.12%). For phenolics, the compounds presented a number of components, such as ethyl α-d-glucopyranoside (1.66%) and 2-hydroxy-5-methoxybenzaldehyde, a TMS derivative (0.26%). In contrast, pinostrobin (0.32%) was the only component of flavonoids. The LC/MS analysis showed the presence of 18 chemicals comprising 97.1% of the total extract composition, represented by 9.17% of terpenoids, 19.49% of phenolics, 5.55% of flavonoids and naphthalene, 7.79% of steroids and benzofurans, 12.91% of fatty acids and glucosides, 6.63% of vitamins, 26.25% of ethylene glycol and 9.31% of other compounds. The main compounds of ethylene glycol were nonaethylene glycol (10.28%) and decaethylene glycol (10.42%). The phenolics namely, acetaminophen (9.9%), 3,5-di-tert-butyl-2-hydroxybenzaldehyde (6.81%) and 6-gingerol (2.75%) were detected. Meanwhile, terpenoids and vitamins each contained only one component namely, ingenol (9.17%) and R-lipoic acid (6.63%), respectively. Based on the 95% EEMC analysis, it was clearly shown that the highest diversity of terpenoids was found among the total identified phytochemical compounds. Our findings were in good agreement with earlier work (Al-Abd et al. 2015) showing that the major phytochemical groups in M. cajuputi were terpenoids, phenolics, flavonoids aromatics and fatty acids. Recently, Isah et al. (2023) compiled and summarized work showing that terpenoids, phenolics, and flavonoids were the major phytocompounds of the solvent extracts and essential oil of M. cajuputi.
Regarding 95% EECV, as shown in Table 4, its compositional variability was in line with those of the 95% EEMC case, showing the highest diversity of terpenoids. Based on GC/MS, the 95% EECV contained 45 intricate chemical compositions consisting of 80.1% of the total extract composition, represented by 30.04% of terpenoids [mainly 1,8-cineole (14%), α-phellandrene (2.04%), γ-sitosterol (1.91%) and L-α-terpineol (1.42%)], 3.47% of phenolics [2'-hydroxy-5'- methoxyacetophenone, 3-methylbutylether (1.7%) and 2,6-di-tert-butyl-4-hydroxy-4-methylcyclohexa-2,5-dien-1-one (1.77%)], 5.27% of flavonoids [prosogerin E (2.06%) and 4',7-di-O-methylnaringenin (1.79%)], 1.49% of aromatic and fatty acids, 3.18% quinones and steroids, including 36.65% of other compounds. Based on the LC/MS, the analysis revealed the presence of 23 phytochemicals comprising 77.06% of the total plant extract composition. A total phytochemical compounds were identified as 6.96% of terpenoids (11-keto-β-boswellic acid), 5.33% of phenolics (metaproterenol), 3.44% of flavonoids (leiocarposide and peonidin cation), 7.57% of the groups of coumarins, alkaloids and steroids, 11.1% of ethylene glycol, 2.07% of vitamins (1,25-dihydroxy vitamin D2), 13.32% of fatty acids (tafluprost, 18-carboxy dinor leukotriene B4, and 16,16-dimethylprostaglandin E2) along with 27.27% of other compounds (nigerose and glu-ile). Based on the 95% EECV analysis, our findings were in good agreement with those of the previous studies by Salem et al. (2017), Ahmad and Athar (2016) and de Oliveira et al. (2014), demonstrating the detection of terpenoids (1,8-cinole), phenolics and flavonoids in C. viminalis.
As shown in Tables 3 and 4, terpenoids were the main compounds in both the EEMC and EECV followed by phenolics, flavonoids and ethylene glycol. The known biological activities (such as antifungal, antibacterial and antiviral activities) of the phytochemical compounds identified in EECV and EEMC were cited and compiled in Tables 3 and 4. Regarding the antimicrobial activities, terpenoid compounds (mainly phytol, 1,8-cineole, α-terpinene, terpinene-4-ol and 11-Keto-β-boswellic acid) were documented to exert potent antifungal activity against Candida albicans (Tadtong et al. 2016), Aspergillus spp. (Morcia et al. 2012; Kim et al. 2018), Alternaria spp. (Morcia et al. 2012; Marei and Abdelgaleil 2018), Botrytis cinerea (Yusoff et al. 2020), Fusarium spp. (Morcia et al. 2012; Marei and Abdelgaleil 2018), Penicillium sp. (Morcia et al. 2012; Marei and Abdelgaleil 2018), and Rhizoctonia solani (Marei and Abdelgaleil 2018). The large amount of terpenoids found in the 95% EEMC (55%) from this analysis tended to support our earlier result regarding the antifungal activity of both plant extracts tested via poisoned food testing, showing the maximum potential inhibition effect of 95% EEMC. Interestingly, our phytochemical analysis also confirmed the presence of vitamin E, 1,25-dihydroxy vitamin D2, nigerose and glu-ile in 95% EECV, which were cited as plant-growth-promoting metabolites (Muñoz and Munné-Bosch 2019; Qiu et al. 2020; Ichimura et al. 2022). Hence, this likely supported the positive effect (such as plant-growth-promoting activity) of EECV on the growth of lettuce in our study found during the phytotoxicity assessment.
Evaluation of EEMC and EECV as an inducer on development of Alternaria leaf spot and induction of defense enzymes in lettuce grown in a hydroponic system
The EEMC effect
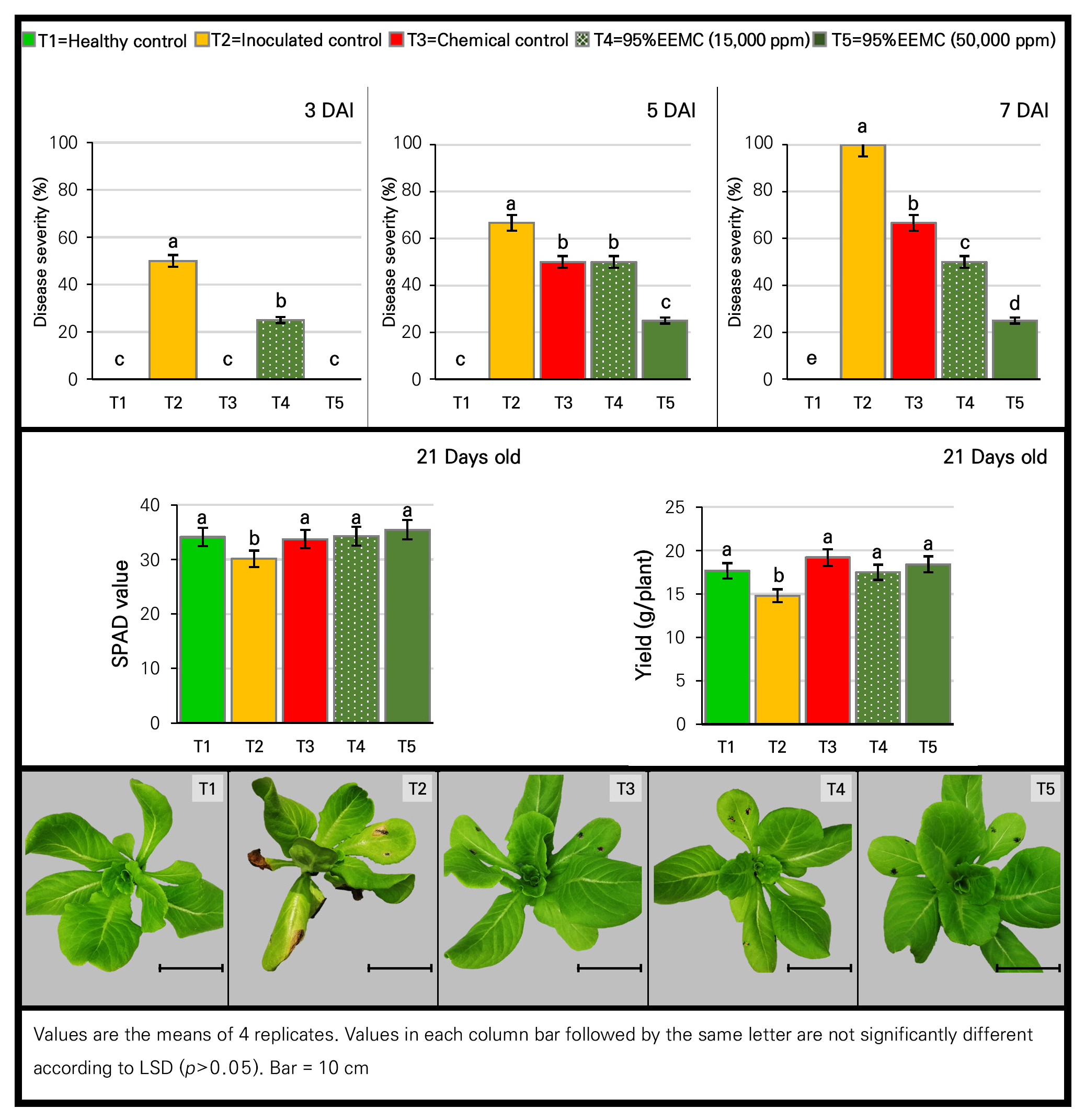
The indirect effect of 95% EEMC at 15,000 and 50,000 ppm against Alternaria leaf spot in lettuce using CRD with 3 replications revealed that the use of the highest concentration (50,000 ppm) of the extract and fungicide led to the complete inhibition of leaf spot at 3 DAI (Fig. 4). In addition, disease progress developed further in all treatments. However, the 2 tested concentrations of the extract as well as the fungicide treatment still significantly (p < 0.05) showed a strong inhibition effect at 5 DAI. At 7 DAI, a statistically significant difference (p < 0.05) in the severity of Alternaria leaf spot was noted among the treatments. Disease severity in the extract treatments was significantly reduced (p < 0.05) by 50–70% compared to an inoculated control, while only the fungicide treatment resulted in a 40% reduction. Importantly, the high concentration (50,000 ppm) significantly (p < 0.05) resulted in the greatest level of disease reduction in this regard. Moreover, the activities of the induced defense enzymes of β-1,3-glucanase, chitinase and peroxidase were monitored along with the disease severity levels at 1, 3, 5 and 7 DAI. When lettuce plants were infected with a pathogen, the enzymatic activities increased with time as the disease developed. On the first day after inoculation, the strongest activity of β-1,3-glucanase at a significant (p < 0.05) level was noted in the 95% EEMC treatments (15,000 and 50,000 ppm) compared to an inoculated control. At 7 DAI, the highest activity at a significant level (p < 0.05) was shown in the 95% EEMC treatments, followed by the inoculated and fungicide control treatment, while the lowest level of activity was recorded in the healthy control. The activities of the chitinase and peroxidase enzymes showed patterns identical to that of β-1,3-glucanase. At 7 DAI, the 95% EEMC treatments resulted in significantly higher activity (p < 0.05) of chitinase compared to the inoculated control and the fungicide treatment, while the highest activity of peroxidase at a significant level (p < 0.05) was also noted in the 95% EEMC treatments (Table 5). This result confirmed that the disease severity was related to the activities of the 3 induced defense enzymes. To be precise, the highest level of enzyme activity was noted in lettuce treated with 95% EEMC showing less severity of Alternaria leaf spot. This implied that 95% EEMC acted as a resistance inducer.
With regard to plant parameters at harvest (21 days old), the 95% EEMC and fungicide treatments showed the highest values of both parameters (SPAD value and yield), which were statistically different (p < 0.05) compared to the inoculated control but not significantly different (p < 0.05) from the healthy control.
Table 5.
Activity of defense enzymes against Alternaria in lettuce treated with 95% EEMC
| Treatment | Activity of defense enzyme | |||||||||||||
|
β-1,3-Glucanase (µmol glucose /g of fresh leaves) |
Chitinase (µmol GlcNAc/g of fresh leaves) |
Peroxidase (unit/ g of fresh leaves) | ||||||||||||
| 1 DAI | 3 DAI | 5 DAI | 7 DAI | 1 DAI | 3 DAI | 5 DAI | 7 DAI | 1 DAI | 3 DAI | 5 DAI | 7 DAI | |||
| T1z | 1.53 dy | 3.53 d | 3.60 d | 3.46 d | 5.50 d | 5.07 d | 5.97 d | 5.63 e | 3.38 e | 4.90 e | 6.52 c | 8.44 c | ||
| T2 | 2.17 b | 5.27 b | 6.17 b | 7.36 b | 9.43 b | 11.25 b | 15.95 b | 17.30 c | 5.37 c | 6.89 c | 8.51 b | 10.43 b | ||
| T3 | 2.20 c | 4.63 c | 5.20 c | 6.20 c | 8.63 c | 9.17 c | 11.03 c | 11.33 d | 4.70 d | 6.22 d | 7.85 b | 9.76 bc | ||
| T4 | 2.55 a | 8.62 a | 7.87 a | 11.66 a | 13.49 a | 15.29 a | 19.23 a | 20.24 b | 7.50 b | 9.02 b | 10.65 a | 12.56 a | ||
| T5 | 2.72 a | 8.72 a | 7.97 a | 11.83 a | 13.49 a | 14.91 a | 19.99 a | 23.12 a | 8.30 a | 9.82 a | 11.45 a | 13.36 a | ||
The EECV effect
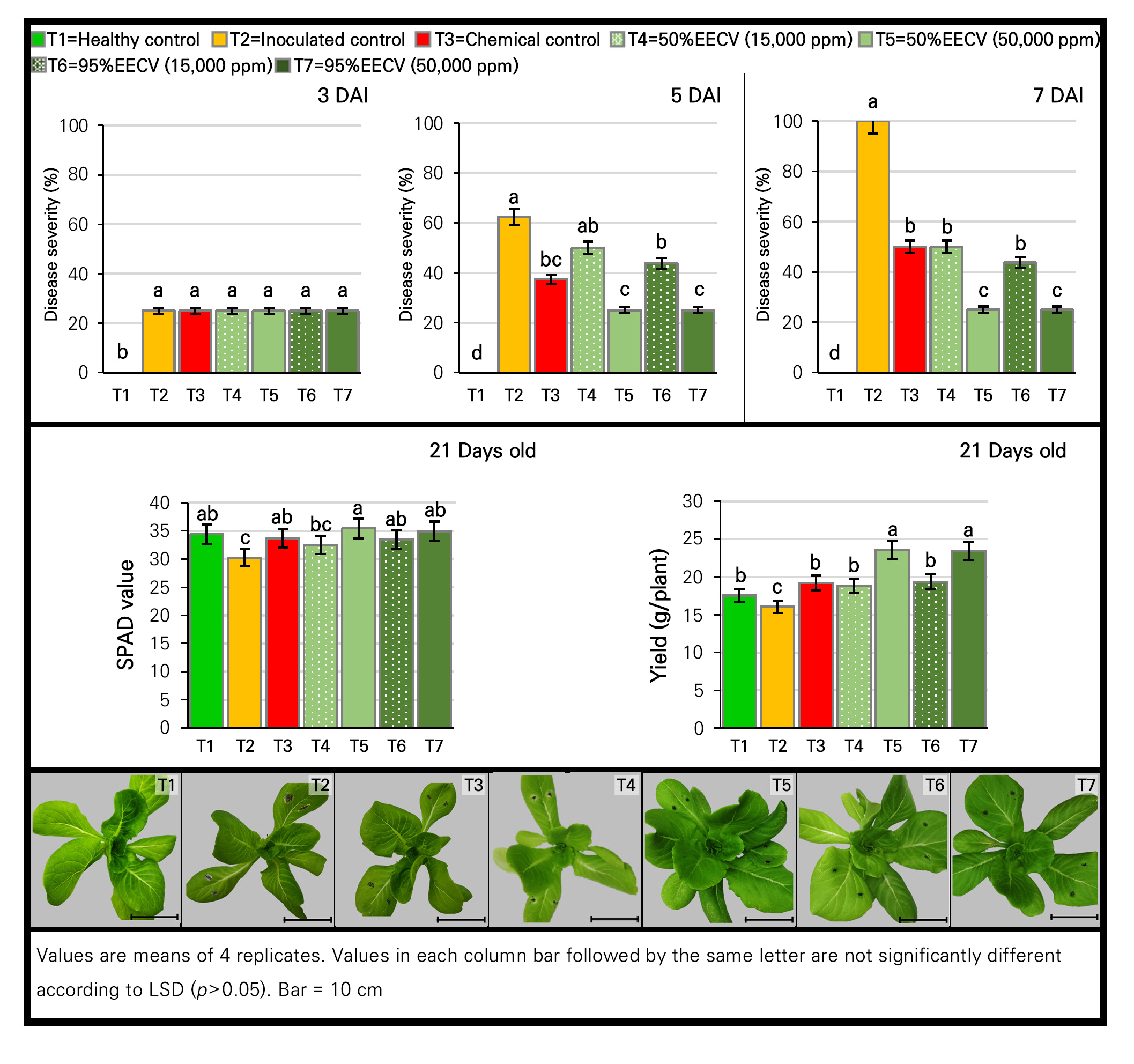
The determination effect of 50% and 95% EECV at 15,000 and 50,000 ppm on disease development and plant defense enzyme induction using CRD with 3 replications revealed that all treatments except for that of the healthy control showed no significant difference with regard to disease severity compared to the inoculated control (at 3 DAI). At 5 DAI, the leaf spot progressed to lesions in all treatment cases, except at the highest concentrations of 50% and 95% EECV, which still showed static lesions. At 7 DAI, the highest concentration (50,000 ppm) of both EECV treatments led to the highest (p < 0.05) disease reduction (75%), followed by the lower concentration (15,000 ppm) and the fungicide control treatments in the range of 43.75–50%. Regarding the induced defense enzymes (Table 6), all presented a pattern similar to those in the 95% EEMC experiment; that is, the enzymatic activities increased over time, and higher levels of enzyme induction were observed in the lettuce treatments with the plant extracts (including the 50% EECV). At 7 DAI, the highest and significant (p < 0.05) enzymes activities of β-1,3-glucanase and chitinase were noted in all 4 EECV treatments compared to the fungicide treatment and the inoculated control. For peroxidase, the highest and significant activity (p < 0.05) was only found in the 95% EECV case at 50,000 ppm. Considering these results, there was a reduction in the severity of Alternaria leaf spot and an increase in defense-related enzymes in the 50% and 95% EECV-treated lettuce. This result was in line with the EEMC experiment implying that EECV acted as a resistance inducer as well.
Table 6.
Activity of defense enzymes against Alternaria in lettuce treated with 50% and 95% EECV
| Treatment | Activity of defense enzyme | |||||||||||||
|
β-1,3-Glucanase (µmol glucose /g of fresh leaves) |
Chitinase (µmol GlcNAc/g of fresh leaves) |
Peroxidase (unit/ g of fresh leaves) | ||||||||||||
| 1 DAI | 3 DAI | 5 DAI | 7 DAI | 1 DAI | 3 DAI | 5 DAI | 7 DAI | 1 DAI | 3 DAI | 5 DAI | 7 DAI | |||
| T1z | 1.53 cy | 3.53 d | 3.60 c | 3.46 d | 5.50 c | 5.07 c | 5.97 c | 5.63 c | 3.33 c | 4.98 e | 5.48 e | 6.45 e | ||
| T2 | 2.17 b | 5.27 c | 9.43 b | 12.86 c | 11.00 b | 14.30 b | 18.67 b | 21.96 b | 6.02 b | 6.47 cd | 6.83 d | 7.45 d | ||
| T3 | 2.20 b | 5.37 c | 9.50 b | 12.43 c | 10.80 b | 15.07 b | 18.60 b | 21.66 b | 6.79 a | 6.54 bc | 7.53 c | 7.99 cd | ||
| T4 | 3.30 a | 8.87 b | 11.20 a | 16.26 ab | 16.00 a | 18.20 a | 21.63 a | 26.46 a | 6.48 a | 7.12 a | 7.50 c | 8.29 cd | ||
| T5 | 3.27 a | 9.30 a | 11.60 a | 15.73 b | 15.50 a | 17.63 a | 22.03 a | 26.63 a | 6.52 a | 6.83 ab | 7.84 b | 8.68 bc | ||
| T6 | 3.30 a | 9.37 a | 11.67 a | 16.93 a | 15.93 a | 17.73 a | 21.67 a | 26.23 a | 6.37 a | 6.74 bc | 6.91 d | 9.52 b | ||
| T7 | 3.47 a | 9.47 a | 11.83 a | 16.2 ab | 15.93 a | 17.27 a | 21.83 a | 26.53 a | 6.22 b | 6.18 d | 8.18 a | 10.97 a | ||
For the plant growth parameters, the 50% and 95% EECV as well as the fungicide treatments gave statistically (p < 0.05) higher SPAD values, which were significantly different compared to the inoculated control but not significantly different from the healthy control (Fig. 5). Interestingly, low concentrations of 50% EECV, 95% EECV and the fungicide treatment gave significantly higher (p < 0.05) yields than that of the inoculated control. Besides, the highest concentration (50,000 ppm) of 50% and 95% EECV significantly (p < 0.05) led to the highest yields and strongest growth stimulation effects on lettuce (about 23.5 g/plant) compared to the other treatments (in the range of 16.04–19.36 g/plant).
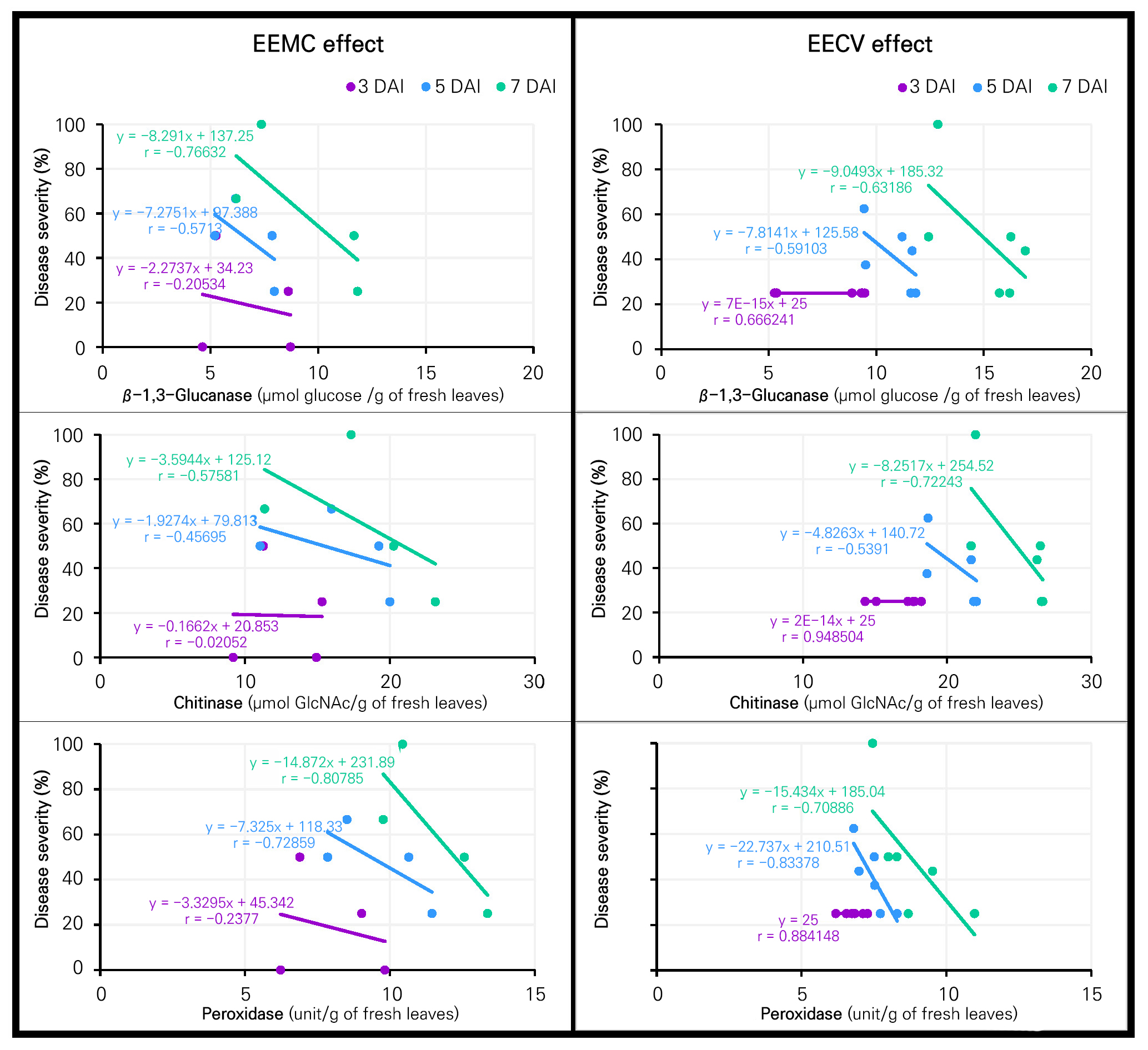
Correlation of disease severity and plant defense enzymes
The correlation between disease severity and plant defense enzymes in lettuce due to the effects of the inducers (EEMC as well as EECV) was assessed at 3, 5 and 7 DAI, as presented in Fig. 6. Moderate to high correlations (r) of –0.766, –0.575 and –0.807 were found between defense-related enzymes of β-1,3-glucanase, chitinase and peroxidase with disease severity in lettuce treated with EEMC at 7 DAI. The corresponding simple regression equation also showed that the increased levels of defense-related enzymes played a negative role in reducing the disease severity. A similar observation of a moderate to high negative correlation (r = –0.631, –0.722 and –0.708) as well as the corresponding simple regression equation outcomes were also found between defense enzymes and disease severity in the EECV lettuce at 7 DAI.
In the experiments here, EEMC and EECV significantly reduced Alternaria leaf spot disease, while significantly higher accumulations of the three enzymes were found in lettuce treated with both extracts. Furthermore, a moderate to high negative correlation was observed between disease severity and plant defense enzymes in lettuce treated with both extracts. These results were likely due to the induction of plant defense enzymes by the plant extracts (Yamunarani et al. 2004; Latha et al. 2009; Gupta et al. 2013; Franzener et al. 2018). These enzymes played a role in suppressing plant diseases, thereby contributing significantly to better overall health and resistance of the plants (Akila et al. 2011; Arzoo et al. 2012; Franzener et al. 2018; Gholamnezhad 2019). β-1,3-glucanase and chitinase sequentially hydrolyzed cell wall components, such as β-1,3-glucans and chitins (Yamunarani et al. 2004; Gupta et al. 2013; Prasannath 2017). Meanwhile, peroxidase was associated with various defense-related processes, including the hypersensitive reactions; lignin biosynthesis; and the cross-linking of phenolics, glycoproteins, and suberin as well as the production of phytoalexins in plants (Prasannath 2017). In addition, our results were in good agreement with the reports from other Myrtaceae plant extracts demonstrating that Corymbia citriodora aqueous extract reduced Fusarium wilt and increased soluble-protein-related β-1,3-glucanase, chitinase and peroxidase levels in tomatoes under greenhouse condition (Arzoo et al. 2012). While Franzener et al. (2018) reported that a water extract of Corymbia citriodora (Myrtaceae plant) reduced the disease severity of Colletotrichum lagenarium and induced 2 enzymes (peroxidase and β-1,3-glucanse) in cucumber, our findings suggested that 95% EEMC as well as 50% and 95% EECV could act as inducers by increasing plant defense enzymes in the plants. These potential plant extracts could be developed into botanical fungicides for the management of leaf spot disease in the near future.
Conclusion
Thai indigenous Myrtaceae plants were tested here as substitutes for chemical fungicides. The 95% ethanolic extracts derived from 4 Myrtaceae leaves, namely Callistemon viminalis (EECV), Melaleuca cajuputi (EEMC), Syzygium jambos (EESJ), and Syzygium malaccense (EESM) exhibited no phytotoxic effects on lettuce. Interestingly, a plant growth stimulation effect on lettuce was detected with the 95% EECV application. Notably, all concentrations (5,000, 15,000, 30,000 ppm) of 95% EEMC significantly demonstrated the highest inhibitory effect, ranging from 85% to 100% on the mycelial growth of A. brassicicola, followed by 95% EECV ranging from 40% to 63%. In the spore germination test, complete inhibition was observed with the application of 95% EEMC (5,000 to 50,000 ppm) and 95% EECV (10,000 to 50,000 ppm). Among the identified phytochemical compounds, terpenoids (such as phytol, 1,8-cineole, α-terpinene, terpinene-4-ol and 11-Keto-β-boswellic acid) were the major bioactive components of both extracts (about 37%–55%) followed by phenolics, flavonoids, ethylene glycols and vitamins. Furthermore, the applications of 95% EEMC, 95% EECV and 50% EECV as inducers reduced the severity of Alternaria leaf spot on lettuce by 50% to 75% but increased the levels of plant defense enzymes (β-1,3-glucanase, chitinase, and peroxidase). Our findings provide evidence of the potential use of EEMC and EECV for controlling Alternaria leaf spot disease in lettuce, which was likely due to their direct effect with the presence of phytochemicals responsible for antifungal activity and the plant-growth-stimulating effect as well as their indirect effects as inducer for plant defense enzymes. Therefore, these M. cajuputi and C. viminalis extracts could serve as alternative potential sources for the development of botanical fungicides in agriculture.