Introduction
Materials and Methods
Setting up the experimental chambers
Preparation of planting materials
Measurements of the physicochemical quality attributes of the fruits
Statistical analysis
Results
Changes in fruit quality attributes in ‘Daewol’ peaches under four different day and night temperatures
Changes of red/brown color development on the flesh of ‘Daewol’ peach under four different day and night temperatures
Flesh red coloration in ‘Daewol’ peaches under four different day and night temperatures
Discussion
Effect of high day and night temperatures on fruit quality attributes in ‘Daewol’ peaches
Effect of high day and night temperatures on red/brown coloration on the flesh of ‘Daewol’ peaches
Reddish pulp disorder as a physiological disorder in ‘Daewol’ peaches
Conclusion
Introduction
Peach (Prunus persica L.) is a deciduous tree belonging to the family Rosaceae that is native to northwest China. Peaches are the third most commercially important temperate tree fruit in the world, following apples and pears, with more than 90% of their production going to the fresh market (Tilahun et al. 2022).
Climate is a major factor determining the geographic range of plants, and plant distributions will likely be significantly impacted by climate change in the upcoming decades. Because plant phenology is highly dependent on the temperature, phenological shifts have been identified as among the earliest indicators of climate change (Zhao et al. 2018). Accordingly, changing the geographic distribution of crops that are significant to the economy will influence agricultural productivity. Anticipating this effect with relevant strategies is crucial to mitigate losses, although some may be unavoidable (Vanalli et al. 2021).
A substantial rise in the average global temperature is anticipated, and on the Korean Peninsula, according to scenario RCP8.5 (RCP 8.5 represents the level of carbon concentration that results in average global warming of 8.5 watts per square meter across the planet), a 0.4°C increase in temperature is projected every decade. This trend is expected to result in a cumulative rise of 4.4°C by the year 2100, assuming a carbon dioxide concentration of 940 parts per million (ppm) (Kim et al. 2021). In several studies, high temperatures and exposure to increased levels of carbon dioxide and ozone have been linked to direct and indirect effects on the yield and quality of fresh fruits. Temperature increases affect membrane-based systems such as respiration and photosynthesis, resulting in changes in sugars, organic acids, flavonoid contents, firmness, and antioxidant activities (Moretti et al. 2010). Additionally, they cause a variety of physiological problems in fruits, including sunburn, malformations, spongy tissues, reddish pulp disorders, skin freckling, and fruit rupture, leading to quality losses (Mulholland et al. 2003).
The generally ideal temperature range for peaches to grow is between 65 and 80°F (18–27°C) (Layne and Bassi 2008). In Korea, peach fruit has recently exhibited flesh discoloration or reddish pulp disorder due to elevated temperatures during the day and night that are associated with climate change (Kim et al. 2020). This phenomenon is commonly observed in orchards with delayed harvesting. Fruits with reddish pulp disorder rated two or higher on specific indexes have low economic value. This problem has mainly been reported in warm production areas in western Japan but is spreading to northern areas, possibly due to global warming. Despite the fact that this situation has been documented in Japan since the early 1990s, there has not been abundant research regarding this disorder in other countries (Hayama 2015; Fukuda et al. 2017). Exposure to high temperatures during the early stages of fruit development shortens the fruit development time of peaches. This can also increase the potential for reddish pulp disorder (Hayama et al. 2007).
Reddish pulp disorder in peaches is attributed to an excess accumulation of anthocyanin, where the flesh of an originally white-fleshed cultivar turns pink to red, with brown discoloration reducing fruit quality significantly and alcoholic stench identified in severe cases (Takata et al. 2005). These symptoms are similar to some extent to ‘water core’ in apples, where the affected areas initially appear to be water-soaked and translucent. This begins around the major vascular bundles and can expand to other cell layers (Hayama 2015). It is difficult to prevent the occurrence of fruit with reddish pulp disorder as it cannot be distinguished by external appearances until the symptoms worsen after harvest, affecting consumer confidence (Fukuda et al. 2017).
However, this disorder diminishes market appeal, resulting in consumer dissatisfaction due to the compromised taste and aroma. These sorts of physiological disorders pose a risk to consistent fruit production and can lead to significant financial losses for peach farmers, as highlighted by Cáceres et al. 2016 and Zhou et al. 2012.
Thus, the present study primarily focuses on identifying the occurrence of flesh red coloration or reddish pulp disorder in peaches due to elevated temperatures. The results emphasize the necessity of mitigating this shortcoming and adduce the urgent need for countermeasures to prevent severe conditions arising from climate change to maintain a consistent production process. The investigation involves analyzing the colorimetric values and various quality parameters of fruit cultivated in controlled chambers, which are regulated at four distinct day and night temperatures.
Materials and Methods
Setting up the experimental chambers
This study was carried out over the years 2022 and 2023, focusing on the ‘Daewol’ peach cultivar grown in artificial chambers called soil-fruit-daylight-system (SFDS) chambers located at the Korea University of Agriculture and Fisheries in Jeonju-si, Jeollabuk-do, South Korea. SFDS chambers allow the user to control the temperature, relative humidity, and CO2 concentration inside the chamber in a manner superior to general chambers due to more detailed programming. Furthermore, as their chamber walls are made of plexiglass, samples placed inside can receive more realistic seasonal light intensity and quality levels compared to the use of closed artificial chambers.
The chambers were subjected to the following four distinct day and night temperature categories: 1; 20°C/10°C; 2; 25°C/15°C; 3; 30°C/20°C; and 4; 35°C/25°C. These temperatures were set starting on June 21, which means approximately two weeks prior to harvesting the fruits each year. During the peach growing season in Korea (Summer season; June-July), the temperature often exceeds 25°C or 30°C in the daytime. Hence, the temperature categories above were selected for practical application. The day (defined as the period between 07:30 and 19:30) and night (defined as the period between 20:30 and 06:30) temperatures were provided in each artificial chamber as mentioned in Table 1, giving priority to 12 hours of daily exposure for daytime.
Table 1.
Temperature processing hours within each SFDS artificial chamber on a daily basis
The chambers also maintained a carbon dioxide level of 400 ppm using carbon dioxide transmitters (GMW86P, Vaisala, Vantaa, Finland, with an accuracy rate of ± 2%) and kept relative humidity at 70%, while temperature and humidity were controlled using chillers and heating systems (Table 1). Irrigation management was maintained depending on the dry condition of each pot in each chamber.
Preparation of planting materials
This experiment used ‘Daewol’ peaches, which are a late-maturing variety known for their white flesh and crunchy texture, typically harvested in July. Three-year-old peaches grown under less vigorous conditions in pots were harvested from artificial chambers under four different temperature conditions for both day and night, as described earlier. (Although the level of vigor is slightly less than that of an average plant, it is still at an acceptable level for the experiment.)
The harvesting date was determined by assessing the color of the apex side (at the point where the redness of the fruit apex no longer increases gradually) and other common parameters, such as the fruit size. The crop load was managed to fit with each peach tree, and fruiting varied from five to ten on one tree. One chamber contained four peach trees as replications. The fruits were harvested from each temperature category on July 5, 2022, and July 6, 2023, the same day in every chamber, to assess the effects of temperature changes on physiological disorders in peaches.
In each temperature category, ten biological samples were collected. All of the fruits were deemed to be of similar status given that the chamber conditions were steadily maintained throughout the period. Fruits were harvested from each tree depending on the fruiting capacity and the uniformity of the fruit size.
The harvested peaches were promptly transported within an hour to the Laboratory of Fruit Science at Gyeongsang National University in Jinju, South Korea, where various quality parameters were measured. All physicochemical characteristics and other quality measurements were acquired from identical sets of replicates with ten biological samples for each chamber in both years.
Measurements of the physicochemical quality attributes of the fruits
Fruit weight
Soon after being transferred to the laboratory, the weight of each fruit was measured separately for ten biological samples from each chamber condition using a digital scale (Electric Indicator Scale, Bas 610, Chiba, Japan). Fruit weight measurements were taken only in 2023 due to potential design limitations identified in the initial year, prompting refinement in the subsequent year, possibly including weight measurements.
Color measurements
According to the CIELAB color space, the skin color values for L*, a*, and b* in the ‘Daewol’ peaches were recorded using a portable colorimeter (Chroma Meter CR-400, Konica Minolta Inc., Osaka, Japan), followed by white calibration. The skin color measurements were obtained from the central area of the right side of the peach when it was positioned on a table with the stem end in contact with the table and the suture facing the observer, in order to ensure consistency with all the fruits. Thereafter, the fruits were cut into two longitudinal halves close to the pit (seed), and color L*, a*, and b* values on the peach flesh for each fruit were obtained from the central part of the same right side. Subsequently, the chroma (C) and hue (h°) values for both skin and flesh were computed using Equations (1) and (2):
The same procedure was carried out for the samples from both 2022 and 2023 to measure the fruit color values from the four distinct chambers separately.
Color development index (CDI) measurements
The color development index (CDI) was computed on each harvest day for the skin and flesh separately in the peach fruit for both years using Equation (3) based on the aforementioned calculated hue angles (Scalisi et al. 2022):
Reddish pulp index
Red/brown color development due to reddish pulp disorder was measured by calculating the reddish pulp index in a manner identical to how the browning index was calculated (Palou et al. 1999; Ruangchakpet and Sajjaanantakul 2007) for peach fruit flesh in both years using previously recorded CIELAB color values according to the following equation:
Firmness
The evaluation of peach fruit firmness was conducted on half of a sliced peach using a rheometer (CR-100, Sun Scientific Inc., Tokyo, Japan) equipped with an 8 mm round flat probe. The probe compressed the fruit to a depth of 3mm, applying a loading rate of 2 mm·s-1. To assess fruit firmness, the half of the peach containing the seed that had been sliced into two longitudinal halves during prior measurements was utilized. Firmness was measured on the central part of a 4 cm2 area where the skin had been removed. The recorded maximum force exerted during penetration served as the firmness measurement, expressed in Newton (N) values (Kumarihami et al. 2020). These measurements were carried out for both the 2022 and 2023 samples.
Soluble solids content (SSC)
The soluble solids contents (SSC) of the peaches were determined by using a hand-held refractometer (Pocket Refractometer, PAL-1, Atago Co., Ltd., Tokyo, Japan). The squared-shaped peeled peach flesh part used for these measurements was covered in four-layer cheesecloth and squeezed by a manual stainless-steel squeezer, with the resulting juice measured for absorbance using the refractometer (Wijethunga et al. 2023). The refractometer was calibrated in °Brix, and the results were expressed as a percentage at room temperature (RT). The refractive index accuracy of the refractometer was ± 0.2, and °Brix (%) could be measured in the range of 0 to 53% with a resolution of 0.1% °Brix at RT. The same procedure was repeated for both years.
Statistical analysis
All of the obtained data was statistically analyzed by JMP Pro (Version 16.1, SAS Institute Inc., Cary, NC, USA) by subjecting it to an analysis of variance (ANOVA). Tukey’s test (p < 0.05) was also conducted to evaluate the significance of the differences between the mean values. All values are denoted as the mean value ± standard error (SE).
Results
Changes in fruit quality attributes in ‘Daewol’ peaches under four different day and night temperatures
Fruit quality parameters, including fruit weight, SSC, firmness, and color, were measured to determine the effects of the different day and night temperatures on the ‘Daewol’ peaches tested here.
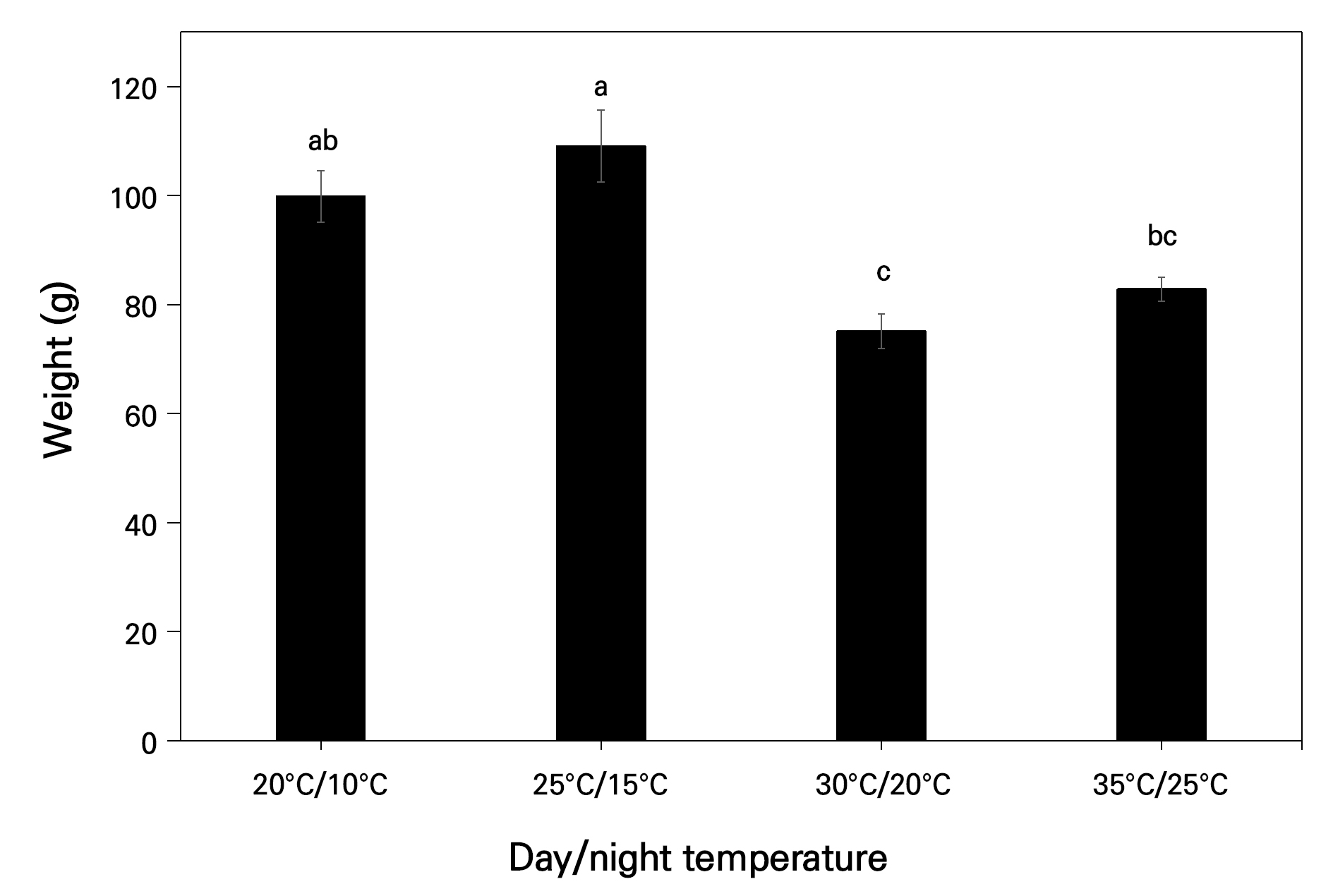
When considering the fruit weight, as depicted in Fig. 1, significantly lower values were noted, in this case 75.1g and 82.8g in the 30°C/20°C and 35°C/25°C chambers, where high day and night temperatures were maintained, compared to the 20°C/10°C and 25°C/15°C chambers (99.8g and 109.1g, respectively), where lower day and night temperatures were utilized (Fig. 1).
Changes in the fruit quality parameters of the SSC, firmness, and color (L*, b*, and chroma values) were also recorded, as presented in Table 2 for the 2022 and 2023 samples. The other color attributes, in this case the a* values, hue angles, and CDI, are addressed in the next section, as they are directly related to the development of red color in a specific fruit.
In the 2022 samples, the highest SSC was reported from the chamber maintained at 35°C/25°C, followed by that maintained at 25°C/15°C with °Brix values exceeding 14. The lowest SSC was observed in the 30°C/20°C and 20°C/10°C chambers, which showed °Brix values of approximately 12. In the 2023 samples, the highest SSC was observed at the 35°C/25°C chamber (11.6°Brix), followed by the 30°C/20°C chamber (10.52°Brix) and 20°C/10°C (10.27°Brix), while the lowest °Brix values of 9.24 was found in the 25°C/15°C chamber (Table 2).
When considering fruit firmness, the results for 2022 showed that they were not significantly different across all chamber conditions and varied by around 50 N. Nonetheless, in 2023, the 30°C/20°C chamber had the lowest firmness, at nearly 40 N, and the 20°C/10°C chamber had the maximum firmness, exceeding 50 N (Table 2).
When measuring the color values, skin chroma values, and flesh L*, b*, and chroma values of the 2022 samples, they were found to be very similar in all chamber conditions, where the highest skin L* value (78.78) was observed in the 25°C/15°C chamber and the lowest (66.92) was found in the 30°C/20°C chamber. The chambers where temperatures of 20°C/10°C and 30°C/20°C were maintained showed the highest and lowest skin b* values (34.09 and 26.86), respectively (Table 2). In the 2023 samples, the skin L* value did not change significantly, but the highest flesh L* value (72.86) was noted in the 20°C/10°C chamber and the lowest flesh L* value (64.36) was found to be 35°C/25°C. The skin and flesh had maximum b* values at 25°C/15°C and 35°C/25°C, respectively, with values higher than 19.2. The chamber that was set to 35°C/25°C had a maximum flesh chroma value of 19.92, whereas the skin chroma did not change significantly among the chambers (Table 2).
Table 2.
Changes in fruit quality characteristics of ‘Daewol’ peaches under four different temperature conditions in artificial chambers during 2022 and 2023
| 2022 Year | |||||||||
|
Day/ Night Chamber Temperature |
SSC (ºBrix) |
Firmness (N) | Skin | Flesh | |||||
| L* | b* | Chroma | L* | b* | Chroma | ||||
| 20°C/10°C | 12.34 ± 0.27 bz | 47.97 ± 2.76 a | 72.78 ± 0.88 ab | 34.09 ± 0.82 a | 34.22 ± 0.80 a | 62.74 ± 0.76 a | 20.60 ± 1.12 a | 21.05 ± 1.04 a | |
| 25°C/15°C | 14.20 ± 0.39 a | 51.17 ± 3.29 a | 78.78 ± 0.63 a | 33.50 ± 2.39 ab | 33.95 ± 2.51 a | 57.22 ± 5.32 a | 19.35 ± 0.97 a | 19.54 ± 0.98 a | |
| 30°C/20°C | 12.25 ± 0.57 b | 47.11 ± 3.60 a | 66.92 ± 3.03 b | 26.86 ± 1.56 b | 32.48 ± 1.33 a | 60.93 ± 1.21 a | 21.01 ± 1.26 a | 21.18 ± 1.20 a | |
| 35°C/25°C | 14.57 ± 0.03 a | 52.41 ± 3.09 a | 73.41 ± 3.31 ab | 28.47 ± 3.19 ab | 31.53 ± 1.18 a | 66.82 ± 0.56 a | 19.91 ± 0.75 a | 20.00 ± 0.70 a | |
| 2023 Year | |||||||||
|
Day/ Night Chamber Temperature |
SSC (ºBrix) |
Firmness (N) | Skin | Flesh | |||||
| L* | b* | Chroma | L* | b* | Chroma | ||||
| 20°C/10°C | 10.27 ± 0.47 abz | 51.33 ± 3.22 a | 63.11 ± 3.29 a | 17.34 ± 0.53 ab | 25.52 ± 0.55 a | 72.86 ± 0.75 a | 15.07 ± 0.42 c | 15.47 ± 0.45 c | |
| 25°C/15°C | 9.24 ± 0.33 b | 46.62 ± 1.08 ab | 68.80 ± 1.56 a | 19.26 ± 0.63 a | 26.13 ± 1.39 a | 68.76 ± 1.47 b | 17.40 ± 0.65 b | 17.50 ± 0.63 bc | |
| 30°C/20°C | 10.52 ± 0.42 ab | 40.73 ± 2.19 b | 63.55 ± 1.47 a | 15.84 ± 0.55 b | 27.9 ± 0.95 a | 66.89 ± 1.13 bc | 18.69 ± 0.33 b | 19.2 ± 0.49 ab | |
| 35°C/25°C | 11.16 ± 0.33 a | 44.17 ± 2.25 ab | 67.21 ± 1.49 a | 18.32 ± 0.97 ab | 24.94 ± 0.87 a | 64.36 ± 0.64 c | 19.78 ± 0.62 a | 19.92 ± 0.62 a | |
Changes of red/brown color development on the flesh of ‘Daewol’ peach under four different day and night temperatures
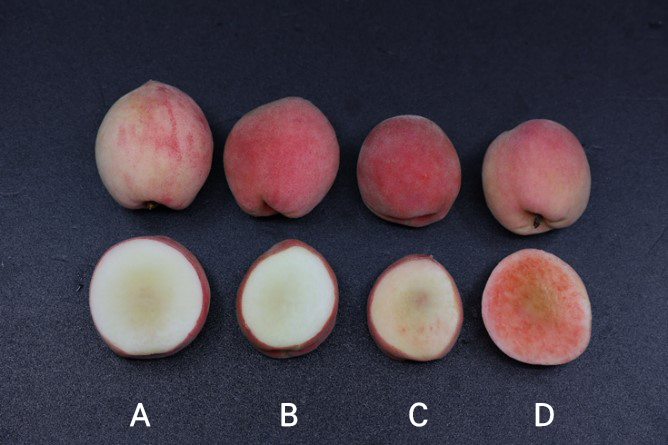
Fig. 2 exhibits the changes in red color development of the flesh in ‘Daewol’ peaches under the four different chamber conditions. Despite the fact that all of the harvested fruits are at the same maturity stage, significant red/brown color development in the peach flesh was observed only in particular chambers, as shown in Fig. 2. The changes were more significant in the 35°C/25°C chamber, followed by the 30°C/20°C case, when compared to the other two chambers where lower day and night temperatures were maintained.
The aforementioned results were confirmed with the CIELAB color values of a*, hue, and CDI. As depicted in Fig. 3A, the highest a* value was observed in fruit skin from the 30°C/20°C chamber in both years (14.88 and 22.70, respectively), but in 2023, there are no significant differences among the values. However, the flesh a* value was significantly higher, showing positive values, which represent red color in the 35°C/25°C chamber, followed by 30°C/20°C in both years, suggesting red/brown color of the flesh (Fig. 3B), whereas the other two chambers showed negative values, indicating yellowish green color.
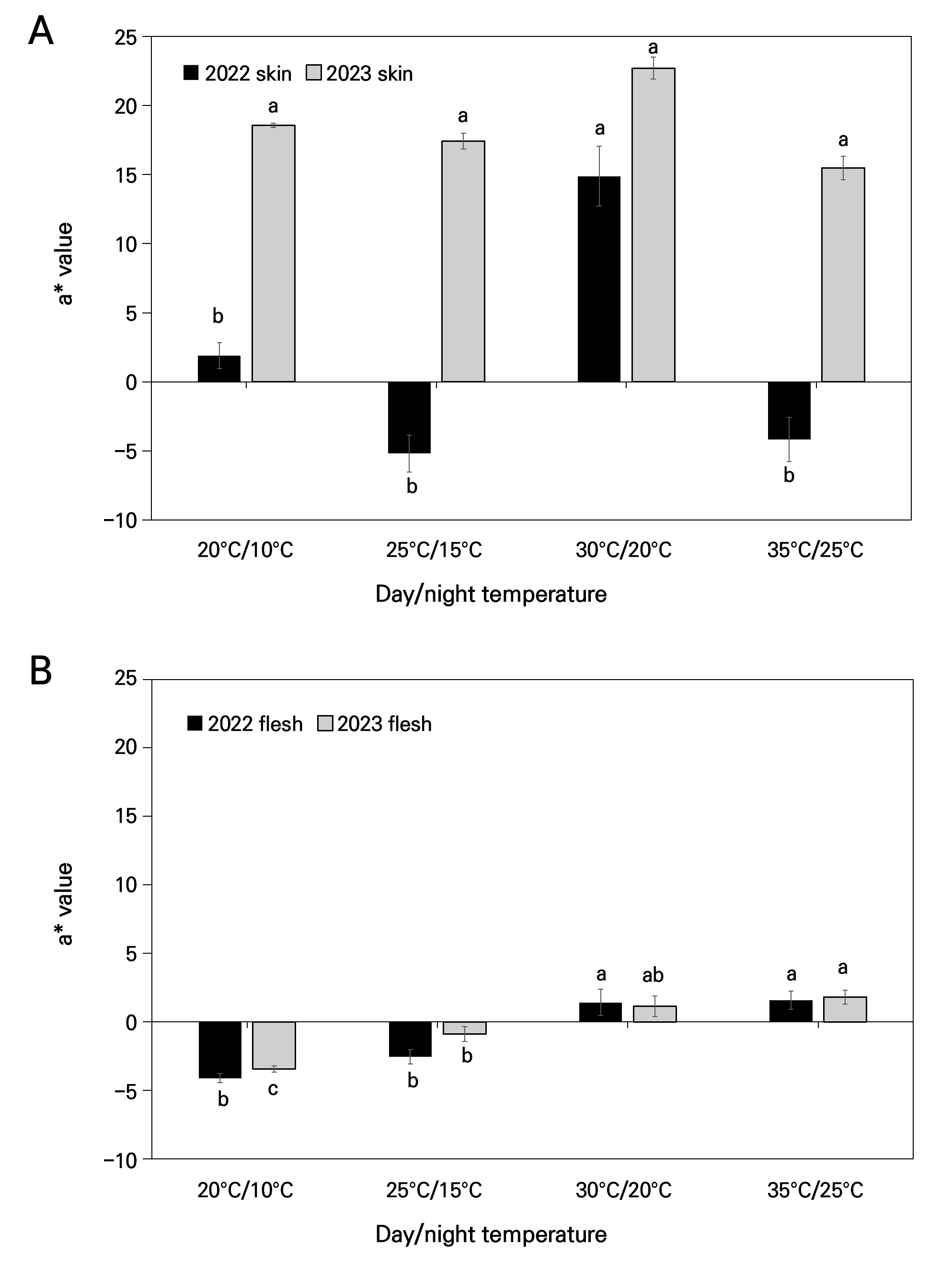
Fig. 3.
Effect of day and night temperatures on the fruit color a* value under four distinct chamber conditions. (A) a* value of skin, (B) a* value of flesh, in 2022 and 2023. Vertical bars indicate mean value ±SE with n=10. Different letters (a-c) among each day and night temperature category are significantly different (p < 0.05) according to Tukey’s test.
When considering the hue values, the lowest hue angles (60.48) in the 2022 fruit skin, at significant levels, were observed in the 30°C/20°C chamber, whereas the 2023 samples remained nonsignificant (Fig. 4A). In terms of the flesh, it is evident that (Fig. 4B), in comparison to the other chambers, the 30°C/20°C and 35°C/25°C chambers had lowest hue values, at the significant levels. They were lower than 90 in both years, whereas the other values exceeded 90, suggesting red/brown color development of the flesh.
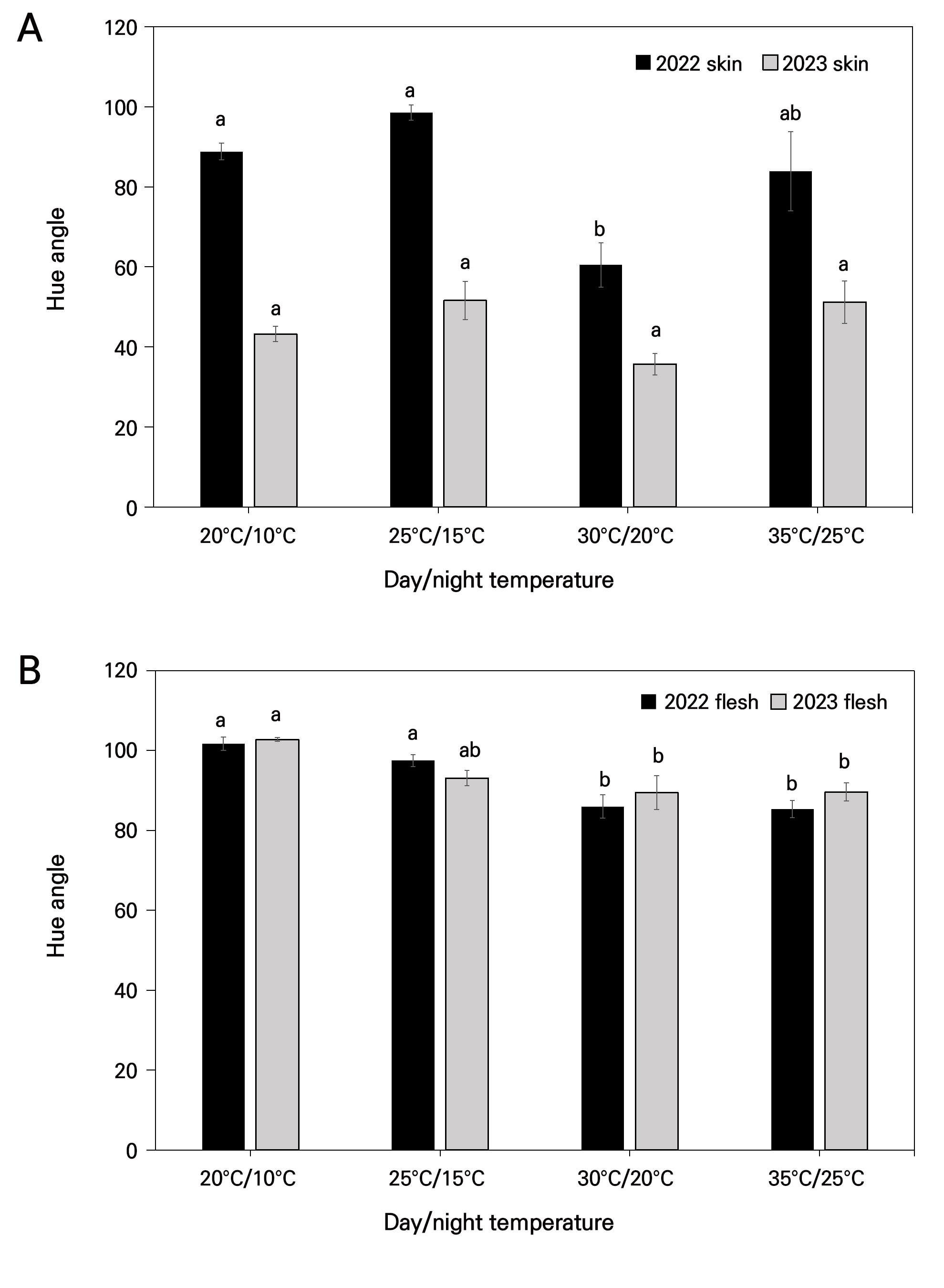
Fig. 4.
Effects of day and night temperatures on the fruit hue angle under four distinct chamber conditions. (A) hue angle of skin, (B) hue angle of flesh in 2022 and 2023. Vertical bars indicate mean value ±SE with n=10. Different letters (a-b) among each day and night temperature category are significantly different (p < 0.05) according to Tukey’s test.
According to Fig. 5A, in 2022, the highest CDI value in skin was observed in the 30°C/20°C chamber (0.66), where the other values varied and were close to 0.5. In 2023, all of the values in the four chambers were very similar to each other. However, the flesh showed the highest CDI values in the 30°C/20°C and 35°C/25°C chambers in both 2022 and 2023, reaching levels of significance. These values were overall higher than 0.5 ± 0.01, revealing maximum yellowness (Fig. 5B), while the other values were less than 0.5 ± 0.01.
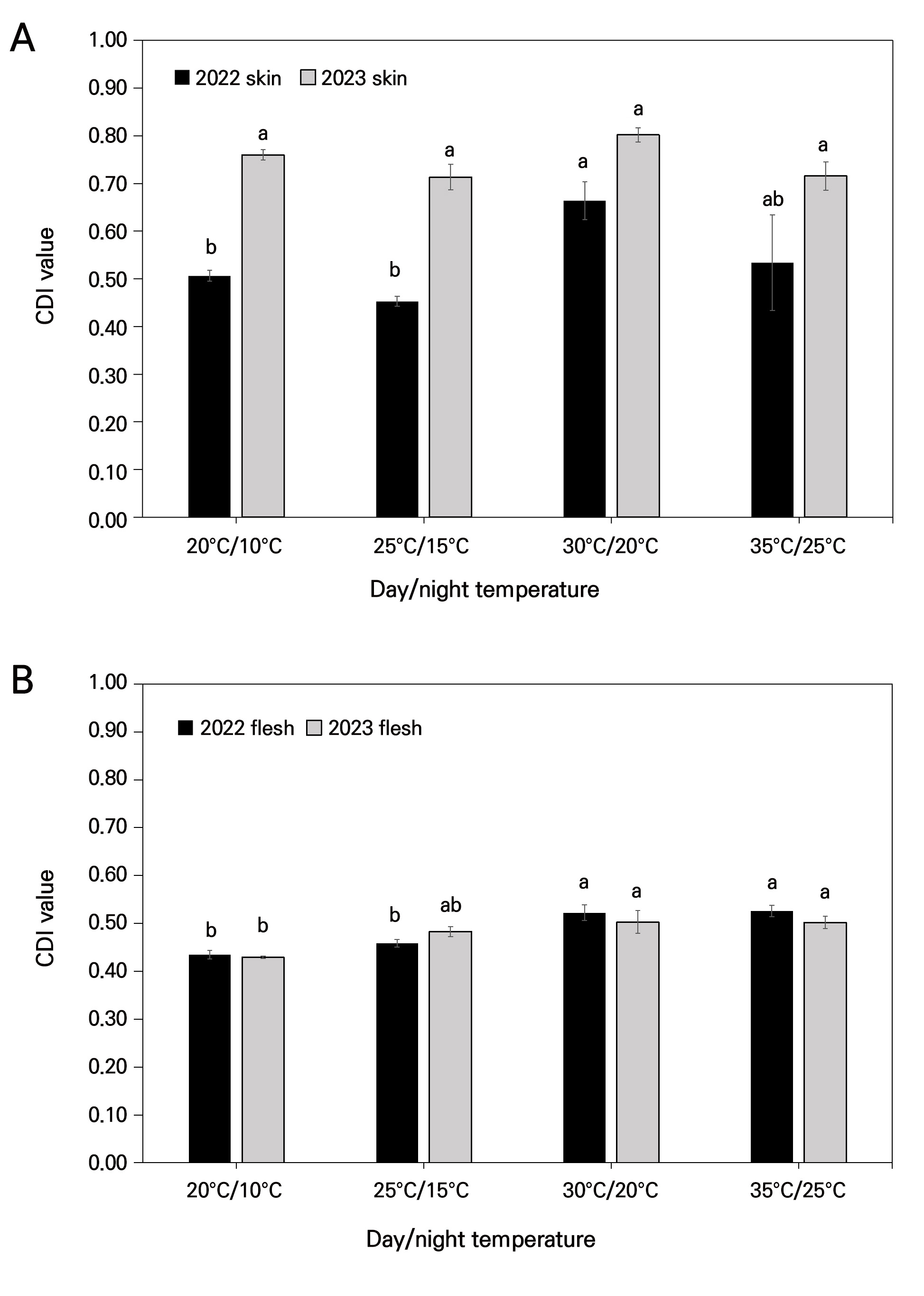
Fig. 5.
Effects of day and night temperatures on fruit color development index (CDI) values under four distinct chamber conditions. (A) CDI of skin and (B) CDI of flesh in 2022 and 2023. Vertical bars indicate mean value ±SE with n=10. Different letters (a-b) among each day and night temperature category are significantly different (p < 0.05) according to Tukey’s test.
Flesh red coloration in ‘Daewol’ peaches under four different day and night temperatures
The reddish pulp index for fruit flesh was computed to assess the impact of elevated temperatures on the flesh discoloration in ‘Daewol’ peaches over two consecutive years. Fig. 6 depicts the highest reddish pulp index, at a significant level, observed in the chambers that mimicked the tropical night phenomenon at 35°C/25°C and 30°C/20°C, with corresponding values of 4.61 and 4.17 in 2022 and 4.98 and 2.63 in 2023. The least amount of red coloration was found in the other two chambers, with some values being negative, indicating zero discoloration.
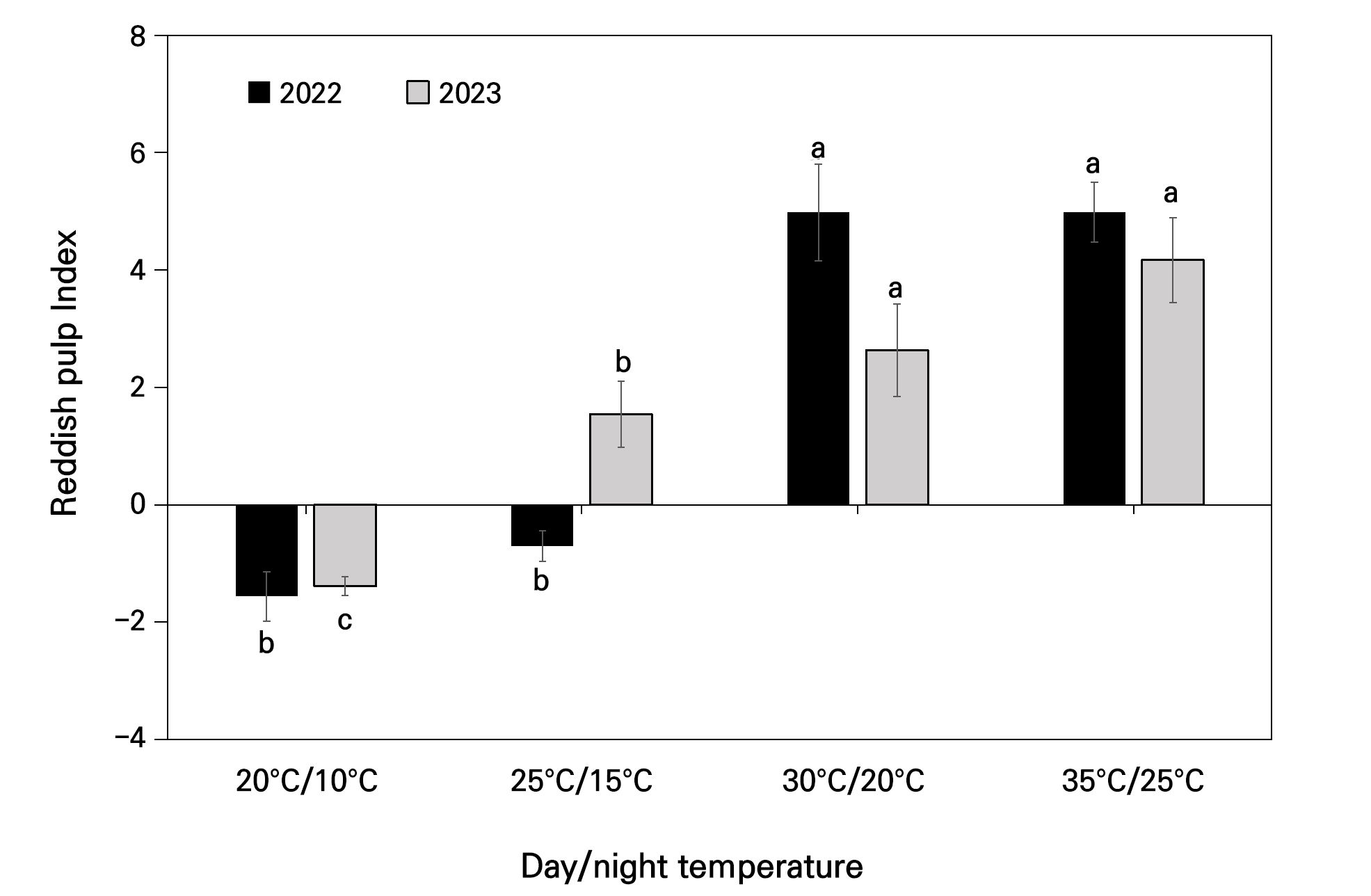
Fig. 6.
Reddish pulp index under each day and night temperature category in the artificial chambers of peach flesh in 2022 and 2023. Vertical bars indicate mean value ±SE with n=10. Different letters (a-c) among each day and night temperature category are significantly different (p < 0.05) according to Tukey’s test.
Discussion
After conducting a two-year study under consistent conditions, the results obtained for fruit flesh displayed a similar pattern in both years, specifically with higher a* values, lower hue angles, and CDI values exceeding 0.5 ± 0.01 under the high-temperature conditions. The reddish pulp index ensures that the high temperatures had a significant influence on the results. The findings from the fruit skin analysis did not show a consistent trend over the two years, indicating that potential disorders or discoloration causes may not be easily identifiable until they become more severe. However, the consistent trends in the data collected suggest that the experimental conditions remained stable throughout the research period, reinforcing the reliability of the study and the validity of the results obtained.
Effect of high day and night temperatures on fruit quality attributes in ‘Daewol’ peaches
This section will focus on the attributes of the fruit, in this case the weight, soluble solids content, firmness, and color parameters, for the L*, b*, and chroma values.
The weight of a particular fruit is likely to be affected by various internal and external factors. For example, a warmer growing season results in the early harvesting of many fruit crops, including peaches and grapes. Furthermore, in some peaches, harvest dates are predicted by monitoring spring temperatures within 30 days of blooming; high temperatures accelerate the rate of fruit growth but reduce the growing period (Lopez and DeJong 2007; Sikhandakasmita et al. 2022). A small temperature increase can lead to an increase in fruit weight through increased fruit development and photosynthetic rates. Conversely, a temperature rise of more than 4°C may be sufficient to decrease the fruit growth period by reducing photosynthetic rates, which in turn reduces the fruit weight (Lee et al. 2022). According to findings by Lopez et al. (2007) using data over a twenty-year period, in major peach cultivars in California (‘Flavorcrest’, ‘Elegant Lady’, and ‘O' Henry’), high spring temperatures reduced the average fruit size.
Research on peaches has shown that cell division during the S1 stage and growth during the S3 stage will determine the ultimate fruit size. Small-fruited cultivars have shorter S1 and S3 phases that can affect cell division and cell expansion. Genetic and environmental variables during fruit development may also influence the ultimate fruit size (Yamaguchi et al. 2004). Positive correlations were discovered between the fruit development duration and the weight and size of peaches and apples by Giovannini et al. (2013) and Sugiura et al. (2003), respectively. Hence, prolonged exposure to high temperatures during fruit development may cause the growing period to shorten in its early stages, reducing the size of the fruit (Sikhandakasmita et al. 2022). The results here corroborate these earlier findings, as the fruit size and weight were reduced under high day and night temperature conditions.
Changes in the SSC and acid content are often related to the growth temperature, light irradiation, and CO2 concentration (Wei et al. 2018; Christopoulos and Ouzounidou 2021). Some research has indicated that the SSC concentration of peaches drops at high temperatures (Lee et al. 2022), and identical results have been shown for strawberries and tomatoes exposed to increased temperatures (Wang et al. 2004; Pimenta et al. 2022). However, the results here were contradictory, showing the highest SSC in the chamber where the highest day and night temperatures were maintained. This situation can be explained as follows: when temperatures rise, the metabolic activity of the fruit increases, leading to the breakdown of complex carbohydrates into simple sugars. This can lead to an increase in the soluble solids content of some fruits. However, excessively high temperatures can also have negative effects on fruit quality, leading to a breakdown of important nutrients and enzymes in the fruit as well as a loss of moisture through evaporation. This can result in a decrease in the overall quality and shelf life of the fruit (Sikhandakasmita et al. 2022). Here, as high temperatures were only during the later stages of fruit ripening, an extreme impact on the SSC may not be observed. In addition, late-flowering fruit tends to have higher levels of soluble solids, suggesting that late-flowering fruits have greater nutrient sink strength than the early-flowering fruits. Therefore, the relationship between temperature and SSC is not straightforward and can depend on several factors, including the type of fruit, the canopy positions, and the stage of development (Fukuda et al. 2017).
Though our results related to firmness did not show an identical pattern over the two years, the temperature significantly impacted the firmness of the fruit, primarily depending on the type of fruit and the corresponding biochemical composition. On average, fruits become less firm as the temperatures increase, though with some outliers (Bourne 1982). Enzymatic activity, which regulates biochemical reactions, may increase at higher temperatures, leading to cell wall degradation and softening. Fruit calcium concentrations also play a role in maintaining membrane integrity, which is linked to a loss of firmness (Lachapelle et al. 2013). The cell wall is a critical component that contributes to the firmness of fruits, and its structure can be altered by the temperature condition (Mulholland et al. 2003). For example, exposure to high temperatures can cause pectin, a key component of the cell wall, to break down, leading to a loss of firmness. Higher temperatures also cause a rise in respiration rates, and this increased metabolic activity can contribute to the softening of fruits (Antunes and Sfakiotakis 2000). Additionally, high temperatures can accelerate transpiration and water movement within fruits, leading to dehydration and potential softening, thereby reducing their firmness. For some fruits, exposure to higher temperatures can accelerate ripening and the production of ethylene. Ripening often involves changes in texture, which can impact firmness (Mulholland et al. 2003). Considering the results of this study, the temperature rise created under these conditions may not be sufficient to cause any changes in the firmness of ‘Daewol’ peaches.
The alteration in color values (L*, a*, b*) is a significant quality factor for assessing color degradation (Kaur et al. 2023). The CIELAB color space values indicate the color changes of the skin and flesh of the peaches under each chamber condition. High temperatures can accelerate chlorophyll degradation and pigment synthesis, leading to changes of color (Cai et al. 2022) in fruits.
The brightness or lightness of an object is denoted by the L* value, which ranges from 0 (representing black) to 100 (representing white) (Urbonaviciene et al. 2012). As fruits ripen, carotenoids accumulate, causing a reduction in lightness or paleness and thus a lower L* value. Identical results were obtained by Lee et al. (2022), who showed reduced L* values with high temperatures (C + 3.4°C treatment, where C is the average temperature). The results related to the peach flesh in our study also displayed a similar pattern, in which peaches grown at high temperatures showed lower L* values, though the skin color did not change significantly.
The b* value represents the yellow-blue color of the object; a positive value represents yellow, and a negative value represents blue (Jayasooriya et al. 2023). Changes in the b* value with temperature in fruits are often associated with the breakdown of certain pigments or chemical compounds responsible for color. When fruits ripen or mature, several chemical reactions occur. In some cases, higher temperatures accelerate these reactions, leading to the breakdown of chlorophyll and the production of other pigments such as carotenoids and anthocyanin (Cáceres et al. 2016). Although there were no significant differences among the 2022 samples, the flesh of the 2023 samples had higher b* values, indicating greater yellowness stemming from the corresponding higher day and night temperatures of the chamber of 35°C/25°C. This suggests that high temperatures caused some color changes in the flesh, while the skin color remained unaffected in fruits at the same maturity stage.
The chroma value refers to the intensity or vividness of a color, often measured in the CIELAB color space (Kortei and Akonor 2015). Extremely high temperatures can affect the color of fruits in several ways, depending on the type of fruit, and the duration and intensity of heat during their growth. As previously discussed, chlorophyll breakdown, which is accelerated by extreme heat, can lead to a loss of green color and can change the composition of fruit, ultimately influencing its color intensity (Arias et al. 2000). The Maillard browning reaction, a chemical reaction between amino acids and reducing sugars (Ribeiro et al. 2020), can also be triggered by high temperatures, affecting the overall color intensity or chroma value. Zhou et al. (2012) showed a rise in the chroma value with pulp discoloration when fruits were in storage, with color changes from white to brownish yellow. Thus, the findings of the 2023 samples related to the flesh also suggest that the chroma values increased due to the brownish color development caused by the high temperatures.
Effect of high day and night temperatures on red/brown coloration on the flesh of ‘Daewol’ peaches
As shown in Fig. 2, the development of significant redness of the flesh in the 30°C/20°C and 35°C/25°C chambers can primarily be explained as a consequence of the high day and night temperatures during the fruit maturity period and excessive water flow to the flesh, considering that all other conditions were held constant. However, the exact physiological mechanism has not been thoroughly clarified. Fig. 2C shows that the anthocyanin accumulation phenomenon, and thereby reddish pulp disorder, begins to occur at this stage, i.e., where 30°C/20°C day and night temperatures are maintained. In the 35°C/25°C temperature chamber, anthocyanin over-accumulation and symptoms of reddish pulp disorder were clearly observed (Fig. 2D). The crucial point is that this greater redness was only found in the chambers that mimicked the tropical night phenomenon. A tropical night occurs when the minimum temperature during the day (including at night) does not fall below 20°C. In midsummer, such warm nights are frequently accompanied by warm temperatures during the day (Choi and Kwon 2005). Accordingly, the anthocyanin accumulation in such cases is assumed to be accelerated by the high temperatures by changing the acidity levels in the fruit pulp and causing the formation of metalloanthocyanin complexes under these changing acidity conditions (Niu et al. 2017).
The CIELAB color a* values and hue values also confirmed the aforementioned phenomenon by showing greater a* values and lower hue angles. The color value a* denotes the redness of a commodity, with positive and negative values representing red and green, respectively (Jayasooriya et al. 2023; Kim et al. 2024). The same results were reported in the study by Lee et al. (2022), showing higher a* values with high day and night temperatures in ‘Mihong’ peach flesh. This a* color increment has a strong positive correlation with anthocyanin accumulation (Whale and Singh 2007), which is essentially responsible for the red and purple color pigmentation in fruits (Lin-Wang et al. 2010). Niu et al. (2017) found that high temperatures, 35°C in their study, accelerate anthocyanin synthesis by increasing respiration and ethylene production and enhancing the enzyme activities of plums. The findings of the present study also indicate that high-temperature conditions can cause reddish/brown flesh in peach fruits as well by stimulating excess anthocyanin production.
Previous research has shown that the hue angle is a strong indicator of color development in the skin and flesh of peaches and nectarines (Scalisi et al. 2020). Generally, the hue angle describes the perceived color of an object and is determined by the dominant wavelength of light reflected or emitted by that object. Red/magenta is a color with a hue angle of 0°/360°, yellow is 90°, green is 180°, and blue is 270°. Some fruits may have decreased hue angles if they are less vibrant or yellow or brown; therefore, the lower the hue value, the more intense the redness of the product (Kortei and Akonor 2015). Thus, the results here indicate that high day and night temperatures cause excess red/brown color development in the flesh of fruit.
CDI is a color index that was developed mainly based on the hue angle in order to study color changes in fruit. CDI = 0 represents green color, CDI = 0.5 represents blue and yellow color, and CDI = 1 indicates red color (Scalisi et al. 2022). According to previous studies, the progression of CDI with the harvesting time is characterized by a polynomial cubic model, in line with the modeling approach used to describe the development of red/brown color attributes. CDI is strongly related to the degradation of chlorophyll and has been found to be correlated with fruit maturity and the development of red color (Scalisi et al. 2022). The fruits utilized for the present experiment were at the same stage of maturity and had the fewest skin color changes, suggesting that other external factors accelerate anthocyanin pigmentation and color development in this case. Thus, elevated temperatures affect the red coloration of fruit flesh, as all other environmental conditions in each chamber remained constant, except for the divergence in the daytime and nighttime temperatures.
Reddish pulp disorder as a physiological disorder in ‘Daewol’ peaches
The reddish pulp index describes the degree of the occurrence of red color development in the flesh of fruit. Flesh discolorations on peaches and nectarines are likely caused by non-oxidation reactions involving anthocyanins, which are present in the fruit cells (Cheng and Crisosto 1994). One type of reaction occurs when anthocyanins undergo a structural transformation at a high pH level, leading to changes in color (Fukuda et al. 2017).
The color of anthocyanin is influenced by the acidity level of the solution. At a low pH (around 1.0), the pigments appear red as flavylium salt. As the pH increases, the pigments change to colorless carbinol pseudo-bases around pH 4 to 5. With higher pH levels, the color shifts to purple, eventually becoming blue above pH 7, as the blue quinoidal base forms (Rumainum et al. 2016).
Another type of reaction involves the formation of metalloanthocyanin complexes at normal pH levels, such as when anthocyanins and cyanidin-3-glucoside interact with metal ions; they can produce blue derivatives and those of other colors. These types of metal ions present also play a role in the formation of these complexes (Rumainum et al. 2016). Understanding how fruit responds to different pH levels and metallic ions is crucial for preventing flesh discoloration (Ruangchakpet and Sajjaanantakul 2007). Previous studies have examined anthocyanin responses in solution systems, but research on fruit skin tissue is lacking.
However, the finding of positive reddish pulp index values only in the high-temperature chambers here indicates that elevated temperatures hasten red coloration in peaches. On the other hand, in the remaining two chambers, the index recorded negative values, indicating that the low temperatures did not have any impact on these fruits. When fruits are exposed to high temperatures of approximately 35°C, molecular vibrations increase, meaning that the water can ionize and form more hydrogen ions. The dissociation of water into hydroxide ions and hydrogen can be represented as H2O (l) H+ (aq) + OH− (aq). Thus, this type of rise in temperature leads to an increase in the number of ions due to the dissociation of molecules while also changing the pH, leading to color changes (Cheng and Crisosto 1994).
In addition, anthocyanin accumulation in fruits and flowers is controlled by developmental processes. The activity of genes involved in anthocyanin biosynthesis is regulated spatially and temporally by MBW complexes made up of R2R3-MYB transcription factors, bHLH transcription factors, and WD40 proteins (Li et al. 2021; Xi et al. 2021). Some of the R2R3-MYB transcription factors known to promote anthocyanin biosynthesis in various plant species include PAP1, PAP2, AtMYB113, and AtMYB114 in Arabidopsis; MdMYBA/MdMYB1/MdMYB10 in apple; VvMYBA1 in grape; and AN2, DEEP PURPLE, and PURPLE HAZE in petunia. These positive regulators belong to subgroup 6 of R2R3-MYBs, which consists of 23 subgroups (Lin-Wang et al. 2010; Yamagishi 2022). As a genetic analysis was not conducted here, further research can be conducted to identify the specific genes involved in anthocyanin biosynthesis in peach fruits.
Overall, this disorder is characterized by the development of watery or translucent tissue in the flesh and an alcoholic aroma accompanied by reddish or brown coloration, leading to a decrease in fruit quality. Therefore, unraveling the mechanisms underlying the disorder and developing techniques to reduce its incidence is necessary.
Conclusion
In this study, four different day and night temperature conditions were evaluated to observe the effect of high temperatures on the quality of peach fruit. Based on the results obtained from four chambers in which specific temperature conditions were maintained, the 30°C/20°C and 35°C/25°C chambers showed a reduction in fruit weight and excess anthocyanin accumulation in ‘Daewol’ peach flesh compared to the 20°C/10°C and 25°C/15°C chambers. Red/brown color development was ensured given the high a* values, lower hue values, and CDI values exceeding 0.5 ± 0.01, signifying maximum yellowness. Thus, the chambers demonstrate the tropical night phenomenon with temperatures exceeding 20°C, emphasizing that this reddish pulp disorder is intensified with higher temperatures, triggered by climate change. Very few changes in skin color values were observed, indicating that these symptoms may not be readily apparent until more severe conditions manifest after harvesting. To ensure a consistent production process, addressing the issue of flesh red coloration in peaches under high-temperature conditions is crucial. The use of strategies such as adequate irrigation, employing shading techniques to shield fruits from intense sunlight, and selecting peach varieties better suited for heat stress has thus become imperative.