Introduction
Materials and Methods
Plant Materials
Color Difference
Petal Pigments
Scent Compounds in Petals
Correlation between Pigments and Scent Compounds
Results
Changes in Morphological Traits with Floral Bud Development
Changes in the Main Pigments and Scent Compounds of Petals during Floral Bud Development
Correlation between Pigments and Scent Compounds in Petals during Floral Bud Development
Discussion
Changes in Pigments and Scent Compounds during Floral Bud Development
Correlation between Pigments and Scent Compounds in Petals
Introduction
Flowers, with polymorphic traits such as form, size, color, scent, and flowering phenology, are the reproductive organs that attract pollinators for fertilization in many plants (van Schie et al., 2006; Delle-Vedove et al., 2011). Flower development is a continuous process and interacts with biotic or abiotic factors such as phytohormones, nutrient state (sucrose content), light, and temperature (O’Neill, 1997; Blázquez, 2000). It has been suggested that flower development is regulated or accelerated by pollination as a biotic factor and results in petal perianth, changes in pigmentation level, and ovary maturation during flowering (O’Neill, 1997). The colored and scented flowers have evolved by the selection of effective animal pollinators that show preferences, learning capabilities, and sensory flexibility for specific colors and scents (Delle-Vedove et al., 2011). The development of pollinated flowers has ecological importance and affects flower quality in ways that change their economic value in horticulture (O’Neill, 1997). The development of floral buds occurs in two steps: cell division and expansion (Weiss, 2000). The flowers of most plants have green petals that result from their chlorophyll content at the cell division stage (Weiss and Halevy, 1989; Weiss, 2000). Petal pigmentation starts at the cell expansion stage (Weiss and Halevy, 1989; Weiss, 2000). The production and accumulation of anthocyanins are indispensable in most flowers and are directly controlled by gibberellins (GAs) that promote petal growth and anthocyanin pigmentation (Weiss, 2000; Kumar et al., 2008). Changes in the pigmentation of petals during floral bud development have been reported in some flowers. The total amount of anthocyanins increases rapidly or gradually until flowers reach the fully open petal stage (Farzad et al., 2002; Dela et al., 2003). Yellow, orange, and red flowers develop carotenoids and become bright, while chlorophyll levels decrease during flowering (Zhu et al., 2002).
Scent compounds have different emission patterns from petals as the floral buds develop. In petunia flowers, some scent compounds, such as benzaldehyde, benzyl alcohol, and isoeugenol, increased, whereas phenylacetaldehyde stayed about the same throughout floral bud development (Zvi et al., 2008). In contrast, p-cresol in petunias decreased with floral bud development (Zvi et al., 2008). Therefore, the composition of scent compounds could change at different flower stages (Shalit et al., 2003; Berougnoux et al., 2007; Sun et al., 2019). Flower senescence, a phenomenon that occurs during flowering, could be led by pollination, which results in the increased production of endogenous ethylene causing the petal color to fade (O’Neill, 1997). Eventually, floral bud development causes a change in both pigments and scent compounds in the petals of many plants (O’Neill, 1997; Zvi et al., 2008).
Roses are important horticultural crops that are highly valued for their ornamental traits, such as flower size and shape, petal color, scent, and recurrent flowering (Bergougnoux et al., 2007; Schulz et al., 2016; Ha et al., 2017). Flower color and scent are the primary ornamental factors expressed mostly in petals, and these qualities make them more appealing to customers (Guterman et al., 2002; Pichersky and Dudareva, 2007; Yue and Behe, 2010; Kanani and Nazarideljou, 2017; An et al., 2018; Lee and Lee, 2018; Fu et al., 2019; Maiti and Mitra, 2019). The amounts of pigments and scent compounds, which are responsible for flower color and fragrance in roses, are floral characteristics with ornamental value (Schulz et al., 2016). Pollination causes changes in the color and scent bouquet as floral buds develop toward flower senescence, which can decrease the value of the flowers (O’Neill, 1997; Dudareva et al., 2000). Some studies have suggested a correlation between pigments and scent compounds that share specific biosynthetic pathways, which express flower color and scent (Delle-Vedove et al., 2011; Dormont et al., 2014). The exact relationship between pigments and scent compounds is complicated because flower color and scent have evolved under the influence of many biotic and abiotic factors (Arista et al., 2013; Chen et al., 2014). To understand the correlation between color and scent of cut roses during synthesis, we investigated the changes in the composition of pigments and scent compounds throughout floral bud development as a biotic factor.
Materials and Methods
Plant Materials
Rosa hybrida ‘Penny Lane’ and ‘Vital’, which were cultivated in commercial greenhouses and traded in domestic flower markets, have bright yellow and red flowers, respectively, without a faded margin on their petals. Two cultivars were used to analyze changes in the composition of pigments and scent compounds throughout floral bud development (Fig. 2), which was divided into five stages with distinct morphological traits of the petals and sepals such as bud diameter, angle of unfolding petals for the peduncle of flowers, petal color (Table 1), and state of bloomed flowers (Fig. 1). We carried out the investigation of morphological traits with six flowers and duplicated the composition analysis of pigments and scent compounds in petals with ten flowers of each cultivar. The roses were grown in an experimental plastic greenhouse at the University of Seoul by a hydroponic system with rockwool cubes (75 × 75 × 65 mm) (KB750, UR Media, Seoul, Korea) and slabs (75 × 200 × 1,000 mm) (V75201, UR Media, Seoul, Korea) from December 2018 to March 2019. Cut roses were irrigated with 380 mL nutrient solution (developed by the University of Seoul) per plant daily 13 times from 8:00 a.m. and managed by electronic conductivity at 1.2-1.4 dS·m-1 and pH 5.7-5.9. ‘Penny Lane’ and ‘Vital’ were exposed to solar irradiation and high-pressure sodium light (217 ± 379.7 µmol·m-2·s-1) for 22 hours per day. Day and night temperatures in the greenhouse were 17 ± 7.3 and 12 ± 3.0°C, respectively, and RH was 36 ± 8.3% (Watchdog-1450, Spectrum Technologies, England).

Fig. 1.
Morphological characteristics of the petals and sepals in five stages of floral bud development of R. hybrida ‘Penny Lane’ (yellow) and ‘Vital’ (red). S1: A and F, floral bud is folded and pigmentation in petals starts; S2: B and G, petals are visible between sepals but not open yet; S3: C and H, petals begin to open and sepals detach from petals; S4: D and I, outer petals are open and sepals are fully detached from petals; S5: E and J, all petals are open and stamens are visible. Scale bars indicate 10 mm.
Table 1.
Changes in the bud size, unfolding petals, and color of R. hybrida 'Penny Lane' and 'Vital' during floral bud development
zColor values measured in the CIE 1976 L* a* b* color space. L*: lightness ranged from 0 to 100; a*: positive value indicates red and negative value indicates green; b*: positive value indicates yellow and negative value indicates blue (a* and b* range from -100 to 100); C*: the hypotenuse of a right triangle formed by joining points (0,0), (a*, b*), and (a*, 0); h*: the angle between the hypotenuse and 0° on the a* axis.
Color Difference
To quantify the color of each cultivar, the surface of the sixth to seventh fresh petal was measured on the adaxial side using a colorimeter (CR-10 plus, Konica Minolta, Japan). The petal color was measured in the CIE L*a*b* color space: L*, lightness of the color within the range from 0 (black) to 100 (white); a*, red (positive value) and green (negative value); b*, yellow (positive value) and blue (negative value); C*, chroma of color (higher values indicate the more luminosity); h*, hue angle of the color within the range from 0-360 (Gitonga et al., 2016). Data were analyzed using Duncan’s multiple range test (DMRT) and analysis of variance (ANOVA) in SAS 9.4 (SAS Institute, Cray, NC, USA).
Petal Pigments
The preconditioning process of the anthocyanin analysis was based on that by Longo and Vasapollo (2005) and Lee and Kim (2015), with modifications. First, 1.0 g (fresh weight, FW) of petals from each of the five developmental stages of the two cultivars was harvested and ground into powder using liquid nitrogen. Powder samples were immersed in 20 mL of 0.1% HCl (v/v) in methanol for 22 hours at room temperature with darkness for extracting the anthocyanins. After centrifuging at 5,000 rpm for 30 min at 23°C, 5 mL supernatant was evaporated with a rotary evaporator at 31°C, and then the remaining fraction was dissolved in 1.5 mL 0.01% HCl (v/v) in methanol. Water-soluble compounds containing anthocyanins were loaded in a C-18 Sep-Pak cartridge (20cc, 6g) (Waters, Milford, MA, USA) activated with 80 mL of 100% methanol and 80 mL of 0.01% aqueous HCl. After adding 15 mL of 0.01% aqueous HCl to the cartridge to get rid of impurities, anthocyanins were then eluted with 10 mL of 0.01% HCl (v/v) in methanol. The acidified methanol was evaporated at 31°C and then the dissolved anthocyanins in 0.01% HCl (v/v) in methanol were filtered through a syringe filter (0.2 µm, PET filter, 15 mm diameter).
The anthocyanin composition was analyzed by high-performance liquid chromatography (HPLC) (LC-20AD, Shimadzu, Japan) using a C-18 column (4.6 × 250 mm, 5 µm) (Zorbax SB-C18; Agilent Technologies, Santa Clara, CA, USA). The 0.1% aqueous trifluoroacetic acid (TFA, solvent A) and 0.1% TFA in acetonitrile (solvent B) were mobile phases at a 1.0 mL·min-1 flow rate. The gradient of the mobile phase was as follows: 0-20 min: 10% B; 30 min: 20% B; 50 min: 30% B. The wavelength was set at 520 nm, and the injection volume was 10 µL. Cyanin, peonin, and pelargonin (Sigma, USA) were used to quantify the anthocyanins.
The preconditioning process of the carotenoid analysis was based on that by Kim et al. (2015) with modifications. First, 1.0 g (FW) of petals from each of the five developmental levels of the two cultivars was harvested and ground into powder using liquid nitrogen. Then, the powder sample with 5 mL of ethanol activated in a water bath at 75°C for 5 min. After adding 1.5 mL of 80% KOH to the sample, it was again heated at 75°C for 10 min in a water bath. Next, the sample was cooled in ice for 5 min, and then 2.5 mL of distilled water and 2.5 mL of hexane were added to the sample for centrifuging at 3,000 rpm for 30 min at 23°C. Five milliliters of supernatant containing carotenoids was collected by repeating the process of adding distilled water and hexane three times. The solvent-containing carotenoids were evaporated by a rotary evaporator at 40°C and then the carotenoids were dissolved in 1.5 mL of dichloromethane:methanol (v:v = 50:50). The sample was filtered with a syringe (0.45 µm, PVDF filter, 13 mm diameter) to extract carotenoids.
The carotenoid composition was analyzed by HPLC (LC-20AD, Shimadzu, Japan) using a C-30 column (4.6 × 250 mm, 3 µm) (YMC Carotenoid C30; YMC, Wilmington, PA, USA). The 92% methanol (v/v) in water containing 10 mM ammonium acetate (solvent A) and 100% methyl tert-butyl ether (Solvent B) were mobile phases at a 0.7 mL·min-1 flow rate. The gradient of the mobile phase was as follows: 0-22 min: 17% B; 23 min: 30% B; 29 min: 41% B; 35 min: 70% B; 40 min: 70% B; 44 min: 17% B; 55 min: 17% B. The wavelength was set at 450 nm, and the injection volume was 10 µL. Lycopene, α-carotene, and β-carotene (Sigma, USA) were used to quantify the carotenoids.
Scent Compounds in Petals
Petals from R. hybrida ‘Penny Lane’ and ‘Vital’ at each of the five developmental stages were immersed for 24 hours in 2 mL of hexane per gram (FW) of petals at room temperature with no light to extract the scent compounds (Bergougnoux et al., 2007).
The composition of the scent compounds was detected by gas chromatography (GC2010plus, Shimadzu, Japan) with a flame ionization detector and a wax column, 30 m × 0.32 mm inner diameter, 0.25-µm film thickness (Stabilwax; Restek, Bellafonte, PA, USA). The column condition was programmed for 3 min at 100°C, with an increase of 5°C min-1 to 220°C and a 10-min hold. Helium (99.999% purity) was used as the carrier gas at a flow rate of 1.0 mL·min-1 in linear mode. The sample was injected in split mode with a 5:1 ratio at 280°C. The detector was set at 280°C. To quantify scent compounds of the five developmental stages of the two cultivars, 13 standards (Sigma, USA) as the main scent compounds in roses (Baldermann et al., 2009) were analyzed (phenolics; 2-phenylethanol, 3,5-dimethoxytoluene, eugenol, and methyl eugenol; terpenes: β-citronellol, β-ionone, geranial, geraniol, linalool, neral, nerol, (‑)rose oxide, and trans-caryophyllene).
Correlation between Pigments and Scent Compounds
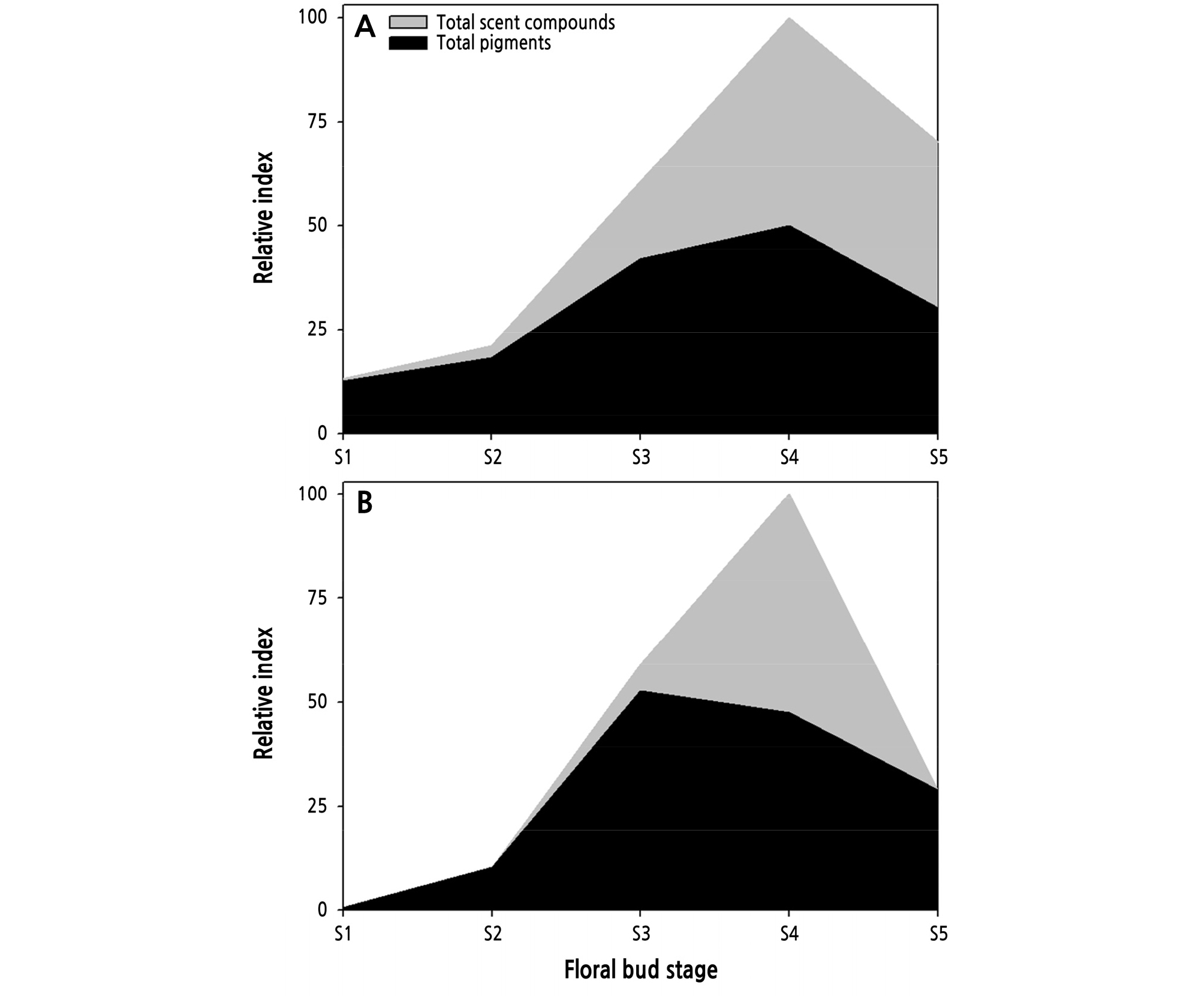
A correlation analysis in SAS 9.4 (SAS Institute) suggested the relationship between the pigments and scent compounds in the petals of cut roses. The relative index of pigments and scent compounds was calculated for the maximum level of total pigment content (‘Penny Lane’ at S4 but ‘Vital’ at S3) and total scent compounds (at S4) across the five stages of floral bud development, which was converted into a percentage, to analyze the concurrent changes of constituents level in each cultivar during flowering.
Results
Changes in Morphological Traits with Floral Bud Development
The morphological traits of the R. hybrida ‘Penny Lane’ and ‘Vital’ floral buds changed in similar ways during flowering. As they developed, their diameters increased to 10 times bigger at S5 than they had been at S1 as the unfolded petals became horizontal (Fig. 1). The petals and sepals of ‘Penny Lane’ and ‘Vital’ were closed at S1 and S2 and then opened gradually from S3 to S5. The petals of both cultivars were open to 20° at S3, 55-60° at S4, and 100-120° at S5 (Table 1). The fully open petals and sepal showing a visible stamen at S5 indicated the senescent stage of rose flowers (Bergougnoux et al., 2007; Feng et al., 2014). Changes in the petal color of ‘Penny Lane’ and ‘Vital’ were apparent to the naked eye during flowering. ‘Penny Lane’ got brighter, with the a* (red), b* (yellow), and C* (chroma) values increasing from S1 to S4 (Table 1). A decrease in the L* (lightness) value and increase in the a* and C* values contributed to the vivid red color of ‘Vital’ that developed form S1 to S4 (Table 1). However, ‘Vital’ lost its red color at S5 through an increase in the L* value.
Changes in the Main Pigments and Scent Compounds of Petals during Floral Bud Development
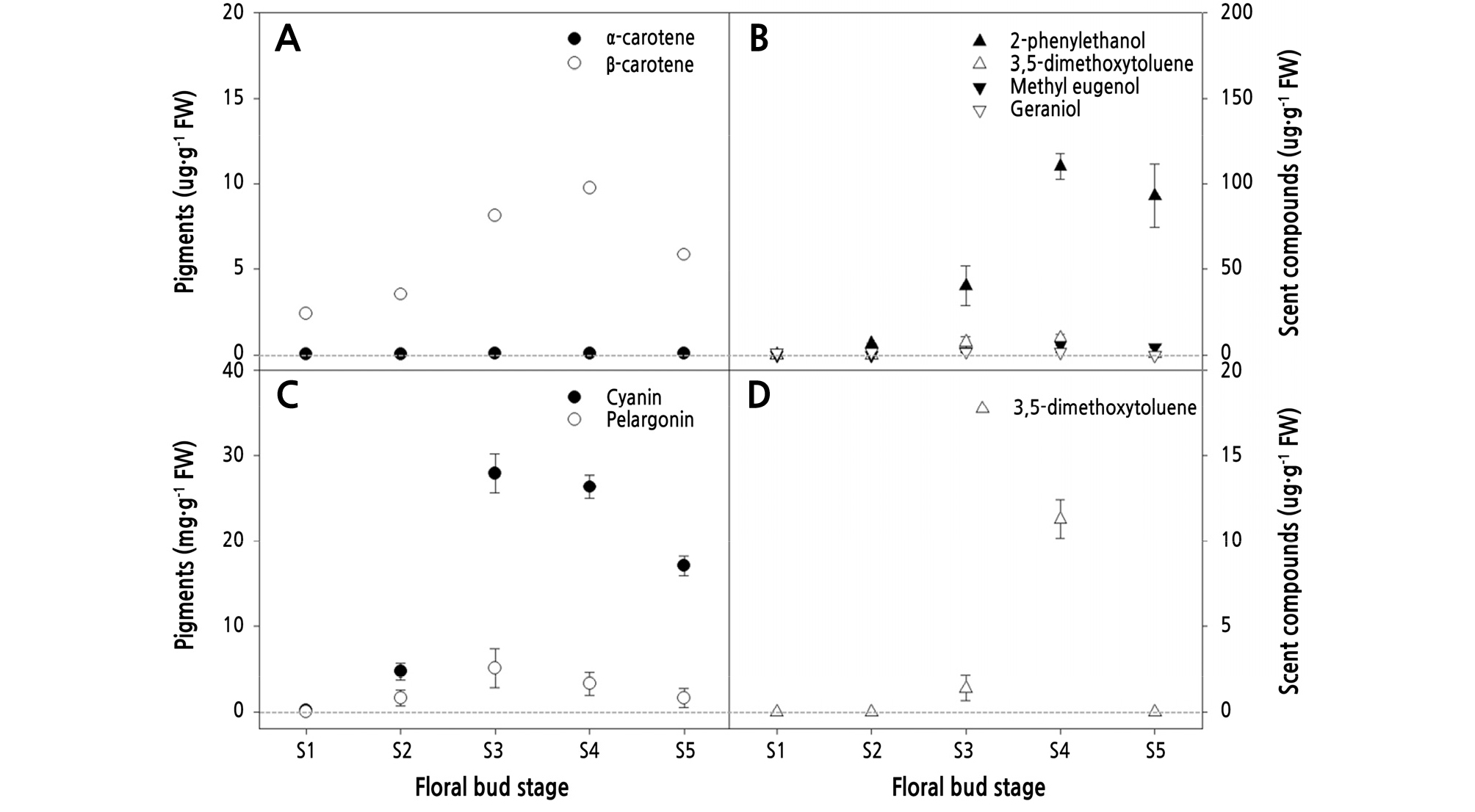
Pigment contents that affect petal colors (Zhao and Tao, 2015) varied with floral bud development in R. hybrida ‘Penny Lane’ and ‘Vital’ (Fig. 2). In ‘Penny Lane’, carotenoids increased significantly to 9.83 µg·g-1FW at S4 and then decreased by 35% at S5 (Fig. 2A). ‘Penny Lane’ did not contain anthocyanins, but total flavonoids were detected at all stages (data not shown). Similar to the change in carotenoid content, the total flavonoid content of ‘Penny Lane’ tripled at S3 compared with the earlier stages, but then the level stayed consistent from S3 to S5 without any reduction. Throughout floral bud development, β-carotene in ‘Penny Lane’ predominated over α-carotene, which appeared in tiny amounts of less than 0.1 µg·g-1FW in all stages (Fig. 2A). In ‘Vital’, anthocyanins increased by 24 times from S1 to S3 and then decreased by more than 43% at S5 (Fig. 2C), which resulted in the purplish-red petals with a higher L* value and lower b* and h* values instead of bright reddish-orange ones (Table 1). Cyanin and pelargonin in ‘Vital’ changed concurrently with floral bud development in similar ways (Fig. 2C). The rate of increase for β-carotene in ‘Penny Lane’ and cyanin in ‘Vital’ was the highest, 230% and 590%, respectively, from S2 to S3.
The petals of ‘Penny Lane’ and ‘Vital’ contained just 4 of the 13 types of scent compounds with 2-phenylethanol, 3,5-dimethoxytoluene, methyl eugenol, and geraniol. All of the scent compounds except geraniol in ‘Penny Lane’ and ‘Vital’ increased during floral bud development to S4 and then decreased by more than 35% at S5, which is similar to the pattern for pigment changes (Fig. 2). However, the rate changes differed for each scent compound during flowering. The compound 2-phenylethanol was first detected at S1, but 3,5-dimethoxytoluene and methyl eugenol emerged only at S3 in ‘Penny Lane’ (Fig. 2B). Geraniol in ‘Penny Lane’ showed a uniform level of 1.12-1.85 µg·g-1FW to S4 and was absent at S5. In ‘Vital’, 3,5-dimethoxytoluene was also first detected at S3 (Fig. 2D). The change point for variation in most of the scent compounds in ‘Penny Lane’ and ‘Vital’ differed with floral bud development. It was found that 2-phenylethanol, 3,5-dimethoxytoluene, and methyl eugenol increased from 20-60° (S3 to S4) as the petals unfolded and then decreased after S4 (Table 1), indicating that the flowers produced most of their scent compounds during petal opening (Fig. 2). Therefore, S3 and S4 were the stages of floral bud development with the highest levels, which then decreased at S5.
Correlation between Pigments and Scent Compounds in Petals during Floral Bud Development
Using the levels of total pigments and scent compounds in ‘Penny Lane’ and ‘Vital’ as a relative index, differences between the changes in color and scent across floral bud development were obvious. Pigment expression was higher and developed faster than scent compound expression in ‘Penny Lane’ and ‘Vital’ at earlier stages between S1 to S3 (Fig. 3). ‘Penny Lane’ showed a slight increase in total scent compounds at the early stages (S1 to S2) of flowering (Fig. 3A), as a result of 2-phenlyethanol production (Fig. 2B), compared to ‘Vital’ (Fig. 3B). Furthermore, the change rate for total pigments was smaller than that for the scent compounds in both ‘Penny Lane’ and ‘Vital’ during flowering. The small changes in pigments across floral bud development indicate that flower color is selected for durability, demonstrating its effect during the whole flowering period. In ‘Vital’, the rapid increase in scent compounds at S4, as the pigments are decreasing, could represent an attempt to compensate for color degradation with odor at a late stage of flowering (Fig. 3B). The correlation analysis between the pigments and scent compounds across floral bud development in ‘Penny Lane’ and ‘Vital’ showed that both the carotenoids and anthocyanins correlated positively with all scent compounds (Tables 2 and 3) because they increased and decreased together during flowering (Fig. 2). In ‘Penny Lane’, the carotenoids had stronger positive correlation with phenolics than a terpene scent compound, geraniol (Table 2).
Table 2.
Correlation between the main pigments and scent compounds in R. hybrida 'Penny Lane' during floral bud development
Discussion
Changes in Pigments and Scent Compounds during Floral Bud Development
Most flowers are composed of sepals, petals, stamen, and pistils (Martin and Gerats, 1993). When flowers mature, the petals unfold to attract pollinators with their color and scent, which are important factors for plant reproductive success (Martin and Gerats, 1993; Zvi et al., 2008; Majetic et al., 2010). Petal development is connected to pigmentation, which is a crucial factor in successful pollination (Kumar et al., 2008). GA transported from the sepals and stamen to the petals induces petal elongation and pigmentation by promoting the uptake of sugars such as sucrose, glucose, and fructose and the expression of anthocyanin-related biosynthetic genes such as chalcone synthase, flavanone 3-hydroxylase, and anthocyanidin synthase (Weiss and Halevy, 1989; Weiss, 2000; Kumar et al., 2008; Ravid et al., 2017). Flower colors get more intense or darker as the amount of pigment increases (Miller et al., 2011). The anthocyanin concentration controls the color variation in petals (Zhao and Tao, 2015). In Viola cornuta, the total anthocyanin level increases by 200 times at the fully open stage compared with earlier stages, which have white petals with no anthocyanins (Farzad et al., 2002). Anthocyanin accumulation during flower opening has also been reported in the R. hybrida ‘Jaguar’, whose total anthocyanin content levels gradually increase to 16-fold higher at the petal open stage than at the initial bud stage (Dela et al., 2003). When flowers are fully open at the senescence stage, rose petals turn bluish and pale because of increased cell sap pH and changes in the amount of phenolic compounds (Schmitzer et al., 2010). Chloroplasts are transformed into chromoplasts as the sexual organs develop, which decreases chlorophylls and increases carotenoid levels such as lutein and β-carotene (Ohmiya et al., 2019). In Sandersonia aurantiaca, the concentration of β-carotene and xanthophylls, such as zeaxanthin and cryptoxanthin, which are produced by β-carotene, increased during floral bud development and then decreased at the tepal wilting stage (Lewis et al., 1998; Zhu et al., 2010). Carotenoid levels in plants are controlled by the rate of accumulation and degradation and the storage capacity of the reserve tissue (Ohmiya et al., 2019). Total carotenoids increased more than 6 times in yellow chrysanthemums as the flowers developed, but they decreased in white ones through the activity of the carotenoid cleavage dioxygenases, which degrade carotenoids at the early stage of flowering (Kishimoto and Ohmiya, 2006; Zhu et al., 2010). The ORANGE protein, which regulates plastid biosynthesis and development, correlated positively with phytoene synthase to produce carotenoids (Kishimoto and Ohmiya, 2006; Zhu et al., 2010).
Changes in petal color with increases and decreases in pigments are sometimes related to pollination (Miller et al., 2011). Marigolds that have both yellow carotenoid at the petal margin and flavonoids at the basal side appear yellow to humans, but bees, which can detect UV light, are led by the yellowish flavonoid to the center for nectar (Miller et al., 2011). In V. cornuta, white flowers at an early flowering stage turn purple after pollination, which is a response indicating pollination and senescence (O’Neill, 1997; Miller et al., 2011).
Flower scent is controlled by the rate of biosynthesis and emission of scent compounds as flowers develop (Guterman et al., 2002; Shalit et al., 2003; van Schie et al., 2006). Scent compounds are maximally produced and emitted when the petals are fully open; closed floral buds with folded petals have no scent in either a strongly scented rose such as R. hybrida ‘Papa Meilland’ or a delicately scented rose such as ‘Black Baccara’ (Guterman et al., 2002; Bergougnoux et al., 2007). Changes in the composition and emission rate of scent compounds could be driven by factors such as flower senescence, pollination, environmental conditions, and circadian rhythms (Negre et al., 2003). When flowers are ready for pollination, they tend to produce peak levels of scent, whereas new floral buds with closed anthers emit little scent (Negre et al., 2003). The mRNA level of terpene synthase, which produces a flower’s scent, is upregulated shortly before petals open (van Schie et al., 2006). Scent compounds increase developmentally in most flowers, and some scent compounds are regulated by a circadian clock related to LATE ELONGATED HYPOCOTYL (Guterman et al., 2002; van Schie et al., 2006; Mohd-Hairul et al., 2010; Ravid et al., 2017). The activity of phenylalanine ammonia-lyase was found to be sensitive to a diurnal rhythm in flowers producing a high level of benzoic acid; however, applying phenylalanine as a precursor to benzoic acid produced modest responses (van Schie et al., 2006). A strongly scented R. hybrida ‘Fragrant Cloud’ emitted germacrene-D at 7-fold higher levels at the petal open stage than at the bud closed stage, with a high level of sesquiterpene synthase activity driven by a farnesyl diphosphate substrate (Guterman et al., 2002). Many flowers substantially lessen their scent emissions after pollination, with snapdragons and petunias discontinuing the production scent compounds when the pollen tube reaches the ovary (Negre et al., 2003).
Correlation between Pigments and Scent Compounds in Petals
Most plants produce their own visual cues and scents to advertise their presence to pollinators, who use those color-scent combinations to find and distinguish flowers (Negre et al., 2003). Both scent and color are controlled by GA in the petals (Weiss and Halevy, 1989; Kumar et al., 2008; Ravid et al., 2017). GA promotes pigmentation through anthocyanins by increasing the expression of genes needed to produce them and downregulates scent compound production by suppressing the transcript level of phenylpropanoid biosynthesis genes such as phenylacetaldehyde synthase and isoeugenol synthase at the closed bud stage (Kumar et al., 2008; Ravid et al., 2017). In petunia flowers, however, GA decreases the emissions of specific scent compounds biosynthesized from phenylalanine and cinnamic acid as precursors for the anthocyanins, but this had no effect on the monoterpenes (Ravid et al., 2017). In ‘Penny Lane’, phenolic scent compounds such as 2-phenylethanol, 3,5-dimethoxytoluene, and methyl eugenol were correlated more strongly with carotenoids than a monoterpene geraniol (Table 2) because phenolic and terpene scent compounds had each precursor in the separated biosynthesis pathway such as the shikimate or mevalonate pathway (Baldermann et al., 2009). The GA activity that controls the levels of pigments and scent compounds in petals could create a time lag by which those levels change in predictable ways at specific stages in flower development, which resulted in the faster accumulation of pigments at the earlier stages in the R. hybrida ‘Penny Lane’ and ‘Vital’ petals during flowering (Fig. 3).
Most flowers produce maximal scent and pigments when they are ready to spread pollen (Negre et al., 2003; Miller et al., 2011). In many plants, flower development is controlled or expedited by pollination to ensure reproductive success (O’Neill, 1997). Pigment and scent compound levels mostly change together for the whole period of flower development, from a closed bud until senescence (Figs. 2 and 3), because the color and scent of flowers work together to attract pollinators as a signal with the synergy effect (Majetic et al., 2007). Pigments and scent compounds decrease after pollination, sending pollinators to nearby, as yet unpollinated flowers (O’Neill, 1997; Negre et al., 2003; Miller et al., 2011). Therefore, the level of pigments and scent compounds in flowers could be the indicator for the pollinated state during flower development (Gil et al., 2020). In this study, we confirmed the concurrent change of pigments and scent compounds in the petals with the increase and decrease of content during flowering in R. hybrida ‘Penny Lane’ and ‘Vital’ (Fig. 3) and determined a positive correlation between them (Tables 2 and 3). Previous studies reported the correlation between petal color and scent in some native flowers such as Calanthe sylvatica, Buddleja davidii, and Orchis (Delle-Vedove et al., 2011; Chen et al., 2014; Dormont et al., 2014), but they did not suggest the composition changes with floral bud development causing the difference in pigments and scent compounds. Furthermore, the composition of floral pigments and scent, as well as vase life of cut roses, also drives consumer decisions because these ornamental traits affect their commercial value (Schulz et al., 2016). Among the five stages of flower bud development in R. hybrida ‘Penny Lane’ and ‘Vital’ in this study, the unfolded petal stages S3 to S4 showed the highest quality cut roses with brilliantly colored and scented petals abundant in pigments and scent compounds (Fig. 3). We expected that the changes in pigments and scent compounds with floral bud development could help determine the optimal stages to harvest high-quality cut flowers and aid in the study of secondary metabolite mechanisms in flowers. Choi et al. (2013) reported that cut roses had the longest vase life when 2-3 outer petals were unfolded at the harvest stage; petals that were unfolded at S4 contained a high level of scent compounds in ‘Penny Lane’ and ‘Vital’ (Fig. 2). However, more study is needed of the difference in colors and scent compounds during vase life because the floral bud stage is continuously changing and because of the incomplete flower opening in cut roses, unlike in intact plants.