Introduction
Materials and Methods
Plant material and cultural conditions
Chlorella treatment
Growth measurements and analysis
Analysis of physiologically active compounds
Statistical Analysis
Results and Discussion
Leaf number
SPAD
Fresh weight and ratio of dry weight
Brix
Internal and external fruit hardness
Total polyphenol and free sugar content
Introduction
Melons are rich in vitamins C, A, and carotenoids and have a sweet taste and scent. The domestic cultivation area of melons has been steadily increasing since the mid-1990s (RDA, 2018). Since 2002, melons have been designated as an export crop, and are exported not only to nearby Asian countries, such as Taiwan and Japan, but also to Europe, the United States, and Central and South America (Youn et al., 2009). Furthermore, the consumer trend toward choosing safe and high-quality fruits (Hong, 2009) has increased the demand for high-quality fruits such as melons, both domestically and internationally (Lee et al., 2020). Currently, most farmers use chemical pesticides to increase the yield and quality, and to control plant diseases, pests, and weeds for melon cultivation. However, there is significant concern that chemical components such as pesticide residues and environmental pollution may negatively impact consumer health and environmental protection. Therefore, organic farming, pesticide-free cultivation, good agricultural practices (GAP), natural enemy utilization, and eco-friendly fertilizers have been used to address these issues in Korea(Siddiqui et al., 2020; Yi et al., 2020). In particular, many farms use chlorella as an biofertilizer instead of chemical fertilizer. chlorella is a freshwater green alga that performs photosynthesis and is a type of plankton and microalga. It can be easily cultured and has simple fertilization methods with no side effects depending on its concentration (RDA, 2016). It contains various nutrients, such as proteins, fats, carbohydrates, and minerals, and is nutritionally superior (Kim, 2004). It is also a healthy food ingredient (Jeong et al., 2006). In addition, chlorella has been reported to boost the immune system (Hasegawa et al., 1997; Hasegawa et al., 1999), exhibit anticancer effects (Umezawa et al., 1982; Morimoto et al., 1995; Morita and Matsueda, 1999), reduce blood pressure (Wang et al., 1981), and detoxify heavy metals (Hagino, 1975; Nagano et al., 1978; Young and Beregi, 1980). In domestic agriculture, it is used as a biofertilizer to increase yield, improve quality, and prevent pests and diseases in several crops, such as cucumbers, peppers, scallions, lettuce, bean sprouts, and strawberries (RDA, 2018). Although many farms use chlorella as a biofertilizer and report positive effects on crops, its full potential for agricultural utilization remains limited, and the related studies on the topic are scarce. Moreover, no specific studies have reported on the appropriate concentration and timing of treatment for crops. Therefore, experiments were conducted to investigate the most effective concentration of chlorella for the optimal growth of melons and the maintenance of fruit quality after harvest.
Materials and Methods
Plant material and cultural conditions
The experiment was conducted on a melon farm inside a 661 m2 greenhouse located at 112-3 Susan-ri, Dopomyeon, Yeongam-gun, Jeollanam-do, South Korea (34.94N, 126.67E). The plant material used in the experiment was a Bose cultivar (PMR Neo Bose, Daenong Seed Ltd , Korea) planted on August 5th, 2020 and the planting distance was set at 25 × 35 cm, and the melons were trained with 1 stem per plant. The cultivation was conducted soil cultivation according to the standard cultivation methods provided by the Rural Development Administration (RDA, 2017).
Chlorella treatment
The chlorella species used in this study was chlorella fusca (CHK0059), supplied by the Rural Development Administration. chlorella fusca, classified as a microalgae, has been reclassified under the genus Scenedesmus, and it has been reported that its genetic, physiological, and biochemical characteristics align with those of Scenedesmus species (Kessler et al., 1996). It was cultured at the Yeongam Agricultural Technology Center using the method described by Kim et al. (2014). To determine the concentration of chlorella after cultivation, we measured the absorbance at 680 nm using a spectrophotometer (UV-vis Spectrophotometer 1201, Shimadzu, Kyoto, Japan) and calculated the number of chlorella cells using a hemocytometer (Hausser Scientific, Horsham, PA, USA). The chlorella stock solution used as biofertilizer for melons was 1 × 107 cells mL-1 and was diluted 0 (Control), 250, 500, and 1,000 times for the treatments. For chlorella foliar application, the prepared suspensions of 0, 250, 500, and 1,000 times were sprayed onto all of the leaves using a spray, separately for each treatment group. The foliar application of chlorella was performed six times, with 5-day intervals, between August 15th to September 10th. It continued until both sides of all leaves on the plant were sufficiently wetted with the chlorella culture solution. All treatment groups consisted of ten selected areas, with five melon plants planted in each area. Seven weeks after transplantation, the melons were harvested, and immediate growth and physiologically active substance analysis were conducted.
Growth measurements and analysis
The number and SPAD values of leaves were measured five times at 7-day intervals starting from August 22nd after transplanting on August 5th. The number of leaves and SPAD of melons according to the chlorella application concentration were investigated five times, every alternate week, between August 22nd to September 19th. The SPAD was measured using a chlorophyll meter (SPAD 502 PLUS, Konica Minolta Optics, Tokyo, Japan) for both the upper and lower leaves. Upper and lower leaves were defined as the third leaf from the top and the third leaf from the bottom of the plant, respectively. After harvesting all the fruits on September 22nd, the fresh weight of fruit was measured, and then the average weight per fruit was calculated. The dry weight was measured after drying for 15 days in a 65-degree dryer. The dry weight percentage was calculated by dividing the dry weight by the fresh weight and expressing it as a percentage. The measurements of fresh weight were repeated 50 times, while the measurements of dry weight were repeated 15 times in the experiment. Brix values and hardness were measured for 45 fruits stored at room temperature after harvest. The Brix values were measured at 2-day intervals, with five randomly selected fruits being divided in half, and each portion was measured twice in a destructive manner. Additionally, the Brix values were measured using a refractometer (PAL-1, Atago, Tokyo, Japan). To measure the hardness, five fruits were selected at a time from the fruits in storage, and measurements were taken at intervals of 3 to 4 days. It was investigated destructively using a hardness tester (CT3 texture analyzer, Brookfield, Middleboro, USA) by applying a pressure of 1 cm at a speed of 1 mm∙s⁻¹, and the peak load values were recorded.
Analysis of physiologically active compounds
The polyphenol and free sugar content of four fruits stored at room temperature for 3 days after harvest were measured once. To measure the total polyphenol content of the fruit, fruit flesh was freeze-dried and ground into powder using a mortar and pestle. The procedure involved mixing 10 mg of the powder with 0.5 mL of distilled water and 125 µl of Follin–Ciocalteu reagent, then adding 1.2 mL of a 7% carbonate solution and thoroughly mixing the resulting mixture. The total volume was adjusted to 3 mL using distilled water for quantification. The mixture was left at room temperature for 90 minute, and the absorbance was measured at 760 nm using a UV-Vis Spectrophotometer (UV-Vis Spectrophotometer 1201, Shimadzu, Kyoto, Japan). To measure the free sugar content of the fruit, a suspension was prepared by grinding 10 g of fresh fruit with 15 mL of distilled water. Thereafter, the suspension was centrifuged at 15,000 rpm for 4oC, and the supernatant was filtered through a filter paper (Filter Paper Qualitative No.2, Advantec, Tokyo, Japan). The extract obtained through the filter paper was further filtered using a membrane filter (SARTORIUS, Sartolab 0.45 µm, Göttingen, Germany) and analyzed using High performance liquid chromatography (HPLC).
Statistical Analysis
The growth data for nine weeks in each group of 10 repetitions were analyzed using one-way ANOVA with Duncan’s post-hoc test at p < 0.05 using SPSS ver.27 (SPSS Institute Inc., USA). The data were graphically represented using Sigmaplot 12.0 (Systat Software, Palo Alto, USA).
Results and Discussion
Leaf number
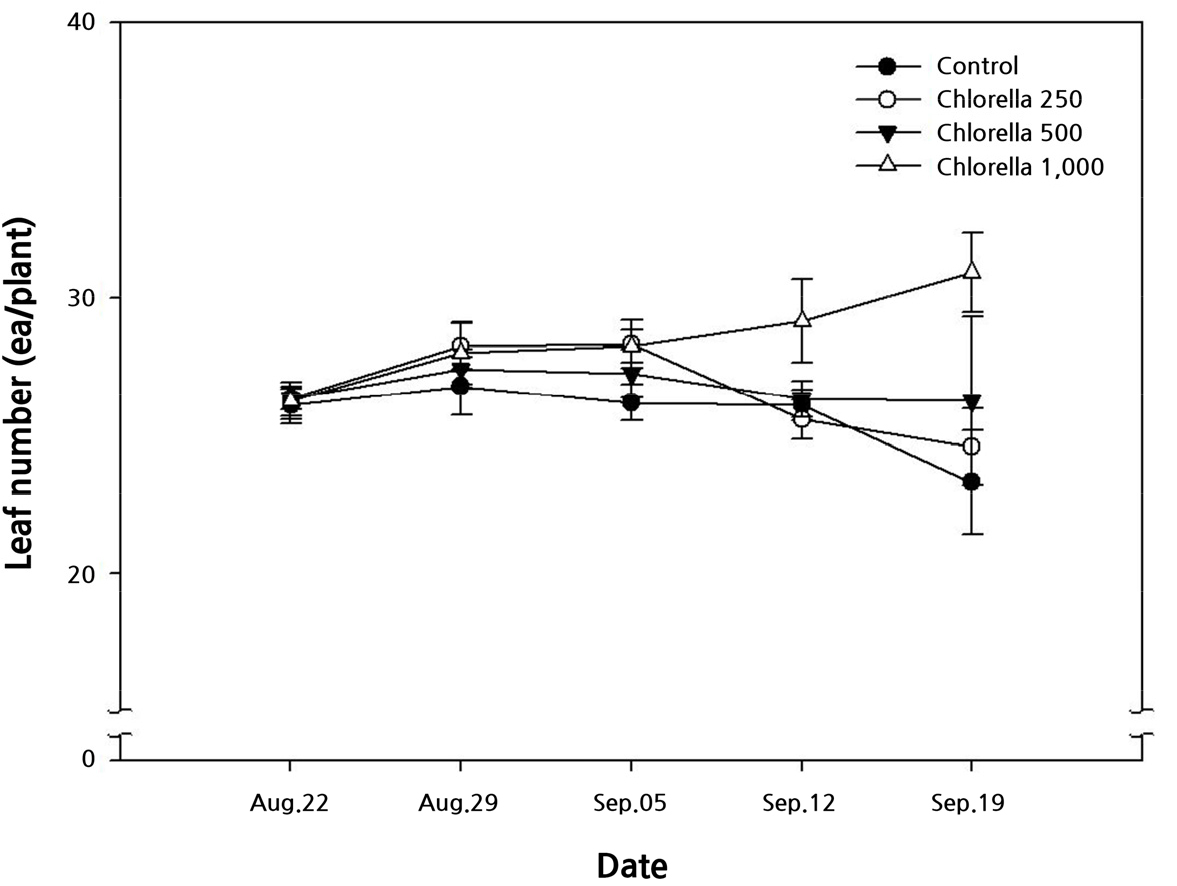
The investigation of leaf number according to the chlorella fertilization concentration revealed that the number of leaves in plants treated with chlorella foliar application was greater than that in those not treated with chlorella, regardless of the concentration of chlorella (Fig. 1). During the early growth stage, there was no difference in the number of leaves according to the chlorella concentration. However, starting on September 12th, the group of 0 (control), 250 and 500 times diluted chlorella solution showed a decrease, whereas the 1,000 times solution group showed an increase. On the final measurement day, September 19th, the number of leaves in the 0 (control), 250, 500, 1,000 times diluted chlorella solution groups was 23.3, 24.6, 26.3, and 30.9, respectively, with the 1,000 times solution group showing the highest number of leaves (Fig. 1). Therefore, it can be interpreted that the plants treated with 1,000 times diluted chlorella biofertilizer showed suppression of leaf drop compared with the other treatment groups. It has been reported that the number of leaves were higher in Italian ryegrass (Seo et al., 2016) and spinach (Kim et al., 2018) treated with chlorella than in the control group. Regarding the chlorophyll, the major pigment in chlorella, and its water-soluble derivatives, Na and Cu of chlorophyll, have been reported to exhibit anti-inflammatory, anti-mutagenic, anti-cancer, and antioxidant properties (Boloor et al., 2000; Cho et al., 2000; Hsu et al., 2005; Kim et al., 2014). Therefore, in this study as well, the number of leaves in plants treated with chlorella was higher than that in the control group. This effect on defoliation suppression is attributed to the antioxidant properties of chlorella.
SPAD
The SPAD of the upper leaves in the melon did not show a significant difference among all experimental groups, except on August 22, and showed irregular changes (Fig. 2). The SPAD of the lower leaves did not differ significantly among the treatment groups during the early growth stages. However, 10 days after the final foliar application of chlorella, the SPAD values in the 1,000 times diluted chlorella solution group were significantly higher than those in the 0 (control) and 500 times solution groups. In particular, the group treated with 1,000 times diluted chlorella solution showed the highest SPAD among all the chlorella-treated groups, with a value of 52.4 (Fig. 2). These results showed a pattern similar to the increase in leaf number as growth progressed, suggesting that the SPAD may also be related to leaf development and the prevention of leaf fall. Studies have reported that the SPAD was higher in ‘Seolhyang’ strawberries (Kim et al., 2020) and Italian ryegrass (Seo et al., 2016) treated with chlorella foliar application compared to the control group.
Fresh weight and ratio of dry weight
The fresh weight of melons fruits was 1.6, 1.8, 1.8, and 1.8 kg at the groups of treated 0 (control), 250, 500, and 1,000 times diluted chlorella solution, respectively (Figs. 3 and 5). The fruits in the 0 (control) times diluted chlorella solution group had the lowest fresh weight compared to the chlorella-treated groups, but there was no significant difference. It has been reported that when chlorella was applied to bean sprouts (Kim et al., 2015), Chinese chives, and spinach (Kim et al., 2018), the groups treated with chlorella showed higher biomass compared to the control group. The chlorella biofertilizer experiment on melons showed a trend similar to the above results. The ratio of dry weight to fresh weight in melons was investigated, and the results showed that the ratios in the groups treated with 0 (control), 500, and 1,000 times diluted chlorella solution were 8.7%, 9.5%, and 8.4%, respectively (Fig. 4). The group treated with 250 times diluted chlorella solution showed the highest ratio of 11.02% (Fig. 4). Assimilates generated through photosynthesis are directly linked to the increase in plant fresh weight and, from the perspective of assimilates balance, lead to dry matter formation (Geelen et al., 2016). Therefore, the plants treated with 250 times diluted chlorella solution accumulated the most photosynthetic products in their fruits. These results can be interpreted to mean that chlorella treatment enhances photosynthetic activity and induces greater assimilates in fruits. It has been reported that the dry weight in the chlorella-treated group increased by 19% compared to that in the control group in perilla leaf cultivation (Ann et al., 2020). Therefore, the results of this study are similar to those of previous studies. However, the dry-to-fresh weight ratio tended to decrease when high concentrations of chlorella were applied.
Brix
The Brix of the melon fruits showed a sharp decrease in all treatment groups starting from the 6th day of storage. In addition, after 12 days of storage, the control group had a lower Brix value than the chlorella-treated groups (Fig. 6). Similar to the results of this study, previous research had reported that the strawberry cultivars ‘YukBo’, ‘Seolhyang’ showed an increase in brix compared to the control when chlorella was used for foliar application (Kim et al., 2014). A study reported that the utilization efficiency of induced potassium (K) and phosphorus (P) in the roots increased due to chlorella fertilization, resulting in the accumulation of phosphorus in the fruits. This led to changes in the nutrient status and improved the Brix of the fruits(Kim et al., 2022). Therefore, it can be inferred that chlorella fertilization may improve the Brix of melons.
Internal and external fruit hardness
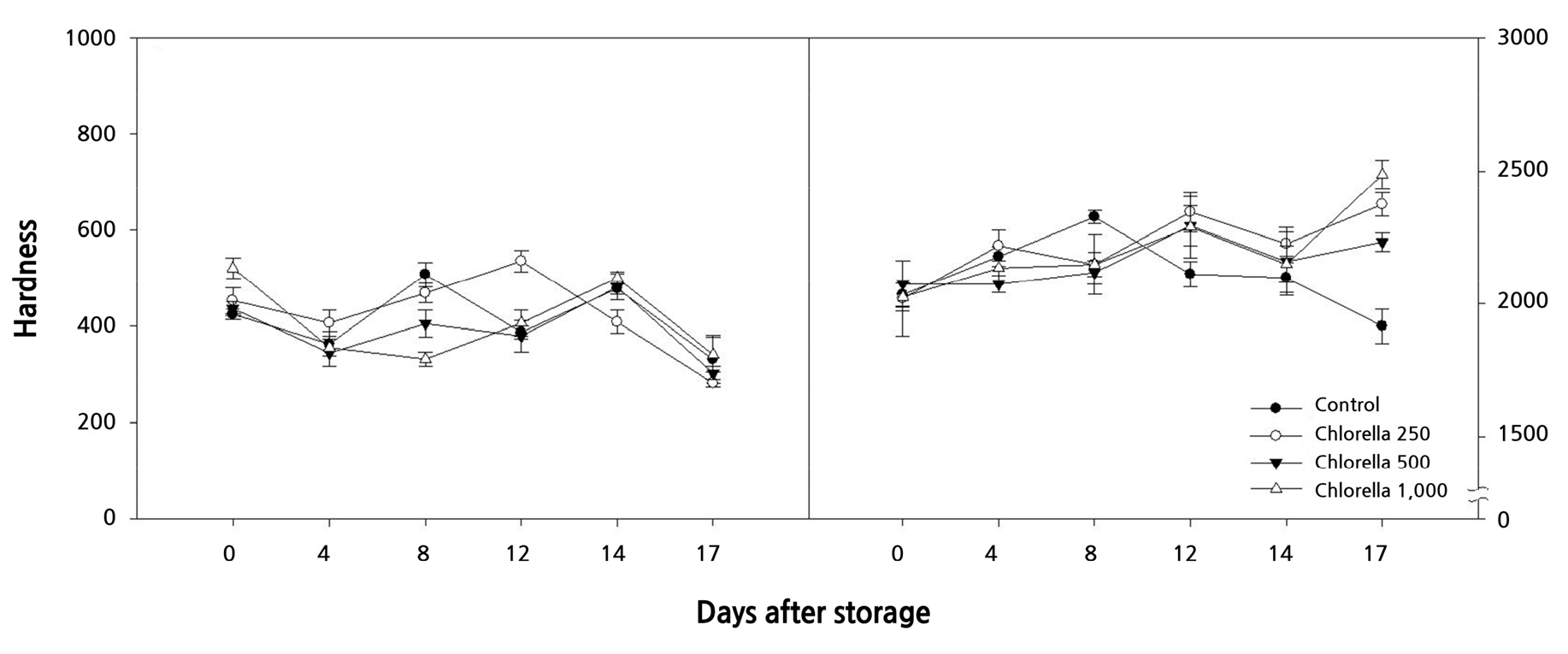
The internal hardness of melons showed irregular changes from immediately after harvest to 12 days of storage overall, it decreased from 14 days of storage onward. On the 17th day of storage, there was no significant difference in the internal hardness among the control group and the groups treated with 250, 500, and 1,000 times diluted chlorella solution fertilizers, with values of 331.2, 282.5, 302, and 341.1 g (Fig. 7A). However, the group treated with 1,000 times diluted chlorella solution showed the highest value. The external hardness of melons in the control group showed a continuous decrease from day 8 to 17 of storage. On the 17th day, it was significantly lower than that in the chlorella-treated groups (Fig. 7B). In contrast, the groups treated with chlorella showed an increasing trend in external hardness. In particular, on day 17 of storage, the external hardness in the group treated with 1,000 times diluted chlorella solution was the highest at 2485.9 g, compared to the other treatment groups (Fig. 7B). When chlorella was used as a biofertilizer for ‘Geumsil’ strawberry cultivation, it was reported that the hardness of strawberries in the chlorella-treated group was much higher than that of the control group (Kim et al., 2022). Furthermore, melon hardness has been reported to be an important factor in extending post-harvest storage life (Nishiyama et al., 2007) and can be used as a quality index to determine freshness (Kim et al., 2011). Therefore, it is expected that chlorella biofertilizer can improve the hardness of melons and extend their shelf life.
Total polyphenol and free sugar content
The total polyphenol content of melon in the groups treated with 0 (control), 250, 500, and 1,000 times diluted chlorella solution were 0.87, 1.01, 1.28, and 1.03 µg·mg-1, respectively (Table 1). All chlorella-treated groups showed higher polyphenol content than the control group. The sucrose, glucose, and fructose levels of melon fruits in the chlorella-treated groups were higher than in the control group, except for the 1,000 times diluted chlorella solution group. These levels in the 250 times diluted chlorella solution group were the highest at 39.35, 23.26, and 19.64 µg·mg-1, respectively (Table 1). Previous studies have reported that applying chlorella as a biofertilizer can increase bean sprouts’ growth and antioxidant capacity (Kim et al., 2015). Phototrophic microorganisms contain trace elements and substances such as plant growth-promoting substances such as phytohormones, vitamins, carotenoids, amino acids, and antifungal substances (Spolaore et al., 2006). Due to these characteristics, phototrophic microorganisms have been reported to not only increase plant growth and crop yield but also enhance the sugar and carotenoid content in tomatoes (Kumari et al., 2011). It has been reported that when photosynthetic microalgae such as Nannochloropsis oculata were applied as biofertilizer to tomatoes, the sugar content concentration in the fruits increased, resulting in improved fruit quality (Coppense et al., 2015). These results indicate that microalgae-based biofertilizers have the effect of increasing the sugar content concentration in melon fruits.
Table 1.
Total polyphenol content and total free sugar content of melon fruits according to chlorella concentration.
| Treatment |
Polyphenol content (µg·mg-1) | Free sugar content (µg·mg-1) | ||
| Sucrose | Glucose | Fructose | ||
| Controlz | 0.87 cv | 30.39 a | 20.39 bc | 17.65 a |
| 250y | 1.01 b | 39.35 a | 23.26 a | 19.64 a |
| 500x | 1.28 a | 33.67 a | 21.78 ab | 18.85 a |
| 1,000w | 1.03 b | 28.52 b | 18.88 c | 17.34 a |