Introduction
Materials and Methods
Plant Materials
Treatments
RNA Extraction and cDNA Synthesis
Quantitative Real-Time PCR (qRT-PCR)
Evaluation of Vase Life and Senescence Symptoms
Experimental Design and Data Analysis
Results
Vase Life and Senescence
Relative Fresh Weight and Flower Opening
Antimicrobial Activity and Water Relations
Leaf Chlorophyll Fluorescence
Expression Pattern of Ethylene Biosynthesis Genes
Discussion
Introduction
Rose is one of the most economically important cut flowers and plays a significant role in the florist trade. However, cut roses are often susceptible to water stress under unfavorable conditions after harvest (Doi et al., 2000). Generally, in cut rose flowers, early senescence symptoms are strongly related to the rise of hydraulic resistance caused by air embolism and physical occlusions by bacteria in the xylem vessels (van Doorn et al., 1995; van Doorn, 2012). Disruption of cut flower water relation causes physiological disorders such as early wilting of flowers and leaves and bending of
peduncle (bent neck), consequently decreasing the vase life of cut flowers.
Despite the strong correlation of vase life with water relations, cut rose flowers are also susceptible to the plant hormone ethylene. In ethylene-sensitive rose cultivars, early senescence symptoms are caused by ethylene synthesis in petals via transcriptional regulation of 1-aminocyclopropane-1 carboxylic acid synthesis (ACS) and 1-aminocyclopropane-1 carboxylic acid oxidase (ACO) genes (In et al., 2017). Exogenous ethylene induces floral organ abscission and wilting and negatively affects the display quality of cut roses (Muller et al., 1998; Ahmadi et al., 2009; In et al., 2017; Pouri et al., 2017; Gong et al., 2018).
The postharvest disorders in cut rose flowers are a major limitation in their ornamental value (Rihn et al., 2014). In order to prolong vase life of cut flowers, many previous studies have been conducted to reduce ethylene damage using various ethylene inhibitors, such as nitric oxide, silver thiosulfate (STS), and 1-methylcyclopropene (1-MCP), which repressed the transcript levels of ethylene biosynthesis and receptor genes (Chamani et al., 2005; Liao et al., 2013; Ichimura and Niki, 2014). Similarly, the bacteria-induced xylem blockage was prevented by using various chemical compounds containing silver ions that have antimicrobial activity to inhibit the growth of microorganism (Ichimura et al., 2008; Cumbal and Alma, 2016). Although STS is widely used as an ethylene inhibitor and antimicrobial agent, it contains a heavy metal, which is a potent environmental pollutant. Thus, other substances with similar preservative effects may be desirable. α-Aminoisobutyric acid (AIB) is an analogue of ethylene precursor 1-aminocyclopropane-1 carboxylic acid (ACC) and is able to inhibit ACO, which is a rate-limiting enzyme in the ethylene biosynthesis pathway (Satoh and Esashi, 1980, 1982). Previous studies showed that AIB retarded petal senescence and prolonged vase life in various cut flowers (Serrano et al., 1990; Shimamura et al., 1997; Onozaki et al., 1998). However, the effects of this compound on the vase life of cut rose flowers have not been studied. 1-MCP, an ethylene binding antagonist, has a higher affinity for the ethylene receptor than ethylene (Serek et al., 1995). Therefore, 1-MCP suppresses ethylene binding to the receptor and inhibits the action of downstream components in ethylene signal transduction. 1-MCP has been used to extend the postharvest longevity of flowers, potted plants, fruits, and vegetables, such as roses, carnations, delphiniums, petunias, Pelargonium peltatum (Sisler et al., 1986; Serek et al., 1995; Cameron and Reid, 2001; Serek et al., 2006; Huang et al., 2017), pears, apples, bananas, avocados, broccoli, and cucumbers (Ku and Wills, 1999; Nilsson, 2005; Watkins, 2006; Xie et al., 2017). Sodium hypochlorite (NaOCl), a chlorine-containing compound, is a strong oxidizer with broad-spectrum antimicrobial activity and works by damaging cell membranes, proteins, and nucleic acids of microbes by oxidative degradation in horticultural crops (van Doorn et al., 1990; Macnish et al., 2010b). NaOCl is an inexpensive and nonresidual chemical that is widely used to reduce bacterial and fungal contamination on fruit and vegetable surfaces, in processing equipment, and in flower vase solutions (van Doorn et al., 1990; Knee, 2000).
Previous studies revealed that the vase life of cut roses varied considerably depending on the ethylene sensitivity level of the cultivar (Macnish et al., 2010a; In et al., 2017). The vase life of SENS flowers is mostly shortened by ethylene perception, whereas that of INSENS flowers is limited by water stress. The cultivar differences in the causes of vase life termination make it difficult to use preservatives to improve postharvest quality of cut roses. Hence, the development of postharvest treatment solutions that can be applied to rose cultivars extensively is a high priority in the cut flower industry. Here, we examined the combination effect of ethylene inhibition (AIB and 1-MCP) and germicide (NaOCl) on the postharvest characteristics of the two cultivars ‘Matador’ and ‘Dolcetto’, which have a different sensitivity to ethylene. We explored effective treatments that improve the postharvest longevity by monitoring water relations, physiological characteristics, and gene expression of cut rose cultivars after exposure to ethylene.
Materials and Methods
Plant Materials
Cut roses (Rosa hybrida L.) ‘Matador’ and ‘Dolcetto’ were selected as SENS and INSENS flowers, respectively, based on previous results (In et al., 2017). Cut flowers at the commercial maturity stage (onset of outer petal reflex) were harvested from a commercial grower in Jangsu, Korea. After harvest, the cut flowers were immediately held in buckets containing tap water and then transported within 3 h to the laboratory. At the laboratory, the rose stems were recut under clean water to 40 cm and with three upper leaves. The flowers were selected for uniformity of size and color as well as freedom from any defects.
Treatments
In order to examine the combination effects of ethylene antagonists and germicides on the postharvest characteristics of cut roses, flowers were treated with the various preservatives shown in Table 1. Cut flowers held in distilled water (DW) were used as control (CON). NaOCl (Yuhanrox, Yuhan Co., Ltd., Seoul, Korea) and AIB (Sigma-Aldrich, Co., MO, USA) were diluted with DW to prepare 10 µL·L-1 and 100 µM solution, respectively. The ethylene binding inhibitor 1-MCP was applied via FreshLongTM sticks (Ecoplants Co., Ltd., Gyeonggi-do, Korea) in the treatment chamber at 25 ± 2°C under dark conditions. To generate 1 µL·L-1 1-MCP gas, the sticks were broken 4 times and immediately placed in the treatment chamber. Air circulation in the chambers was provided by a small fan inside of the chamber. After 12 h of 1-MCP treatment, three flowers among fifteen flowers in each treatment were used for RNA isolation before ethylene treatment. Fresh weight and flower diameter of cut flowers were also determined before ethylene exposure.
Table 1. Preservatives, concentrations, and abbreviations used in this study 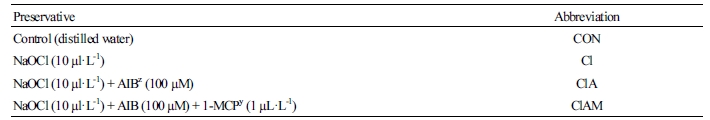 | |
zα-Aminoisobutyric acid. y1-Methylcyclopropene. | |
For ethylene treatments, cut flowers were enclosed in the treatment chambers at 25 ± 2°C. Ethylene gas was injected into the chamber to achieve a final concentration of 2 µL·L-1. Two beakers containing 100 mL of 1 M NaOH were put in the chamber to prevent the accumulation of CO2 released by respiration during the ethylene treatment. After 24 h of the treatment, six flowers among twelve flowers per treatment were held under control conditions at 25°C, 50 % relative humidity, and 30 µmol·m-2·s-1 fluorescent lighting for 12 h for vase life evaluation. The remaining six flowers in each treatment were used for RNA extraction and chlorophyll fluorescence detection after ethylene treatment.
RNA Extraction and cDNA Synthesis
For RNA extraction, outermost petals were taken from cut rose flowers before ethylene treatment at day 0 and after ethylene treatment at day 4. The detached petals were immediately frozen in liquid nitrogen and stored at -80°C for RNA extraction. Two petals (~ 300 mg) were ground with liquid nitrogen into a fine powder using a prechilled mortar and pestle. The total RNA was extracted from petals using the GeneJET plant RNA Purification Mini Kit (Thermo Fisher Scientific Baltics UBA V.A. Graiciuno 8, LT-02241 Vilnius, Lithuania) with slight modifications to the manufacturer’s procedure. The concentration of RNA was determined at 260 nm/280 nm using a NanoDrop spectrophotometer (NanoDrop One, Thermo Fisher Sci., Madison, USA). Purified total RNA was treated with sterile water to 9.5 µL in the RNase-free tube and used for the first strand of cDNA synthesis using Power cDNA Synthesis Kit (INTRON Biotechnology, Inc., Seongnam, Korea). Oligo (dT)15 primer (1 µL) was added and mixed with 9.5 µL of sterile water and 0.1 µg of total RNA. The mixture was incubated at 75°C for 5 min and quickly cooled for at least 1 min. The following reagents were then added in the following order and mixed gently: RNase inhibitor, 5x RT buffer, dNTP, DTT, and AMV RT enzyme in a final volume of 20 µL. The reverse reaction was performed in a SimpliAmp Thermal Cycler machine (AB Applied Biosystems, Singapore) for 60 min at 42°C followed by 5 min at 70°C to terminate the reaction.
Quantitative Real-Time PCR (qRT-PCR)
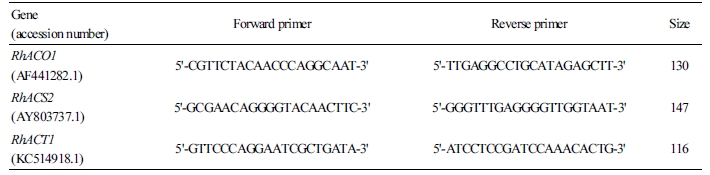
The sequences of primers using in this study were listed in Table 2. The gene-specific primers for the ethylene biosynthesis genes were designed by Primer 3 and synthesized by Cosmogenetech (Seoul, Korea). Rosa hybrida actin (RhACT1) was used as an internal control. qRT-PCR was conducted using the StepOnePlusTM real-time PCR system (Applied Biosystems, CA, USA). The reaction mixture for qRT-PCR analysis included 1 µL of cDNA as template, 2 µL of 0.5 µM forward and reverse primers, and 10 µL of 2X Maxima SYBR Green/ROX qPCR Master Mix (Applied Biosystems, CA, USA) in a final volume of 20 µL dispensed in an optical 96-well plate. The qRT-PCR reaction was conducted following the fast thermal cycles: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. The th잉reshold cycle (Ct) values were automatically measured for each reaction by the qRT-PCR system with default parameters. The final Ct values were calculated from three independent biological replications, and the coefficient of variance (CV) was also detected for each gene. The relative expression of the genes was calculated as the absolute integrated absorbency normalized to the relative actin.
Evaluation of Vase Life and Senescence Symptoms
The treatment effects in cut roses were evaluated by measuring changes in vase solution uptake, relative fresh weight, and flower diameter daily at 10:00 am. The flower diameter was assessed by measuring the largest diameter of an individual flower and the diameter perpendicular to it using calipers (CD-20APX, Mitutoyo Corporation, Kanagawa, Japan). Water balance was measured by deducting transpiration from solution uptake daily. To determine water stress status of cut flowers, chlorophyll fluorescence parameters in the leaves were analyzed with an imaging fluorometer (FluorCam 700MF, Photon Systems Instruments, Brno, Czech) with the following light sources: measuring flash, actinic 1, actinic 2, and saturating pulse. Minimal fluorescence (F₀) was measured in 30 min dark-adapted leaves, and maximal fluorescence (Fm) was also measured in the same leaves in full light-adapted conditions. Maximal variable fluorescence in dark-adapted states (Fv = Fm - F₀) and the maximal quantum yield of dark-adapted PSII (Fv/Fm) were automatically calculated from measured parameters. The water stress status of cut roses was reflected in the Fv/Fm values.
In order to evaluate the bacterial contamination at the base of the rose stems, the total microbial population was determined after 4 days of ethylene treatment. The microbial counts were performed using swab samples taken from the basal 2 cm of the cut ends. Each sample was diluted in 10 mL of sodium chloride buffer (3M Pipette Swab; 3M Health Care, St. Paul, MN, USA), and 1 mL of each diluted sample was poured into aerobic count plates (Petrifilm 6400; 3M Health Care, St. Paul, MN, USA). After 2 days of incubation at 37°C, the microbial count was measured by counting the number of colonies generated on the plates.
The vase life of cut roses was evaluated as the time from the placement of cut flowers in the vase in the environmentally controlled room after ethylene treatment to the end of vase life. Vase life was considered ended when the cut flowers showed at least one of following senescence symptoms: bluing (≥50% blue petals), petal abscission (≥3 petal drop), and wilting of petals (≥50% petal turgor loss).
Experimental Design and Data Analysis
Vase life experiments were arranged as a completely randomized design with six replicates for each treatment. Chlorophyll fluorescence and qRT-PCR analysis were performed with three biological replicates. Data were presented as mean ± standard error (SE). One-way analysis of variance (ANOVA) was carried out using SPSS 16.0 (IBM, Somers, NY, USA). When significant effects were detected, post-hoc pairwise comparisons of group means were performed by Duncan's multiple range test, with a significance level of p ˂ 0.05.
Results
Vase Life and Senescence
The vase life of cut roses ‘Matador’ and ‘Dolcetto’ was significantly prolonged by treatment with Cl, ClA, and ClAM compared with that of control flowers (Fig. 1A and B). Flowers treated with ClAM showed the longest vase life (9 d in ‘Matador’ and 14.3 d in ‘Dolcetto’), followed by those treated with ClA and Cl.
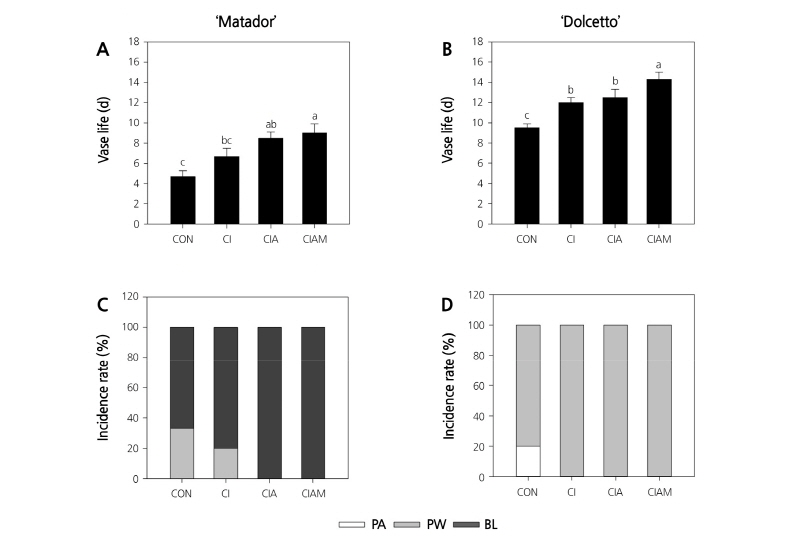
Fig. 1. Changes in vase life and senescence symptoms in ‘Matador’ (A and C) and ‘Dolcetto’ (B and D) cut roses. Cut flowers held in vases containing DW or preservatives were subsequently incubated in chambers with 2 µL·L-1 of ethylene for 24 h at 25°C. CON, control (DW); Cl, NaOCl; ClA, NaOCl + AIB; ClAM, NaOCl + AIB + 1-MCP; PA, petal abscission; PW, petal wilting; BL, bluing. Different letters (a-c) among treatments indicate statistically significant differences at p < 0.05 based on Duncan’s multiple range test. Vertical bars represent standard errors of the mean (n = 6).
The primary cause of vase life termination was bluing (67%) and petal wilting (33%) in ‘Matador’, whereas it was wilting (80%) and abscission (20%) of petals in ‘Dolcetto’. Interestingly, treatment with the ethylene inhibitors AIB and 1-MCP completely decreased petal wilting in ‘Matador’ and petal abscission in ‘Dolcetto’ (Fig. 1C and D).
Relative Fresh Weight and Flower Opening
Fresh weight (FW) of control flowers in both cultivars reached a maximum at day 3 and subsequently decreased thereafter (Fig. 2A and B). The reduction of FW during the vase period was effectively prevented by preservative treatments in both cultivars. The maintenance time of initial FW was extended by 2 - 4 days by ClAM compared with control (Fig. 2A and B).
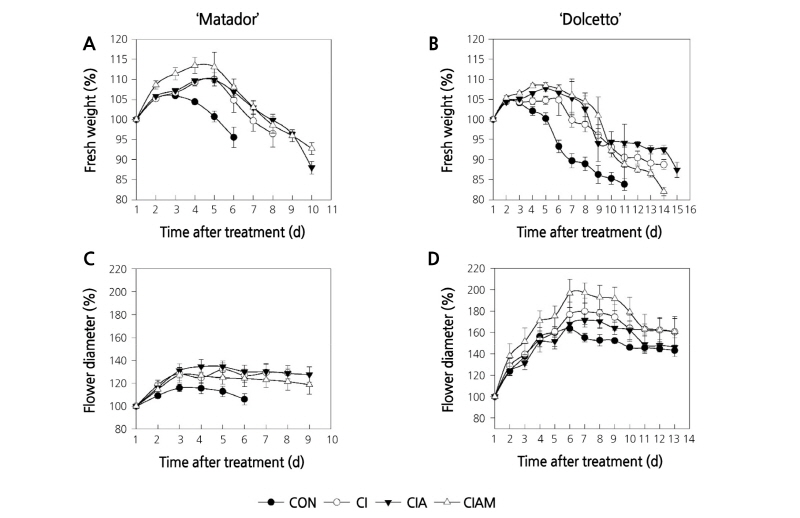
Fig. 2. Changes in fresh weight and maximum flower diameter in ‘Matador’ (A and C) and ‘Dolcetto’ (B and D) cut roses. CON, control (DW); Cl, NaOCl; ClA, NaOCl + AIB; ClAM, NaOCl + AIB + 1-MCP. Cut flowers held in vases containing DW or preservatives were subsequently incubated in chambers with 2 µL·L-1 of ethylene for 24 h at 25°C. Vertical bars represent standard errors of the mean (n = 6).
Changes in flower diameter (FD) of cut flowers mirrored the changes in FW. Treatments with Cl, ClA, and ClAM increased FD of cut roses in the both cultivars, compared with control groups. The FD of cut flowers during the vase period increased the most by ClA in ‘Matador’, but it increased the most by ClAM in ‘Dolcetto’ (Fig. 2C and D).
Antimicrobial Activity and Water Relations
The total bacterial population at the base of the cut stems in the both cultivars was reduced by all treatments. ClAM significantly inhibited the bacterial proliferation after 4 days of ethylene treatment (p < 0.05) (Fig. 3A and B).
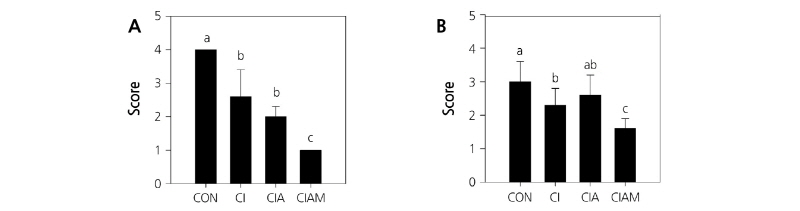
Fig. 3. Effects of preservative treatments on the bacterial population at the base of the cut stems in ‘Matador’ (A) and ‘Dolcetto’ (B) cut roses after 4 days of treatment. CON, control (DW); Cl, NaOCl; ClA, NaOCl + AIB; ClAM, NaOCl + AIB + 1-MCP. Cut flowers held in vases containing DW or preservatives were subsequently incubated in chambers with 2 µL·L-1 of ethylene for 24 h at 25°C. Score: (1) colony; (2) 1 to <10 colonies; (3) 10 to 50 colonies; and (4) >50 colonies. Different letters (a-c) among treatments indicate statistically significant differences at p < 0.05 based on Duncan’s multiple range test. Vertical bars represent standard errors of the mean (n = 6).
Treatment with preservatives improved solution uptake during vase life of cut roses ‘Matador’ and ‘Dolcetto’ (Fig. 4A and B). Among the preservative treatments, ClAM effectively improved solution uptake (p < 0.05). Cut rose flowers of the both cultivars treated with ClAM showed the highest solution uptake at day 3, whereas control flowers showed lowest solution uptake (Fig. 4A and B).
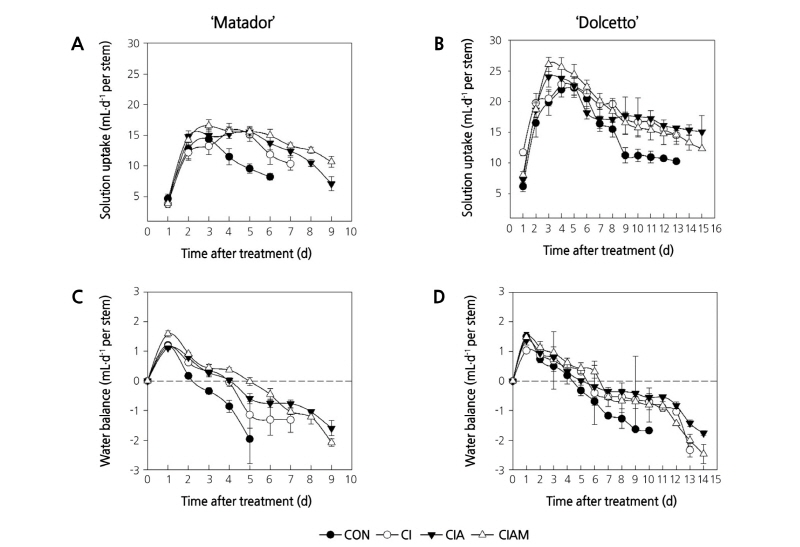
Fig. 4. Changes in vase solution uptake and water balance in ‘Matador’ (A and C) and ‘Dolcetto’ (B and D) cut roses. CON, control (DW); Cl, NaOCl; ClA, NaOCl + AIB; ClAM, NaOCl + AIB + 1-MCP. Cut flowers held in vases containing DW or preservatives were subsequently incubated in chambers with 2 µL·L-1 of ethylene for 24 h at 25°C. Vertical bars represent standard errors of the mean (n = 6).
Water balance (WB) of cut roses was determined by the difference between water loss from leaves and water uptake by stems. ClAM greatly increased the time that flowers retained a positive WB by 3 d in ‘Matador’ and 2 d in ‘Dolcetto’ compared with control flowers (Fig. 4C and D). Cl and ClA treatments also increased the time that flowers retained a positive WB than control flowers (Fig. 4C and D).
Leaf Chlorophyll Fluorescence
Water stress of cut flowers generally leads to wilting of petals and leaves, and the plants emit a decreased chlorophyll fluorescence (CF) ratio in response to the stress conditions. To evaluate the effects of preservatives on water stress status of cut roses, the CF ratio (Fv/Fm) were measured in leaves of cut flowers. Fv/Fm in the leaves was most reduced in control in response to ethylene, whereas the reduction in Fv/Fm was significantly prevented by the treatment with Cl, ClA, and ClAM in the both cultivars (Fig. 5A and B). The results also showed that Fv/Fm was the highest in the flowers treated with ClAM. Meanwhile, control flowers showed considerable wilting and senescence in accordance with the reduction in Fv/Fm at day 4 in ‘Matador’ and at day 9 in ‘Dolcetto’ (Fig. 5A-D), indicating that the CF ratio may be closely related to flower wilting.
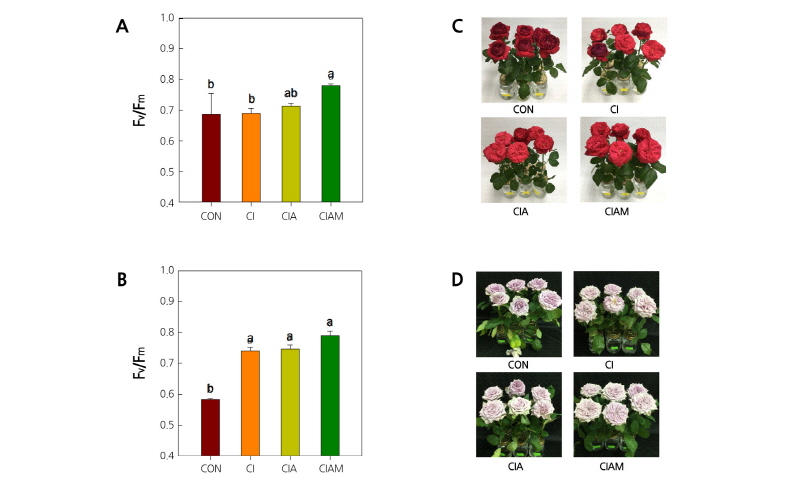
Fig. 5. Changes in chlorophyll fluorescence ratio (Fv/Fm) and photographs of the cut roses ‘Matador’ at day 4 (A and C) and ‘Dolcetto’ at day 9 (B and D). CON, control (DW); Cl, NaOCl; ClA, NaOCl + AIB; ClAM, NaOCl + AIB + 1-MCP. Cut flowers held in vases containing DW or preservatives were subsequently incubated in chambers with 2 µL·L-1 of ethylene for 24 h at 25°C. Different letters (a-b) among treatments indicate statistically significant differences at p < 0.05 based on Duncan’s multiple range test. Vertical bars represent standard errors of the mean (n = 3).
Expression Pattern of Ethylene Biosynthesis Genes
Previously, we found that among ACS members, RhACS2 showed a similar expression pattern with RhACO1 in response to ethylene and during flower senescence (In et al., 2017), indicating that RhACS2 is possibly involved in autocatalytic synthesis of ethylene during climacteric phase of flowers. In this study, the expression of RhACS2 and RhACO1 genes was detected before ethylene treatment at day 0 and after ethylene treatment at day 4 to examine the effects of preservative solutions on ethylene biosynthesis in cut roses.
Generally, the expression levels of RhACS2 and RhACO1 in control flowers greatly increased after ethylene exposure; however, the ethylene-inducible increase of the genes was suppressed by ClA and ClAM in both cultivars (Fig. 6A-D). Interestingly, ClAM most strongly inhibited the ethylene-inducible increase of RhACS2 and RhACO1 in both rose cultivars, suggesting that inhibition of ethylene synthesis simultaneously with ethylene action is more effective in preventing flower response to ethylene.
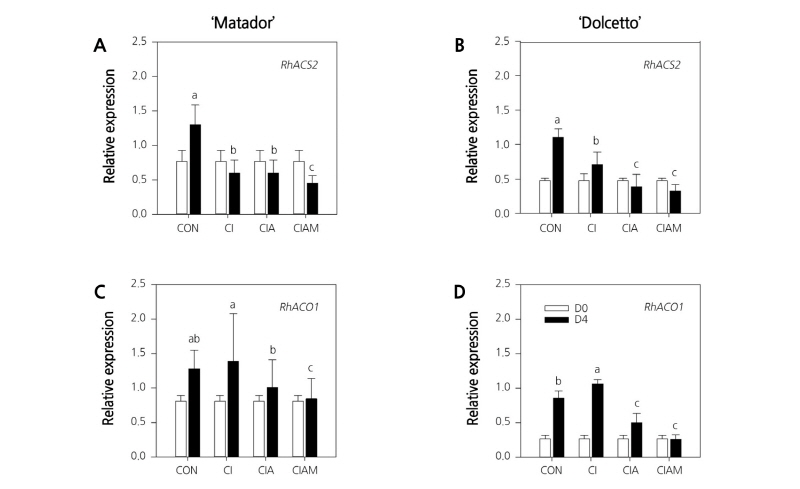
Fig. 6. Expression patterns of RhACS2 and RhACO1 genes in ‘Matador’ (A and C) and ‘Dolcetto’ (B and D) cut roses. CON, control (DW); Cl, NaOCl; ClA, NaOCl + AIB; ClAM, NaOCl + AIB + 1-MCP. Cut flowers held in vases containing DW or preservatives were subsequently incubated in chambers with 2 µL·L-1 of ethylene for 24 h at 25°C. Expression of RhACS2 and RhACO1 was detected before ethylene treatment at day 0 (D0) and after ethylene treatment at day 4 (D4). Different letters (a-c) among treatments indicate statistically significant differences at p < 0.05 based on Duncan’s multiple range test. Vertical bars represent standard errors of the mean (n = 3).
Discussion
The longevity of cut flowers is determined by multiple factors, including ethylene and water deficit. In cut rose flowers, ethylene induces abscission and wilting in leaves and petals, leaf yellowing, and flower opening (Macnish et al., 2000; Macnish et al., 2010a). The water deficit in cut flowers also often occurred in an early stage of flower development during the postharvest period (Mayak et al., 1974). Therefore, the most consistent ways to extend the vase life of cut flowers is through ethylene control and improvement of water relations using preservative solutions.
This study revealed that preservative solutions had significant effects not only on vase life but also on the postharvest quality of cut roses. Our results showed that ClAM effectively extended the vase life by 4.3 d (202 %) in ethylene-sensitive flowers (‘Matador’) and 4.8 d (150 %) in ethylene-insensitive flowers (‘Dolcetto’) compared with control groups. The considerable extension of the vase life in ClAM flowers presumably resulted from the combination effects of improving water relations and inhibiting ethylene damage.
It is well known that bacterial proliferation in vase solution leads to xylem plugging, consequently impairing water flux in the stems and resulting in a short vase life of cut flowers (van Doorn et al., 1990). In the present study, ClAM effectively inhibited bacterial growth after 4 days of treatment. This could be explained by the role of NaOCl as an antimicrobial agent. NaOCl is used in the cut flower industry to suppress the growth of bacteria in vase solutions (van Doorn et al., 1990; Joyce et al., 1996). Treatment with ClAM increased solution uptake, resulting in a relatively longer retention period of positive water balance and initial fresh weight in both rose cultivars compared with other preservative solutions. This observation is consistent with a previous study showing that treatment of cut flowers with NaOCl allowed water absorption in flower tissues and reduced water loss, resulting in increased longevity of cut rose flowers (Tilahun et al., 2015). Water absorption from the preservative solution helps maintain a longer positive water balance and fresh weight and protects the flowers from early wilting, resulting in prolonged vase life.
In the present study, the vase life of control flowers rapidly decreased due to an early failure of water relations after exposure solely to ethylene. The CF ratio is often used for evaluating physiological characteristics and water stress conditions in cut flowers (Baker and Rosenqvist, 2004; Pompodakis et al., 2005; Calatayud et al., 2006). In this work, ClAM effectively inhibited the Fv/Fm reduction in leaves and water stress of cut roses. Ethylene often accelerates the chlorophyll degradation and Fv/Fm reduction in leaves (Ferrante et al., 2012). 1-MCP delays chlorophyll degradation by inhibiting ethylene production and suppressing chlorophyll degradation pathway-related genes, which results in high chlorophyll content and an effective maintenance of Fv/Fm ratio in plants (Blankenship and Dole, 2003; Cheng et al., 2012).
Petal abscission is associated with leaf abscission, which is caused by ethylene production in cut flowers. Here, ClAM treatment effectively prevented the petal abscission that occurred during the vase life of ‘Dolcetto’ cut roses. Ethylene biosynthesis and action inhibitors are effective in delaying senescence of many flowers (Serek et al., 2006). RhACS2 and RhACO1 transcripts were correlated with flower senescence in cut roses and were up-regulated by treatment with ethylene (In et al., 2017). In this study, the expression levels of RhACS2 and RhACO1 were increased in control flowers after ethylene treatment. ClAM treatment effectively inhibited the accumulation of RhACS2 and RhACO1 transcripts in rose petals and consequently delayed flower senescence in the both rose cultivars. This result is consistent with previous studies showing that treatment of cut flowers with 1-MCP or AIB combined with sucrose interrupted the senescence process in cut flowers (Shimamura et al., 1997; Daneshi Nergi and Ahmadi, 2014). Since AIB has been shown to inhibit ethylene synthesis by inhibiting ACS activity (Satoh and Esashi, 1980, 1982), the effect of AIB on flower senescence is probably attributed to the inhibition of ethylene production. 1-MCP, on the other hand, binds to the ethylene receptors, blocks the ethylene signal, and consequently suppresses ethylene responses in cut flowers (Sisler and Serek, 1997). The results from the present study revealed that inhibition of ethylene synthesis simultaneously with ethylene action using AIB and 1-MCP effectively prevents ethylene damage and thereby improves the postharvest quality of cut flowers.
Overall, the treatment with ClAM most effectively prolonged the vase life and improved the water relations in the ethylene-sensitive and ethylene-insensitive cultivars. The efficacy of ClAM on postharvest quality of cut roses could be explained by its role in inhibiting proliferation of bacteria, sustaining good solution uptake, and suppressing ethylene responses in flowers.