Introduction
Materials and Methods
Plant Materials and Skin Color Measurement
Isolation of RNA and cDNA Synthesis
Quantitative RT-PCR
Statistical Analysis
Results and Discussion
Skin Color Change
Expression Levels of Carotenoid Biosynthesis Genes
Expression Levels of Anthocyanin Regulatory and Structural Genes
Introduction
In apples (Malusdomestica), fruit skin color is an important trait that influences consumer preference and market acceptance (Saure, 1990). Apple skin color is determined by the presence and concentration of various compounds, including chlorophyll, carotenoids, and anthocyanins (Lancaster and Dougall, 1992). As fruits mature, chlorophyll is degraded, while carotenoids and anthocyanins accumulate in their skin (Kim et al., 2018). Major carotenoid biosynthesis genes related to the coloration of ripe apple skin include ζ-carotene isomerase (ZISO), carotene isomerase (CRTISO), and epsilon lycopene cyclase (LCY-ε) (Ampomah-Dwamena et al., 2012). Anthocyanin is synthesized through the flavonoid pathway, which includes both regulatory and structural genes (Rehman et al., 2017). Structural genes can be distinguished as early biosynthetic genes (EBGs) and late biosynthetic genes (LBGs). EBGs include chalcone synthase (CHS), chalcone isomerase (CHI), and flavonoid 3’-hydroxylase (F3’H), and LBGs include dihydroflavonol reductase (DFR) and UDP-glucose: flavonoid 3-O-glucosyltransferase (UFGT) (Rehman et al., 2018). These genes are regulated by the MBW complex and transported by glutathione S-transferase (GST), leading to anthocyanin accumulation in the vacuoles (Petroni and Tonelli, 2011). Five main anthocyanins contribute to skin coloration in red apples and cyanidin 3-galactoside (cy 3-gal) accounts for 80% of the total anthocyanin (Treutter, 2001; Tsao et al., 2003; Ben-Yehudah et al., 2005).
The ‘Fuji’ apple cultivar, a cross between the ‘Ralls Janet’ and ‘Red Delicious’ varieties, is valued for its high quality and visual appearance and is widely grown (Yoshida et al., 1995; Yoo et al., 2018). ‘Fuji’ apple is categorized into the striped group (Masseron et al., 1995; Iglesias et al., 2012). ‘Fuji’ apple skin differs from other varieties due to the presence of randomly distributed red and green areas. The coloring is usually yellow-green with red highlights, but can sometimes have either a pinkish blush or be nearly all red. Depending on the strain, the ‘Fuji’ apple skin is either striped or blush over a yellow-green background (Marquina et al., 2004).
Late-season harvests frequently result in poor skin coloration of the ‘Fuji’ cultivar, which is associated with decreased light availability (Jakopic et al., 2007). Interventions, such as fruit bagging, using reflective film, and chemical treatments for improving the red skin color, are labor-intensive and not economically justifiable (Ju et al., 1999;Lim et al., 2019). The best solution for preventing the lack of skin color in apples grown late in the season is to grow ‘Fuji’ apple strains that are less affected by decreasing light. There are several ‘Fuji’ cultivars (bud sports) with enhanced red coloration compared to the standard ‘Fuji’ cultivar (Parker and Guerra, 2008; Iglesias et al., 2012; Olivares-Soto et al., 2020). For example, the ‘Mishima’ cultivar, identified in 2001, shows a significant improvement in fruit skin color at harvest. However, little is known about the molecular mechanisms underlying the differences in pigment accumulation in apple skin (Stanger et al., 2017).
Here, we characterized the skin of ‘Fuji’ and ‘Mishima’ apples at five fruit development stages. We compared the expression patterns of three carotenoid biosynthesis genes and eight anthocyanin biosynthesis genes to determine which pigment biosynthesis genes induce the superior skin color in ‘Mishima’ compared to its original cultivar, ‘Fuji’.
Materials and Methods
Plant Materials and Skin Color Measurement
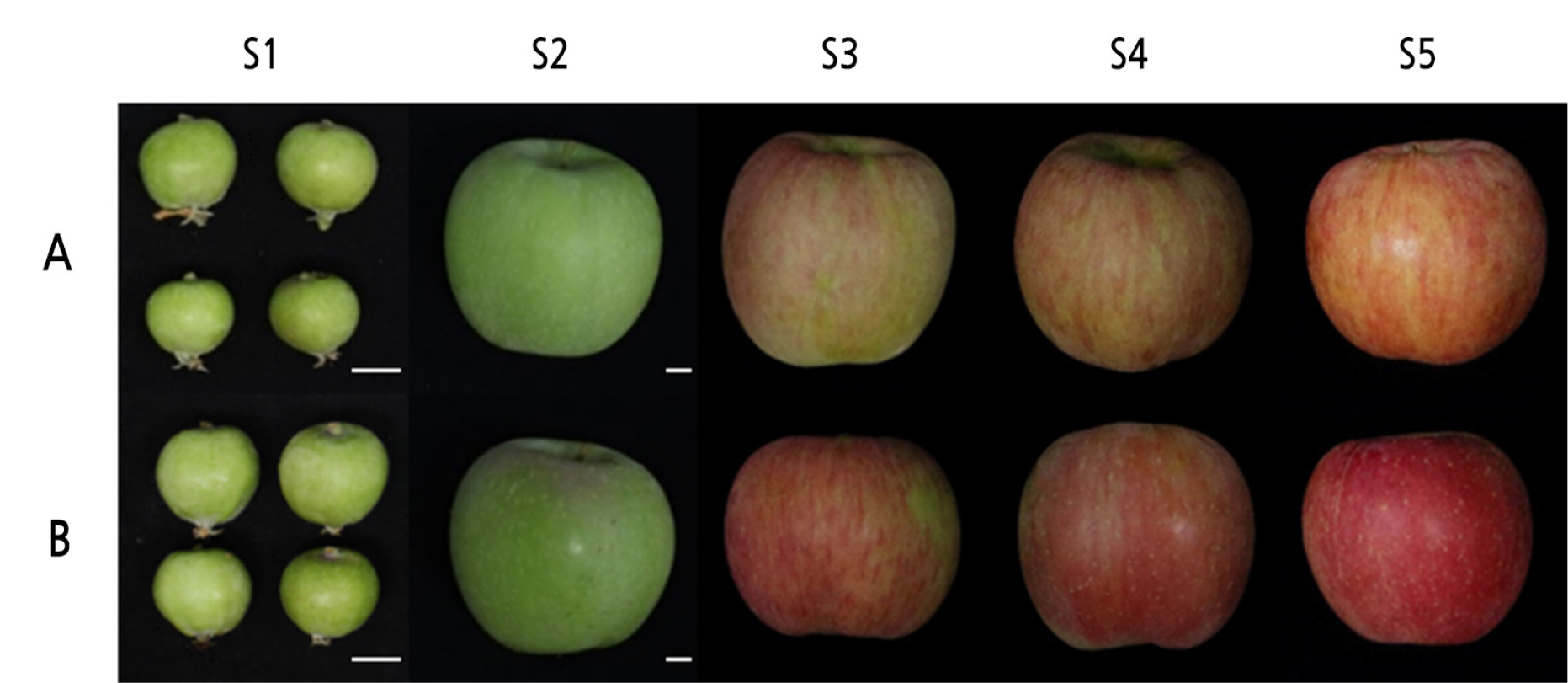
‘Fuji’ and ‘Mishima’ apples (Malusdomestica) were collected at the Apple Research Institute in Gunwi, Korea (36°16' N, 128°28' E), and full bloom was recorded as April 28, 2016. For each cultivar, a total of nine fruit were randomly harvested from three trees at each of five fruit development stages: stage 1 (S1: young fruit stage at 23 days after full bloom (DAFB)), stage 2 (S2: 87 DAFB), stage 3 (S3: 155 DAFB), stage 4 (S4: 164 DAFB), and stage 5 (S5: 177 DAFB) (Fig. 1). After sampling, the skin color of the fruit was immediately analyzed using a CR-400/410 colorimeter (KONICA MINOLTA, Japan) after calibration with a white calibration plate. The chromaticity was recorded in CIELAB and color was expressed as three values: L* for the relative lightness with a range from 0 (black) to 100 (white); a* from green (-) to red (+); and b* from blue (-) to yellow (+), extending from -60 to 60. The ratio of a* and b* values was then used to calculate hue (a*/b*). For each fruit, the mean color measurements were calculated from three points taken from the fruit’s equator. After measuring, the peel (less than 1 mm in thickness) was carefully removed, frozen in liquid nitrogen, and stored at -80°C until use.
Isolation of RNA and cDNA Synthesis
Apple peels were finely ground using a 6870 Freezer/Mill (SPEX SamplePrep, USA) with the continuous addition of liquid nitrogen to maintain -80°C. Total RNA was isolated using the modified CTAB method as described (Chang et al., 1993), and cDNA was immediately synthesized using a QuantiTect Reverse Transcription Kit (QIAGEN, Germany). The first reaction to eliminate genomic DNA was at 42°C for 10 min and 4°C for 5 min. The second reaction to reverse transcribe RNA by polymerase chain reaction (PCR) was at 42°C for 20 min and 95°C for 3 min. The synthesized cDNA was stored at -20°C and used subsequently for quantitative real-time PCR. The concentration (µg·µL-1) and quality (A260/A280 ratio) were tested using a PLUS Spectrophotometer (Bio-Rad, USA). RNA degradation and contamination were assessed on a 0.8% agarose gel.
Quantitative RT-PCR
Quantitative real-time PCR was performed using 2× qPCRBIO SyGreen Blue Mix Lo-ROX (BioD, Korea). Primers for carotenoid and anthocyanin biosynthesis genes were reported previously and used for qRT-PCR in this study (Ampomah-Dwamena et al., 2012; El-Sharkawy et al., 2015). They are listed in Table 1. Reactions were performed in triplicates on a Rotor-Gene Q (QIAGEN, Germany) at 95°C for 2 min followed by 40 cycles of 95°C for 10 s, 60°C for 15 s, and 72°C for 20 s. The transcript levels were measured, and relative quantification was performed using the cycle threshold (Ct) 2-△△Ct method. MdAct and MdACT amplicons served as the endogenous controls for normalizing expression between samples.
Table 1.
Primers used for quantitative real-time PCR
Statistical Analysis
Statistical analysis was performed using the Student’s t-test method and IBM SPSS Statistics 23 (p < 0.05 and 0.01).
Results and Discussion
Skin Color Change
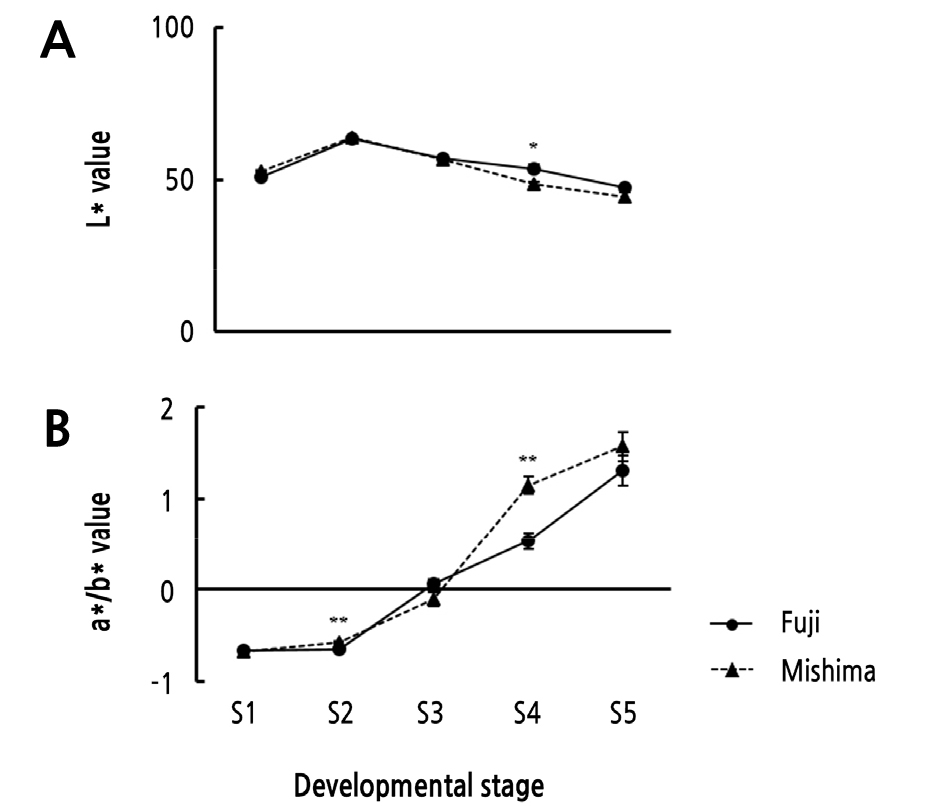
Although ‘Fuji’ and its bud sport cultivar, ‘Mishima’, have similar genetic backgrounds, they show notable differences in the percent and intensity of red color in their fruit skins (Parker and Guerra, 2008). The chromaticity values of ‘Fuji’ and ‘Mishima’ fruits are shown in Fig. 2. The chromaticity values of the two varieties showed a similar pattern of overall change during fruit ripening, as expected because they are both red-fruit cultivars. As the fruit skin color progressively changed at five DAFB, the value of L* increased up to S2 and then decreased, while the a*/b* ratio continued to increase. The two values remained similar in both ‘Fuji’ and ‘Mishima’ fruit skins until S2, but there were differences after S3. The S3 ‘Mishima’ fruit showed a lower L* value and a higher a*/b* value than S3 ‘Fuji’ fruit, and the differences were greatest at S4. Our results show that the a*/b* ratio corresponded with the intense red pigmentation in the skin of ‘Mishima’ apples and can be used to estimate the anthocyanin concentrations (Singha et al., 1991).
Expression Levels of Carotenoid Biosynthesis Genes
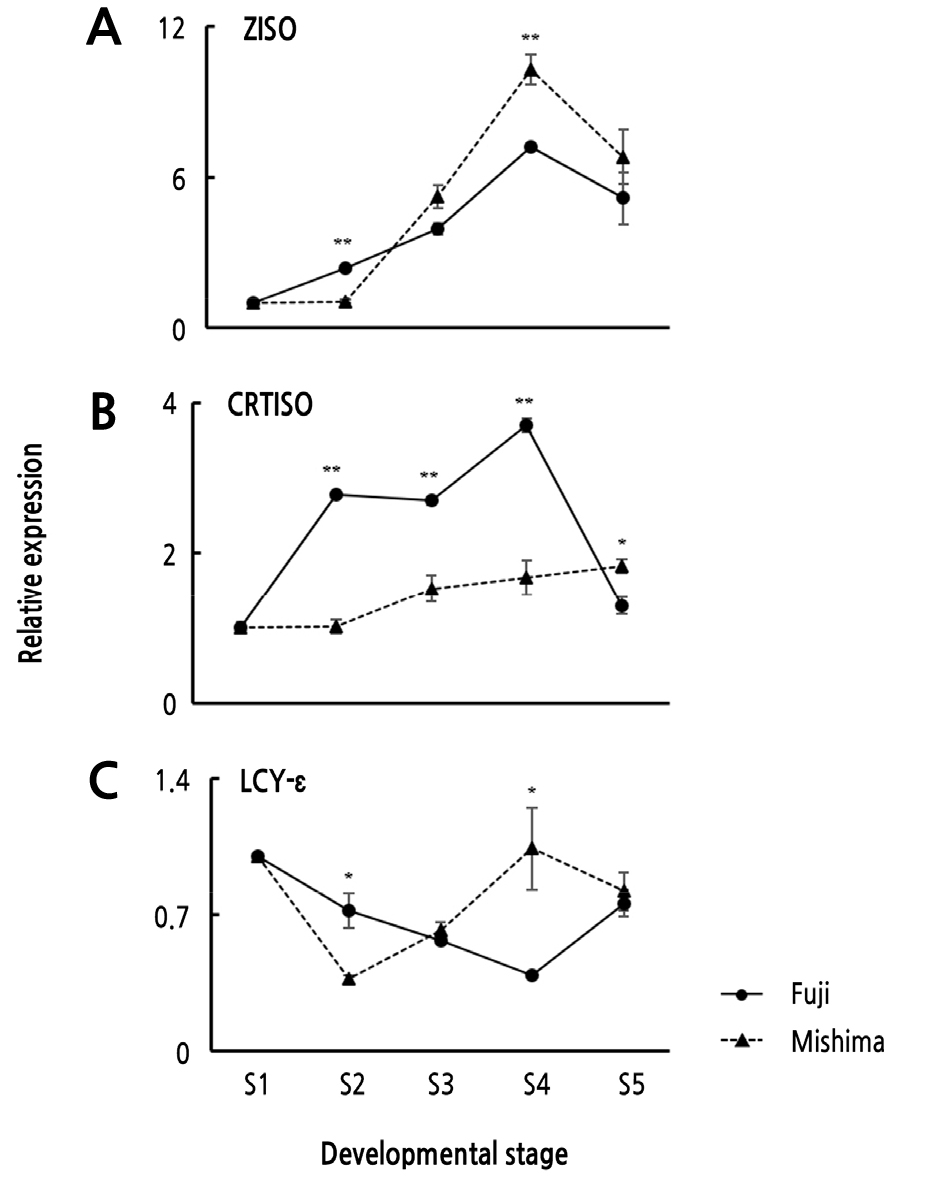
RNA was isolated from the fruit skin samples of the two cultivars at five developmental stages and cDNA was synthesized immediately for quantitative real-time PCR. The ‘Fuji’ and ‘Mishima’ apples are categorized in the striped pattern group, so their fruit skin coloration is determined by a blend of carotenoids and anthocyanins (Kim et al., 2003). We analyzed the expression of carotenoid biosynthesis genes, including two genes encoding isomerase enzymes, MdZISO and MdCRTISO, and MdLCY-ε, which is known to have a positive influence on apple skin color at the ripe stage, and evaluated their expression relative to skin color (Ampomah-Dwamena et al., 2012). In addition, we compared the expression of the carotenoid biosynthesis genes in ‘Mishima’ to the expression levels in ‘Fuji’ (Fig. 3).
The expression levels of MdZISO, MdCRTISO, and MdLCY-ε gradually increased with ripening in both apple cultivars, but a higher level of MdZISO and MdLCY-ε expression was observed in the ‘Mishima’ fruit skin than in ‘Fuji’ fruit skin. The expression levels of MdZISO and MdLCY-ε peaked in the S4 fruit. The relative expression level of MdZISO was 10.30 in ‘Mishima’ and 7.22 in ‘Fuji’, with the mean level in ‘Mishima’ about 1.43 times greater than that in ‘Fuji’. A similar pattern was observed for MdLCY-ε at S4. The relative expression level of MdLCY-ε was 1.04 and 0.39 in ‘Fuji’ and ‘Mishima’, respectively, and the mean level in ‘Mishima’ was about 2.67 times greater than that in ‘Fuji’. A previous study showed that accumulation of lutein, which is responsible for yellow coloration, is controlled by MdLCY-ε, which is expressed at higher levels in fruit skin than in the flesh in apple (Delgado-Pelayo et al., 2014). These two genes were expressed at significantly higher levels in S4 fruit and likely correlate with the lower L* value of the CIELAB data. Taken together, our data suggest that mature ‘Mishima’ fruit skins accumulate more carotenoids than ‘Fuji’ fruit skins.
Expression Levels of Anthocyanin Regulatory and Structural Genes
Genes related to the anthocyanin pathway in apples have been well-characterized (Kim et al., 2003). To explore which genes are associated with the improved red skin color in ‘Mishima’ fruit, we investigated the expression levels of three regulatory genes (MdMYB10, MdWD40, and MdGST) and five structural genes (MdCHS, MdCHI, MdF3’H, MdDFR, and MdUFGT) in ‘Fuji’ and ‘Mishima’ fruit (Fig. 4).
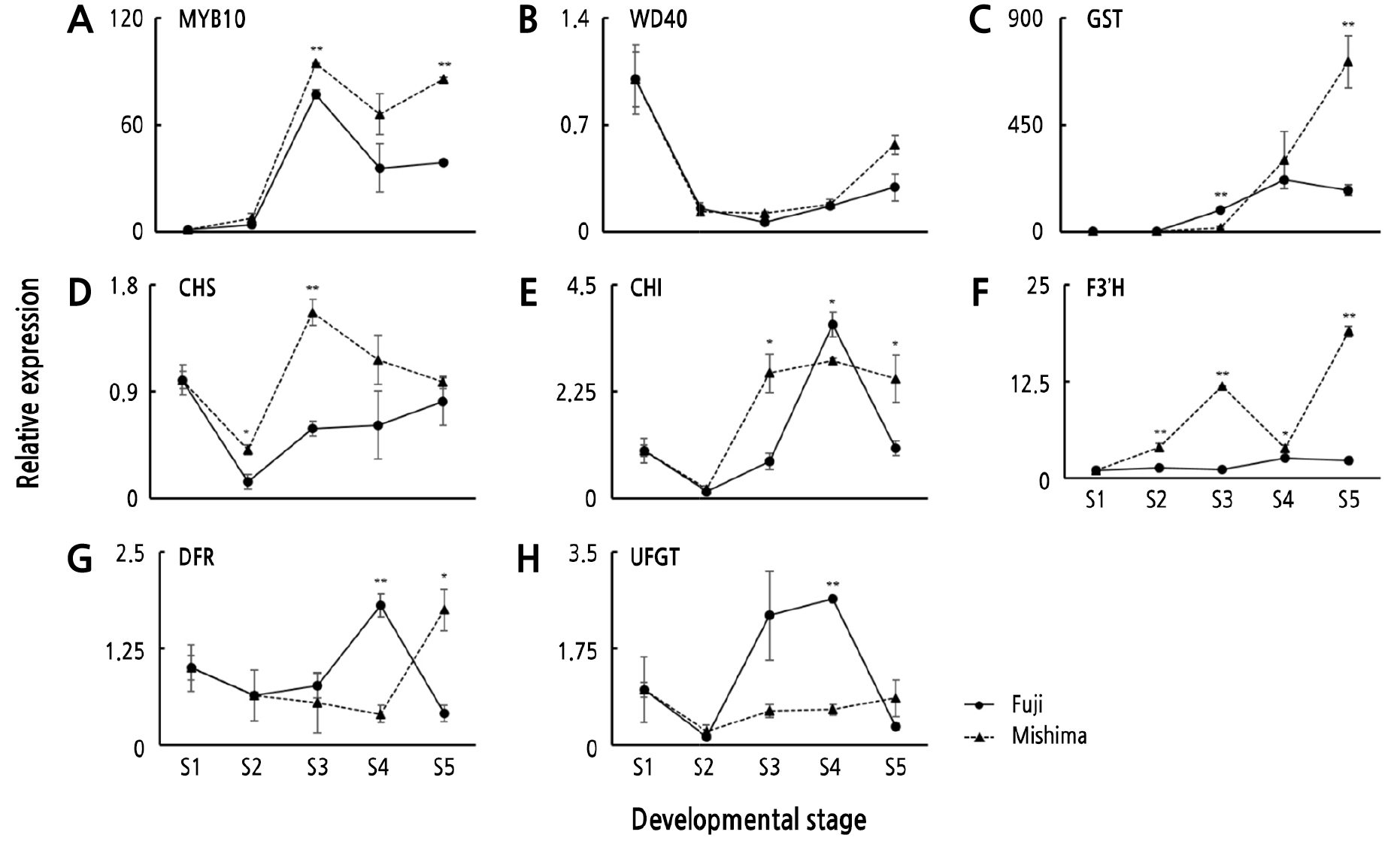
Fig. 4.
Expression patterns of eight anthocyanin biosynthesis genes (A) MdMYB10, (B) MdWD40, (C) MdGST, (D) MdCHS, (E) MdCHI, (F) MdF3’H, (G) MdDFR, and (H) MdUFGT in the skins of ‘Fuji’ and ‘Mishima’ fruit at the five fruit development stages (S1-S5). Error bars represent mean ± SE of triplicates. * and ** indicate p < 0.05 and 0.01, respectively.
Anthocyanin accumulation in apples has two peaks, the first peak occurs at the young fruit stage and the second peak occurs at the ripening fruit stage because fruitlets younger than 30 DAFB have partially red skin contrary to other stage fruit (Meng et al., 2016). Most anthocyanin biosynthesis genes were upregulated in both cultivars, which was expected because they are red-colored apple cultivars. Transcripts of the two transcription factor genes MdMYB10 and MdWD40, whose products form the MBW complex were increased, and the MdGST transport gene was expressed 4 times higher in ‘Mishima’ apple skins (716.97) than in ‘Fuji’ (174.20) especially in S5. A previous study also reported a markedly rapid increase of MdMYB10 and MdGST transcript levels in the blushed-pattern mutant of ‘Fuji’ apple (Cho et al., 2020). The high expression of MdMYB10 and MdGST has a positive effect on the overall anthocyanin biosynthesis process, including on the expression of anthocyanin structural genes (Petroni and Tonelli, 2011). Similar expression patterns were also observed in structural genes. The expression levels of early anthocyanin biosynthesis genes, such as MdCHS, MdCHI, and MdF3’H, were continuously upregulated in S1 to S5 fruit in ‘Mishima’ relative to ‘Fuji’, but MdDFR and MdUFGT were only upregulated in S5 fruit skins. In particular, the expression level of MdF3’H was 8 times higher in the S5 fruit skins of ‘Mishima’ (19.01) relative to ‘Fuji’ (2.27). Similarly, upregulated expression of early biosynthesis genes, like MdANS and MdF3H, was important in apple mutants having deep-red-skinned fruit (Jiang et al., 2019). The CIELAB data showed that ‘Mishima’ fruit color intensified from S4 to S5, while the L* value decreased and the a*/b* value increased significantly. A significant correlation between the a*/b* ratio and anthocyanin biosynthesis gene expression at the ripening stages suggests that anthocyanin biosynthesis genes are upregulated in intense-red-skinned apples. These results show that expression levels of anthocyanin biosynthesis genes are closely related to anthocyanin accumulation in red apple cultivars (El-Sharkawy et al., 2015). Anthocyanins accumulate in ‘Mishima’ apple skins in stripes, or uniformly to produce an intense red color (Marquina et al., 2004). In this study, genes in the anthocyanin biosynthesis pathway were highly expressed in a fruit color-dependent manner in the ‘Mishima’ cultivar, indicating that anthocyanin biosynthesis genes are involved in regulating red fruit color in apples. These results add to our understanding of the molecular basis of the enhanced skin color of ‘Fuji’ bud sport apple varieties.