Introduction
Diversity of Colors and Scents in Flowers
Floral Pigments in Phenols and Terpenes
Volatile Scent Compounds in Phenols and Terpenes
Changes in Color and Scent with Flower Development
Preference of Pollinators for Color and Scent
Color-Scent Linkage in Biosynthetic Pathways
Complementary Expression of Color and Scent
Linkage between Color and Scent in the Phenol-Synthetic Pathway
Linkage between Color and Scent in the Terpene-Synthetic Pathway
Expression of Color and Scent with Environmental Factors
Conclusion
Introduction
Flowers contribute to plant reproduction and have economic value in the floriculture industry because their various colors, shapes, types, and scents are attractive to people (Abreu et al., 2009; Burchi et al., 2010). Behe et al. (1999) reported that flower colors were the most critical trait that affected the purchasing decision compared with other marketable characteristics such as leaf variegation, size, or price. Also, fragrance is a factor that has aesthetic and emotional values. In particular, plant essential oils are widely used as natural materials in various industries, such as cosmetics, foodstuffs, beverages, and medicine (Pichersky and Dudareva, 2007; Nasiri et al., 2010). Essential oils extracted from various plant organs, such as flowers, leaves, fruits, and roots, emit specific smells and by blending produce new scents (Ali et al., 2015). The essential oils extracted from lavender and rose flowers have a relaxing effect and can mitigate depression and anxiety (Setzer, 2009). For example, damask rose (Rosa × damascena) are known for their delicate fragrance. They are commercially harvested for rose oil “rose otto” or “rose absolute,” which are used to make rose water and rose concrete. These essential oils, which contain several aromatic compounds, such as 2-phenylethanol, citronellol, geraniol, and nonadecane, have relaxing effects that help lower blood pressure (Setzer, 2009).
Previous studies over the past two decades have reported correlations between color and scent biosynthesis pathways and the critical roles they have played in ecology and the horticultural industry (O’Neill, 1997; Delle-Vedove et al., 2011; Dormont et al., 2014; Yeon and Kim, 2020a). Floral pigments and scent compounds constantly change their composition during flowering as a result of GA activity controlling flower development and creating a time gap for the biosynthesis of pigments and scent compounds (Yeon and Kim, 2020b). Light-colored flowers have stronger scents and emit more diverse scent compounds than dark and bright flowers, while color is the dominant trait over fragrance for attracting pollinators (Cseke et al., 2007). However, few reports have clearly explained the linkage between color and scent of flowers because of the complex evolution of various biotic and abiotic factors (Arista et al., 2013; Chen et al., 2014). In this study, we reviewed the relevant references to clarify the shared biosynthetic pathways of flower colors and scents and their functions and changes in response to biotic and abiotic factors (Table 1). We suggest a novel schematic diagram of the shared biosynthetic pathways for floral pigments and scent compounds in flowering plants, classified as phenolics and terpenes.
Table 1.
Literature cited on the linkage between floral color and scent in flowering plants
Diversity of Colors and Scents in Flowers
Plants reproduce by inducing plant-pollinator interactions with flowers, which is affected by various traits, including shape, color, size, scent, and flowering time (Fig. 1) (van Schie et al., 2006; Koski, 2020). Flower color and scent are critical factors for plant reproductive success, and variations in floral traits occur at the individual and population levels in most flowering plants (Majetic et al., 2010). When flowers mature and are ready to provide pollen, the petals unfold to attract pollinators with pigmentation and scent emission (Martin and Gerats, 1993; Negre et al., 2003; Zvi et al., 2008; Majetic et al., 2009). In general, plants don’t attract pollinators and stop nectar production after pollination due to changes in the color and scent of flowers (Lucas-Barbosa, 2016). Because pollinators have preferences and sensory flexibility for specific colors and scents, they can discriminate changes in color and scent and avoid pollinated flowers that provide little reward (Lucas-Barbosa, 2016). Pollinators recognize whether or not flowers have been pollinated by detecting color and scent, and the two traits work synergistically (Cseke et al., 2007; Dormont et al., 2010). Pollinators are better able to differentiate among flowering plants with combinations of color and scent than if the two traits are independent (Delle-Vedove et al., 2011).

Fig. 1.
Polymorphism of flower traits in flowering plants. A: Iris japonica; B: Medicago arborea; C: Dahlia ‘Vdtg57’; D: Lantana camara; E: Hydrangea serrata; F: Monarda fistulosa; G: Platycodon grandiflorus; H: Echium hierrense; I: Impatiens hybrida ‘New Guinea’; J: Spiraea japonica; K: Grevillea rosmarinifolia; L: Zinnia elegans.
Most flowers are categorized into four groups by pollinator type: entomophilous, ornithophilous, anemophilous, and hydrophilous. Wind- and water-pollinated flowers rarely have brightly colored petals. In contrast, animal-pollinated flowers use petal color as a signal to attract pollinators, and thereby the color ensures a flower’s reproductive success (Miller et al., 2011). Petal colors such as white, yellow, orange, pink, red, purple, and blue vary depending on the composition of floral pigments (Warren and Mackenzie, 2001). The primary floral pigments are divided into flavonoids, carotenoids, and betalains (Table 2). These floral pigments accumulate at several locations within cells and have various functions in plants (Timoneda et al., 2019). Flavonoids and carotenoids are prevalent in most flowers, while the betalains are generally limited to some flowers, such as Portulaca grandiflora, Gomphrena globosa, and Mirabilis jalapa, and fruits in the Caryophyllales order (Strack et al., 2003). Plants produce various pigments, and some that are categorized as flavonoids and terpenes have a connection with scent compounds.
Table 2.
Functions of representative floral pigments in flowering plants (Tanaka et al., 2008; Miller et al., 2011; Timoneda et al., 2019)
Floral Pigments in Phenols and Terpenes
Phenolic Pigment
Flavonoids are polyphenolic pigments from the shikimic acid (shikimate, SA) pathway, which provides amino acids such as phenylalanine and tyrosine. These amino acids are used for protein synthesis and also serve as a substrate for secondary metabolite biosynthesis to produce compounds such as phenolic acids (Gitonga et al., 2016). Anthocyanins are water-soluble pigments localized in the vacuole of epidermal cells in reproductive and vegetative tissues (Katsumoto et al., 2007). Some anthocyanidins, which are differentiated by the substituents in A-, C-, and B-rings (Fig. 2), have been identified in plants (Tanaka et al., 2008). These pigments express orange, red, pink, purple, violet, or blue. The color expressed varies depending on vacuole pH and accumulation of co-pigments or metal ion complexes in some flowers, such as petunia, hydrangea, and cornflower (Glover and Martin, 2012). Pigment content regulates flower color, depending on the balance between biosynthesis and decomposition (Liu et al., 2018). Flavones and flavonols act as co-pigments with anthocyanins, making the anthocyanins darker (Katsumoto et al., 2007). Although humans perceive flavones and flavonols to be colorless because they absorb ultraviolet (UV) light, some pollinators detect UV light, leading them to white-yellowish flowers with nectar (Miller et al., 2011).
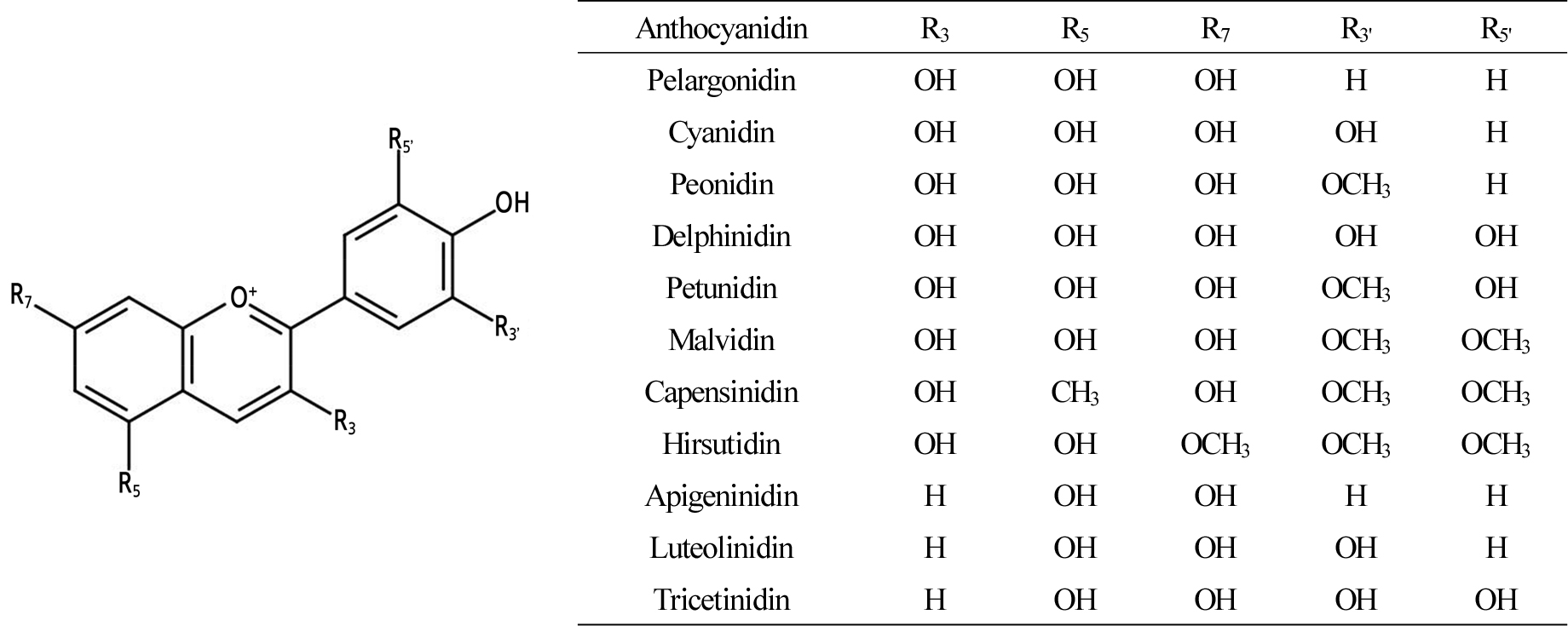
Fig. 2.
Chemical structure and substituents of anthocyanidins in flowering plants (Tanaka et al., 2008).
Some plant species’ flowers are limited to specific colors due to key regulating enzymes or genes. For example, roses and carnations are representative floricultural crops that lack blue and violet cultivars. They lack the flavonoid 3',5'- hydroxylase, the critical enzyme needed to produce blue pigments in biosynthetic flux (Katsumoto et al., 2007). Fukui et al. (2006) reported that Rosa hybrida ‘Rhapsody in Blue’ looks blue because it accumulates small amounts of the pigment rosacyanin, a derivative of cyanin, not delphinidin. Petunias and cymbidiums have rare red or orange flowers that result from substrate specificity of dihydroflavonol-4-reductase (DFR), which does not use dihydrokaempferol as a substrate (Katsumoto et al., 2007).
Terpene Pigment
Carotenoids are long-chained pigments formed by C5 (isoprene unit) in the chloroplasts and chromoplasts of plants and the most prevalent pigments after chlorophylls (Hannoufa and Hossain, 2012). The carotenoids are structured as a polyene chain consisting of 9 to 13 double bonds terminating with rings, and they are biosynthesized from the methylerythritol 4-phosphate (MEP) pathway. After lycopene biosynthesis, cyclization with β- or ε-rings forms two groups: carotenes and xanthophylls. The carotenes are hydrocarbons, and the xanthophylls contain both hydrocarbons and oxygen, changing excess solar energy into thermal energy (Demming-Adams and Adams, 2002; Zhu et al., 2010).
Many flowers colored by carotenoids contain different types of carotenoids, even if they have the same color. For example, the bright yellow flowers of Gentiana lutea are abundant in β-carotene and xanthophyll. Meanwhile, Asiatic hybrid lilies (Lilium) contain lutein and xanthophyll in their yellow petals (Zhu et al., 2010). Tomatoes (Solanum lycopersicum), which have yellow flowers, accumulate xanthophylls such as violaxanthin and neoxanthin (Zhu et al., 2010). Like anthocyanin, carotenoids can coexist with other pigments such as chlorophyll and anthocyanins (Miller et al., 2011). Degradation and accumulation of carotenoids affect flower color. Yellow chrysanthemums accumulate lutein as the primary pigment in their petals (Kishimoto et al., 2004). Inhibition of lutein in the late stage of development through the expression of carotenoid cleavage dioxygenases (CCDs) produces white chrysanthemums by degrading the yellow carotenoid pigments (Zhu et al., 2010). The expression of carotenoid biosynthetic genes in white petals is similar to that of yellow petals. CCDs contribute to expressing the white color in the petals by controlling the accumulation of carotenoids (Ohmiya et al., 2019). CCDs (CCD1 and CCD4) are the main contributors for controlling the fragrance of flowers and fruits such as petunias, roses, and papayas because they can produce scent compounds such as α- and β-ionone and 6-methyl-5-hepten-2-one (Ohmiya et al., 2019).
Volatile Scent Compounds in Phenols and Terpenes
Plants produce many volatile scent compounds from leaves, flowers, fruits, and roots for pollinator attraction and defense against herbivores and insects (Baudino et al., 2007). Pollinators perceive flowers with the invisible cue “floral scent” from long distances and at night (Dudareva and Pichersky, 2000; Pichersky et al., 2006; Stashenko and Martínez, 2008; Majetic et al., 2009; Feng et al., 2014). A flower’s scent bouquet is variable because it represents a mixture of volatile compounds that are mainly divided into three classes, terpenes, phenylpropanoids, and fatty acid derivatives, that have different content levels and physicochemical traits of volatility, solubility, and diffusivity (Effmert et al., 2005; Farré-Armengol et al., 2014). Vanda emits a combination of volatile compounds including terpenes, phenolics, indole, and formanilide (Mohd-Hairul et al., 2010). Some floral volatiles in phenolics and terpenes are associated with pigments sharing specific biosynthetic pathways.
Volatile Phenolic Compounds
Phenylpropanoids and benzenoids originate through the SA pathway, which produces various phenolic compounds and in which benzene rings are oxidized, acylated, or methylated to contribute to flower scent (van Schie et al., 2006; Baldermann et al., 2009). Most phenolic scent compounds use chorismic acid, l-phenylalanine, and trans-cinnamic acid as precursors (Baldermann et al., 2009). Phenylpropanoids greatly contribute to flower scent (Mohd-Hairul et al., 2010). More than 300 scent compounds, such as benzyl acetate, eugenol, methyl benzoate, phenylethyl acetate, and phenyl ethanol, are phenylpropanoids (Baldermann et al., 2009; Mohd-Hairul et al., 2010). Phenylpropanoids are distributed and released differently from each part of the flower. For example, while whole Clarkia breweri flowers emit eugenol, isoeugenol, methyl eugenol, and methyl isoeugenol, the petals produce on average 2.5, 1.8, and 0.5 µg·flower-1·day-1 of methyl eugenol, methyl isoeugenol, and eugenol, respectively (Tan and Nishida, 2012). In the cosmetics industry, 2-phenylethanol, the typical rose scent that is dominant in Rosa damascene, is widely used to make fragrant chemicals because it has a high odor threshold (Etschmann et al., 2002; Bendahmane et al., 2013). Also, 3,5-demethoxytoluene and 1,3,5-trimethoxybenzene with tea scent are the major scent compounds from Chinese rose (Scalliet et al., 2008).
Volatile Terpene Compounds
Terpenoids are subdivided by the number of C5 groups into monoterpenes (C10), sesquiterpenes (C15), and diterpenes (C20) (Baldwin, 2010). All volatile terpene compounds are formed through the mevalonate (MVA) or MEP pathway in the cytosol or plastid, respectively (van Schie et al., 2006). The MVA pathway in the cytosol produces mainly sesquiterpenes, and the MEP pathway in the plastid produces monoterpenes and diterpenes (Fig. 3). Each volatile terpene compound has a specific precursor: geranyl diphosphate (GPP) or farnesyl diphosphate (FPP), which become monoterpenes, sesquiterpenes, and diterpenes (Degenhardt et al., 2009). Each volatile terpene compound contributes a different amount to the overall floral scent. Monoterpenes, such as geraniol, nerol, citronellol, and linalool, compose 60% of rose oils quantitatively; however, their contribution to the scent is only 6% because they have high odor thresholds of 6–300 µg·kg-1 in water (Baldermann et al., 2009). CCDs produce some scent compounds derived from carotenoids such as β-ionone and damascenone in yellow or orange flowers (Huang et al., 2009). β-Ionone and damascenone are biosynthesized in small quantities and comprise less than 1% of the scent compounds. Nevertheless, they have a significant effect on flower scent because of their low odor thresholds of 0.007–0.009 µg·kg-1 in water (Baldermann et al., 2009).
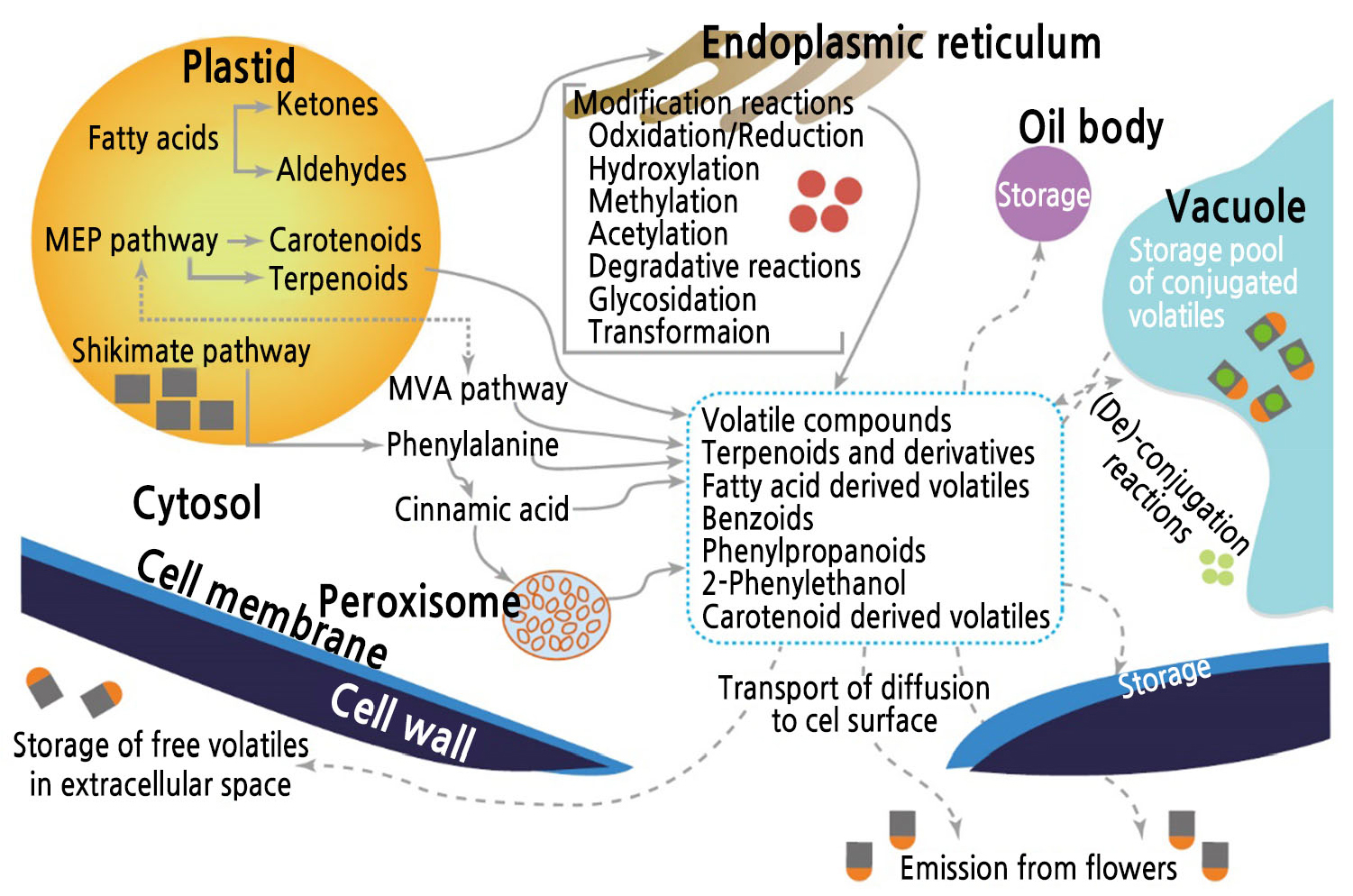
Fig. 3.
Emission processes for scent compounds produced in rose flowers by biosynthetic pathways. Redrawn from Baldermann et al. (2009).
Changes in Color and Scent with Flower Development
Flowers develop continuously to senescence, and the process is affected by biotic and abiotic conditions, such as growth stage (nutrients), phytohormones (GAs), light and temperature conditions, and pollination (O’Neill, 1997; Blázquez, 2000; Kumar et al., 2008). Petals, which are the primary source for color and scent, unfold with the increase of floral pigments and scent compounds that occurs when flowers are ready for pollination (Miller et al., 2011; Yeon and Kim, 2020b). For pollinator selection, flowers emit scent compounds developmentally and rhythmically in accordance with visitor activity and irradiation (Helsper et al., 1998; Muhlemann et al., 2014). Petunia axillaris that was pollinated by a nocturnal insect moth emitted more scent compounds during the night than Petunia integrifolia that was pollinated by diurnal insect moths (Muhlemann et al., 2014). Narcissus flowers and roses changed the emission rate of scent compounds in accordance with the circadian rhythm, and they emit more scent compounds during the day than at night (Helsper et al., 1998; Ruíz-Ramón et al., 2014).
Flowers at an early stage show green color because both carotenoids and chlorophylls are accumulated in chloroplasts (Ohmiya et al., 2019). Carotenoids in petals increased during flowering and decreased at the wilting stage in gentian, sandersonia, chrysanthemum, rose, and petunia flowers, which showed a change in the activity of CCDs producing carotenoid-derived scent compounds (Lewis et al., 1998; Zhu et al., 2002; Simkin et al., 2004; Huang et al., 2009; Zhu et al., 2010). Pollination accelerates flower senescence by producing increasingly endogenous ethylene and changing the composition of pigments and scent compounds to avoid revisitation (O’Neill, 1997; Lucas-Barbosa, 2016). Farzad et al. (2002) reported that the malvidin level in the petals of Viola cornuta increased gradually during flower development. The flowers then changed color to pale lavender or purple after pollination. Rosa damascene, which opens folded petals within hours, showed changes in the composition and increased emission levels of scent compounds at a petal opening stage early in the morning. There was a 40% decrease in emission levels at the fully open stage with visible stamens (Oka et al., 1999). The concentration of both floral pigments and scent compounds increased during flowering and declined sharply at the senescence stage (Fig. 4), but the rate of change in different varieties had a time gap (Yeon and Kim, 2020b). The GAs produced at the sepal and stamen control the expression of floral pigments and scent compounds in petals, which results in pigmentation coinciding with the suppression of scent compound accumulation in green petals at the elongation stage (Weiss and Halevy, 1989; Weiss, 2000; Kumar et al., 2008; Ravid et al., 2017; Yeon and Kim, 2020b).
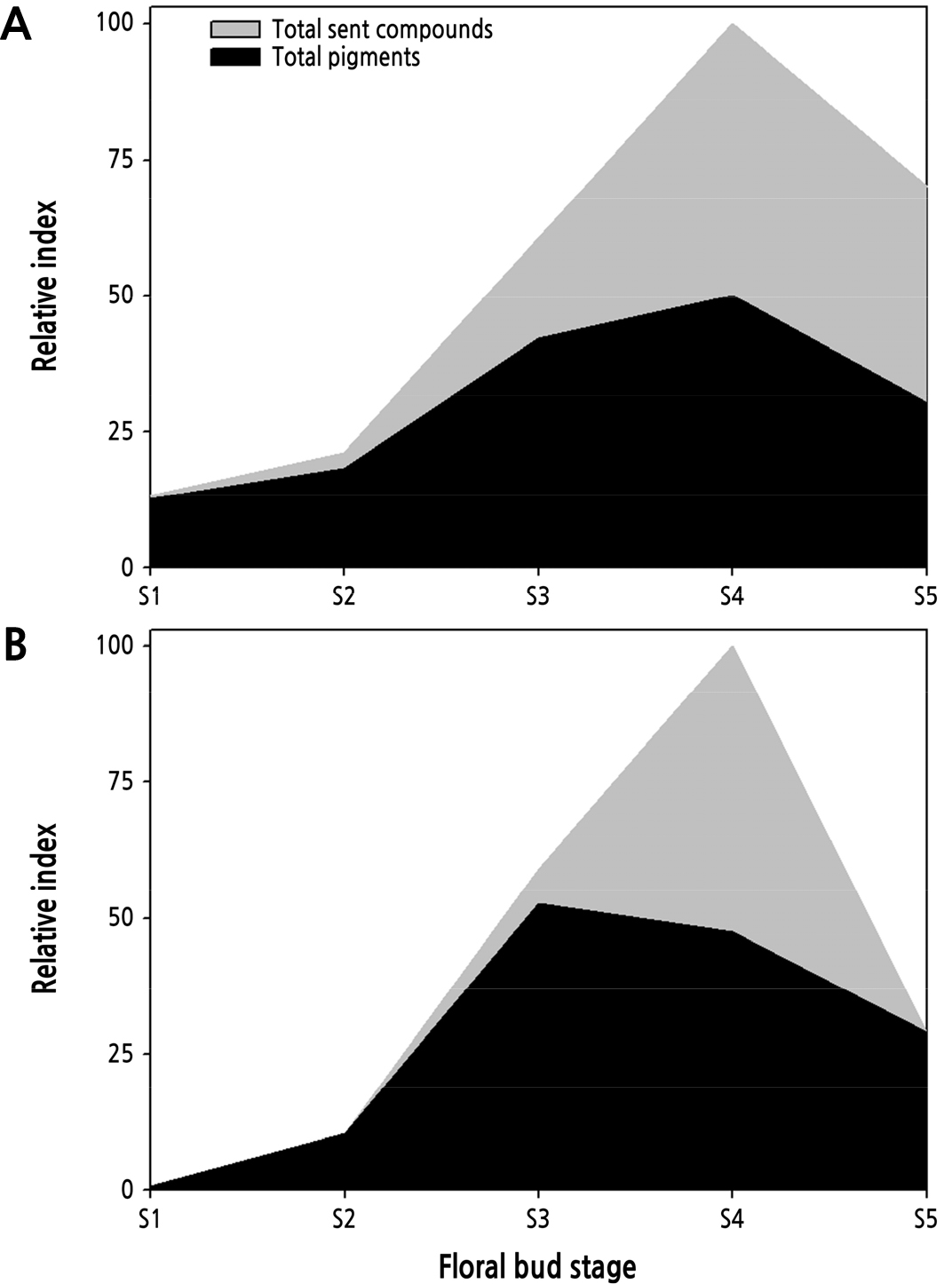
Fig. 4.
Changes in floral pigments and scent compounds in Rosa hybrida ‘Vital’ (A) and ‘Penny Lane’ (B) during flowering (Yeon and Kim, 2020b).
Preference of Pollinators for Color and Scent
Animal pollinators prefer specific combinations of various floral traits, such as color, scent, or shape, which have evolved through pollinator-mediated selection using learning capabilities and sensory flexibility (Delle-Vedove et al., 2011). Pollinators are inclined to visit flowers having the same morph as flowers just visited because they perceive rewarding flowers with certain phenotype combinations (Dormont et al., 2010). It is known that a visual cue color attracts more halictid bees, while an olfactory cue scent attracts butterflies and anthomyia flies to flowers (Ômura and Honda, 2005; Koethe et al., 2020). Campbell et al. (2010) reported that petal color is a priority trait over flower shape for some pollinators, and most Leioproctus spp. preferred yellow rather than white. Many butterfly, bumblebee, and honeybee species prefer certain flower colors such as yellow, blue, purple, or white, but yellow and blue are usually the most attractive visual cues (Sheehan et al., 2012). Although phenolic scents such as benzaldehyde and methyl salicylate are an effective signal that attracts pollinators, yellow color has a higher priority than scent in Vanessa indica, indicating that visual cues are the primary factor for attracting visitors (Sheehan et al., 2012). Specific floral scent bouquets and volatile compounds help pollinators distinguish different colored flowers (Raguso, 2008). However, some visitors may learn the association between flower traits and rewards rather than preferring a particular trait (Raguso, 2008).
Color-Scent Linkage in Biosynthetic Pathways
Some studies have suggested the possibility of a correlation between pigments and scent compounds because they share some biosynthetic pathways (Dormont et al., 2014; Raymond et al., 2018). We have created a novel schematic diagram to explain the biosynthetic association between color and scent (Fig. 5). For example, the SA pathway produces phenylpropanoids, including anthocyanins and benzenoid volatiles (Fig. 5A) (Dudareva and Pichersky, 2000; Zuker et al., 2002; Maffei, 2010). In comparison, the biosynthesis of carotenoids and some terpene volatiles takes place from the MEP and MVA pathways, which begin with condensation between isopentenyl diphosphate and dimethylallyl diphosphate (Fig. 5B) (Zhu et al., 2010; Magnard et al., 2015; Tholl and Gershenzon, 2015). However, previous studies on the connection between flower color and fragrance have reported discordant results. The exact relationship between flower color and scent is complicated because they have evolved under the influence of many biotic and abiotic factors, such as the preferences of specific pollinators and random genetic drift (Arista et al., 2013; Chen et al., 2014).
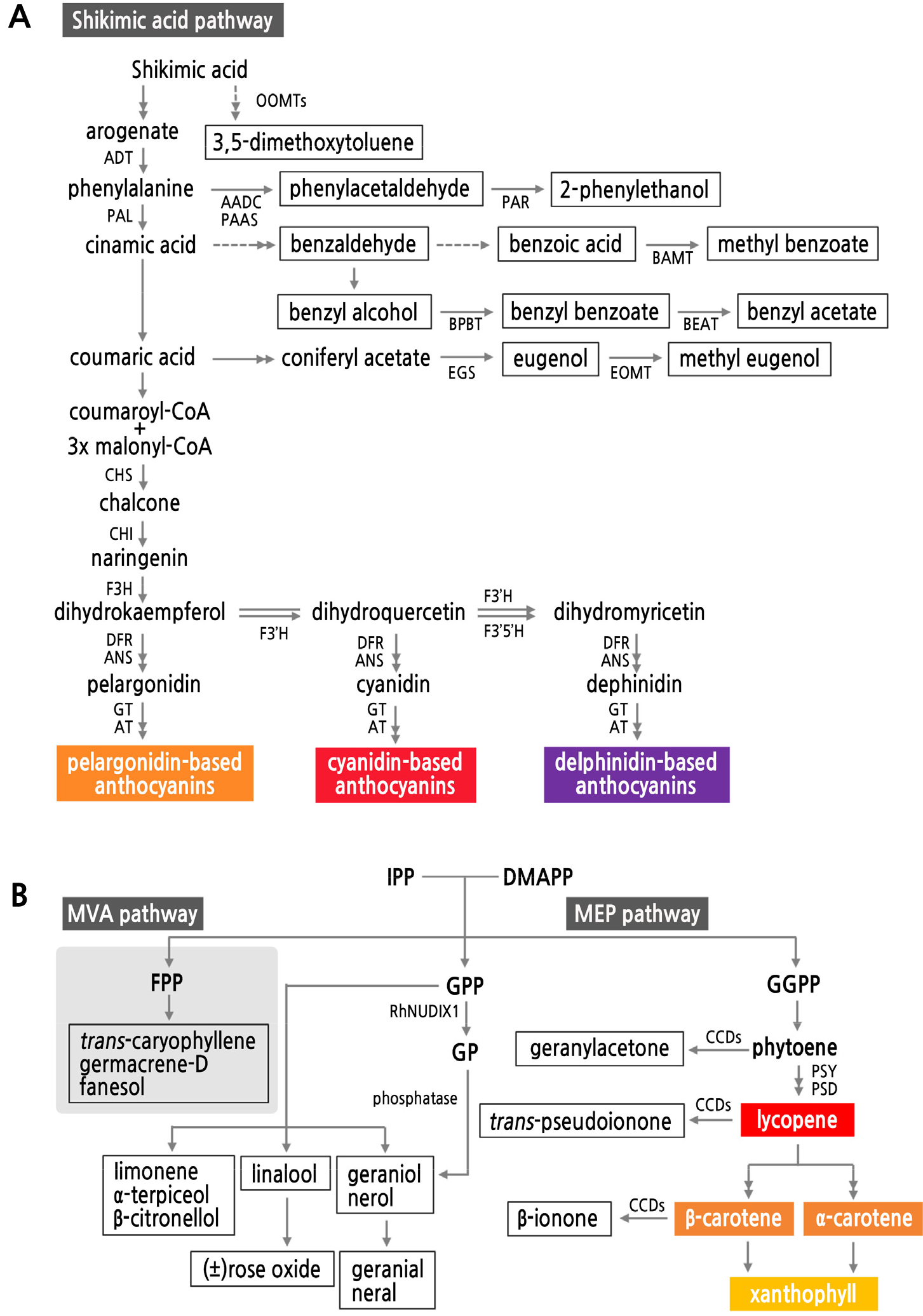
Fig. 5.
A schematic diagram of shared biosynthetic pathways for pigments and scent compounds in flowers classified as phenolics (A) and terpenes (B). Colored boxes are pigments, and white boxes are scent compounds. A: a pathway for anthocyanin and phenolic scent compounds; B: a pathway for carotenoids and terpenes. Chemicals in frames are scent compounds; those in colored frames are floral pigments. AADC: l-aromatic amino acid decarboxylase; ADT: arogenate dehydratase; ANS: anthocyanidin synthase; AT: anthocyanin acyltransferase; BAMT: benzoic acid carboxyl methyltransferase; BEAT: acetyl-CoA:benzylalcohol acetyltransferase; BPBT: benzoyl-CoA:benzylalcohol/2-phenylethanol benzoyltransferase; CCD: carotenoid cleavage dioxygenase; CHS: chalcone synthase; CHI: chalcone flavanone isomerase; DFR: dihydroflavonol-4-reductase; DMAPP: dimethylallyl diphosphate; EGS: eugenol synthase; EOMT: eugenol O-methyltransgerase; F3'H: flavonoid 3'-hydroxylase; F3'5'H: flavonoid 3',5'-hydroxylase; FHT: flavanone 3-hydroxylase; FLS: flavonol synthase; FPP: farnesyl diphosphate; GP: geranyl monophosphate; GPP: geranyl diphosphate; GGPP: geranylgeranyl diphosphate; GT: anthocyanidin glucosyltransferase; IPP: isopentenyl diphosphate; MEP: methylerythritol phosphate; MVA: mevalonate; OOMTs: orcinol O-methyltransferases; PAL: phenylalanine ammonia lyase; PAR: phenylacetaldehyde reductase; PAAS: phenylacetaldehyde synthase; PDS: phytoene desaturase; PSY: phytoene synthase.
Complementary Expression of Color and Scent
Petals are the primary source of color and scent in most plants (Guterman et al., 2002; Mohd-Hairul et al., 2010). Flower color is expressed by floral pigments such as anthocyanins and carotenoids, while volatile bouquets emit from epidermal cells in the petals (Bergougnoux et al., 2007; Abdelali and Hossain, 2012; Liu et al., 2018). Some studies suggested that white flowers usually have a strong scent (Campbell et al., 2010). In orchids, white flowers emit more benzenoids than colored flowers and the combined scent composition of colored flowers, and this resulted from sharing of and competition for precursors of phenolic pigments and scent compounds in biosynthetic flux (Delle-Vedove et al., 2011; Dormont et al., 2014). Principal component analysis (PCA) with primary floral pigments and scent compounds in eight cultivars of Rosa hybrida created three groups, and red morphs had fewer scent compounds in petals than anthocyanin-colored cultivars (Yeon and Kim, 2020a). Although carnation flowers with a cream color morph produced by suppressing flavanone 3-hydroxylase (F3H) emitted more methyl benzoate and 2-hydroxy methyl benzoate than a basic cultivar with a reddish-orange color, both color flowers produced similar amounts of β-caryophyllene, a sesquiterpene (Zuker et al., 2002). When the transcription factor anthocyanin pigment 1 (Pap1) was inserted in petunia to produce more floral pigment, phenylpropanoids (such as benzaldehyde and phenylacetaldehyde) and anthocyanins also increased because they share the biosynthetic pathway (Majetic et al., 2010). Salzmann and Schiestl (2007) reported that the different scent compositions of red and yellow flowers in Dactylorhiza romana occurred because the red morph had two times more linalool than the yellow morph. However, silencing chalcone synthase (CHS), which is active in the early stages of anthocyanin production, decreased the anthocyanin content in petunia petals without affecting the scent compound content (Zvi et al., 2008). Therefore, it was not easy to determine the apparent association between them because the biosynthesis of floral volatiles is more diverse and complex than that of floral pigments and involves pleiotropy of those compositions and the genetic background (Dormont et al., 2019).
Linkage between Color and Scent in the Phenol-Synthetic Pathway
Flavonoids and phenolic scent compounds are synthesized at the cytosol of some plant organs, such as leaves, flowers, fruits, stems, and roots (Pichersky et al., 2006). In the SA pathway, phenylalanine is the core precursor. Some floral volatiles, such as 2-phenylethanol, methyl benzoate, and benzyl acetate, use phenylalanine as a substrate that is transformed to cinnamic acid or decarboxylated and then reduced to produce flavonoid and scent compounds before the production of chalcone, which is a crucial intermediate for anthocyanin (Fig. 5A) (Zvi et al., 2008; Maeda and Dudareva, 2012; Muhlemann et al., 2014). Because scent compounds have lower molecular weights than pigments, phenolic volatiles (group C9) are synthesized upstream of anthocyanin production (group C15), which results in competition between secondary metabolites for the application of phenylalanine (Dudareva and Pichersky, 2000; Sinopoli et al., 2019; Joh et al., 2020). Bright- and deep-colored flowers emit fewer scent compounds than light- and pale-colored flowers, and some pollinators visit flowers with a visual signal first, which may indicate that flower color is dominant in biosynthetic flux (Zuker et al., 2002; Ômura and Honda, 2005; Salzmann and Schiestl, 2007; Sheehan et al., 2012; Yeon and Kim, 2020a).
Linkage between Color and Scent in the Terpene-Synthetic Pathway
Few studies about the biosynthetic association between carotenoids and terpene volatiles have been conducted. Yeon and Kim (2020a) revealed that the correlation between carotenoids and scent compounds was not evident in cut roses because of the complexity of the terpene-synthetic pathway. Unlike phenolics, terpenes have relatively less vertical biosynthetic flux (Fig. 5B). Carotenoids and monoterpenes are produced at the plastid through the MEP pathway, but sesquiterpenes are made at the cytosol through the MVA pathway (Fig. 3) (Iijima et al., 2004; van Schie et al., 2006; Baldermann et al., 2009; Degenhardt et al., 2009). GPP is the primary substrate for monoterpenes such as limonene, linalool, and geraniol. FPP is the substrate for sesquiterpenes such as trans-caryophyllene and germacrene-D (Degenhardt et al., 2009). Also, nudix hydrolase 1 (NUDX1) catalyzes GPP from the plastid into geranyl monophosphate (GP) in the cytosol. GP also biosynthesizes geraniol, producing other monoterpenes at the cytosol (Chen and Viljoen, 2010; Magnard et al., 2015; Tholl and Gershenzon, 2015). Geranylgeranyl diphosphate (GGPP) synthesizes carotenoids through the critical substrate phytoene in the MEP pathway (Zhu et al., 2010). Some pigments are cleaved into carotenoid-derived volatiles such as geranyl acetone, trans-pseudoionone, β-damascenone, and β-ionone by CCDs, which turns yellow/orange flowers into white- or cream-colored flowers (Huang et al., 2009; Zhu et al., 2010; Bendahmane et al., 2013). Further studies are needed to determine the association between pigments and scent compounds for predominance in production and expression.
In summary, the primary pigments in rose flowers are flavonoids and carotenoids, which are mostly related to the SA and MEP pathways. Scent compounds are mainly produced from the SA, mevalonate (MVA), or MEP pathway (Fig. 5). Some phenolic scent compounds (e.g., 2-phenylethanol, methyl benzoate, and eugenol) are biosynthesized through the SA pathway, which ultimately provides flavonoid floral pigments (e.g., flavonol and anthocyanin). The MEP pathway provides monoterpenes and carotenoids from different substrates such as GPP and GGPP. These examples show that flowering plants choose the color or fragrance of flowers through interrelations between their biosynthetic pathways in accordance with petal development.
Expression of Color and Scent with Environmental Factors
Climate change reduces the plant-pollinator interaction, which results in declines of the reproductive rate, population, and availability of plants and threatens the ecosystem and food security (Forrest, 2015). Plants have evolved in accordance with diverse environmental conditions, resulting in responses to abiotic signals when they grow and bloom (Cna’Ani et al., 2015). Floral color and scent are the primary sources of ornamental factors, and their production and expression depend on environmental factors such as light, temperature, and humidity (Hu et al., 2013; Lee and Kim, 2015; Hoang and Kim, 2018; Lee and Kim, 2018). Changes in pigments and scent compounds affect flower quality and cause economic damage in the global horticulture industry (Yeon and Kim, 2020c). However, the effect of environmental factors on the expression of color and scent varies depending on their composition, and few studies are currently being conducted on this subject. To improve the flower quality of both color and scent, we need to understand their responses to environmental conditions.
Changes in pigments because of environmental conditions are better documented than changes in scent compounds. In general, temperature is the primary factor that affects the regulation of floral pigment with high temperature inducing a reduction and lower temperature conditions enhancing the accumulation of anthocyanin by reducing the biosynthetic rate and stability (Dela et al., 2003; Zhao and Tao, 2015). The effect of temperature conditions varies depending on the composition of the floral pigments and their role in plants. At the pigmentation stage, accumulation of anthocyanin was disturbed, while there was increased carotenoid in petals, protecting plant tissues (thylakoid membrane) from thermal side effects that degrade both pigments after pigmentation (Yeon and Kim, 2020c). Degradation of anthocyanin content by heat stress did not correspond to the expression of biosynthetic genes, such as CHALCONE SYNTHASE, CHACONE FLAVONONE ISOMERASE, and FLAVONOID 3'-HYDROXYLASE (Fig. 6), which suggests that another regulating factor, the MYB-bHLH-WD40 complex, activated anthocyanin production (Raymond et al., 2018). Temporary heat stress during flowering has different effects on the pigments and scent compounds depending on treatment timing. Yeon and Kim (2020c) reported content reduction and composition changes of both pigments and scent compounds in rose petals after pigmentation because heat stress at an earlier stage with no accumulation of scent compounds induced a decrease in total anthocyanin content. This resulted from the activity of GA, which created a time cap for production and accumulation of these compounds. To prevent the decline of flower quality of color and scent, growers should manage the temperature conditions in greenhouses. Super-optimal high temperature affects the emission rate and production of scent compounds and limits insect pollinator activity (Cna’Ani et al., 2015). In some flowers, elevated day and night temperatures change the composition of scent compounds in petals because of the downregulation of many scent-related genes, including PHENYLALANINE AMMONIA LYASE (PAL), PHENYLACETALDEHYDE SYNTHASE (PAAS), BENZOYL-CoA: BENZYLALCOHOL/2-PHENYLETHANOL BENZOYLTRANSFERASE (BPBT), and EUGENOL SYNTHASE (EGS). However, the high-temperature range is different for each flower species (Hu et al., 2013; Cna’Ani et al., 2015).
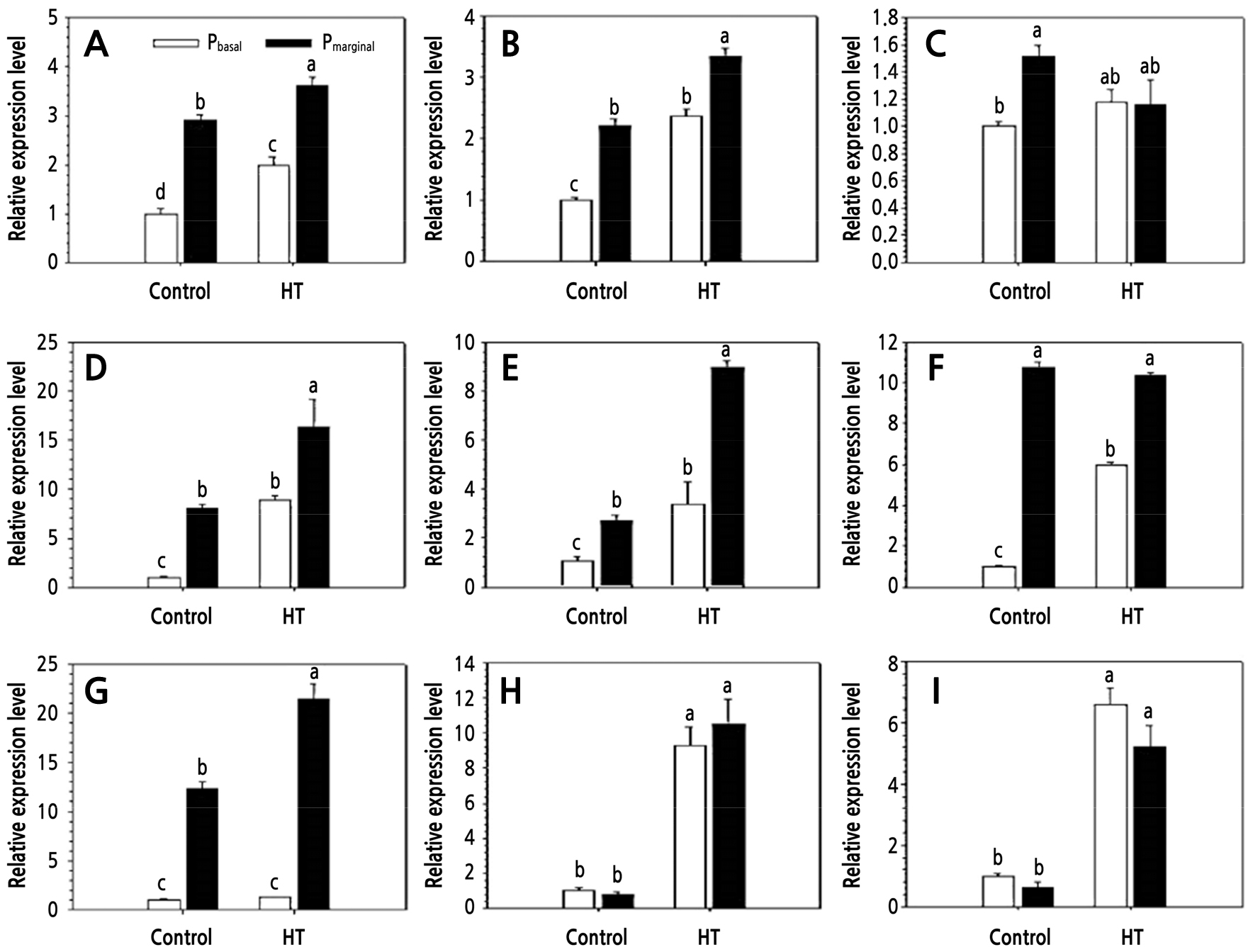
Fig. 6.
Changes in relative expression of some genes concerning anthocyanin biosynthesis in petals of Rosa hybrida ‘Pinky Girl’ under heat stress conditions for 3 days. Control: 25/18°C; HT: high-temperature treatment (39/18°C). Pbasal: basal part of petal; Pmarginal: marginal part of petals. A: CHS; B: CHI; C: F3H; D: F3'H; E: DFR; F: FLS; G: ANS; H: 3GT; and I: 5GT (Lee and Kim, 2015).
Light conditions, such as intensity, quality, and circadian rhythms, also control the production of pigments and scent compounds in flowers (Zhao and Tao, 2015; Fenske and Imaizumi, 2016; Chuang et al., 2017; Lee and Kim, 2018). Hu et al. (2013) reported that inadequate light intensity (less or more) limited scent emission in Lilium, which resulted from daylength-dependent release. To control the biosynthesis of benzenoids, such as benzaldehyde, eugenol, and methyl benzoate, the expression of genes such as LATE ELONGATED HYPOCOTYL, CIRCADIAN CLOCK ASSOCIATED1, and PAL is up- and down-regulated by light period (Fenske and Imaizumi, 2016). Although many abiotic conditions besides light and temperature change the composition of pigments and scent compounds, it is difficult to determine the concurrent effect of abiotic stress on them because of separately conducted studies.
Conclusion
Flower breeding research has mostly focused on commercial traits such as color and form rather than scent, resulting in a lack of information on fragrance and its relation to color (Guterman et al., 2002; Cherri-Martin et al., 2007). Recently, demand for flowers rich in scent has been increasing, especially for strongly sweet-scented flowers, which are mainly pollinated by bees and moths (Dudareva and Pichersky, 2000; Zvi et al., 2012). The evolutionary process may indicate that flowering plants have chosen floral color or scent as a mutual substitute by sharing their biosynthetic pathways to balance pollination and growth. Our review clarified that the color and scent of flowers have a biochemical connection and are a selective choice during flowering development (Fig. 5).
Nevertheless, there is still insufficient information to determine the correct correlation between pigments and scent compounds in flowering plants because of the complexity in expression and biosynthesis and the relationship with various biotic and abiotic environmental conditions. A comprehensive understanding of the biochemical linkage between the color and fragrance of flowers is critical for developing new varieties with novel traits. This paper could guide further studies on this complex relationship.


