Introduction
Materials and Methods
Application to Shoot Apexes
Application to Shoot Apexes with Oryzalin + Tween20
Seed Soaking
Stomata Observation
Chromosome Observations
Results and Discussion
Application to Shoot Apexes
Application to Shoot Apexes with Oryzalin + Tween20
Seed Soaking
Morphological Changes in Leaves
Characteristics of Stomata
Chromosome Observations
Introduction
Watermelon (Citrullus lanatus L., 2n = 2x = 22), originated in South Africa, and is an economically important fruit crop, like cucumber (Cucumis sativus L.), melon (Cucumis melo L.), and pumpkin (Cucurbita moschata) (Anonymous, 2012). In Korea, watermelon is grown over an area of 2,726 hectares with an annual production of 92,736 tons. Watermelon is the third largest consumed fruit crop in Korea after strawberries and dried peppers (MAFRA, 2017). Desired characteristics of horticultural crops include plant size, disease resistance, environmental stresses tolerance and seedless fruits introduced through breeding and polyploidization (Lapins, 1975; Cockerham and Galletta, 1976; Ranney, 2006). Polyploidization is an important way in desired plant development which is often associated with introduction of novel traits (Levin, 2002). Polyploidy in gymnosperms is rare, while 70% of naturally occurring angiosperms are polyploid (Khoshoo, 1959; Koshoo, 1961; Goldblatt, 1980; Masterson, 1994; Ahuja, 2005). Usually, polyploid organisms exhibit heterosis, increased vigor, higher yield, produce higher quality products and are more tolerant to both biotic and abiotic stresses than their diploid counterparts (Fawcett et al., 2009; Sattler et al., 2016).
Seedlessness is one of the major goals of modern watermelon breeding, and seedless watermelon is preferred by consumers due to higher sugar content (Marr and Gast, 1991), superior quality, and higher yield (Henderson, 1977). Many techniques are used to develop seedless watermelons, with the most commonly used antimitotic alkali agent being colchicine, which prevents spindle formation (Eigsti, 1971; Henderson, 1977; Miguel and Leonhardt, 2011), and prevent nuclear cell division (Ganga and Chezhiyan, 2002). Lower concentrations (0.5 mM) of colchicine inhibit microtubule formation, resulting in reducing fertility, while higher concentrations (5 mM) induce microtubule polymerization to develop spindle in c-metaphase cells (Caperta et al., 2006). Though efficient in inducing polyploids, colchicine is a highly toxic, carcinogenic compound which is not only harmful to humans but can also cause infertility and chimeras due to abnormal cell division in crops (Morejohn et al., 1984; Wan et al., 1989). In recent studies, oryzalin, trifluralin, amiprophos-methy1 (APM), and caffeine have been used as herbicides as well as substitutes for colchicine (Dhooghe et al., 2011; Miguel and Leonhardt, 2011). Moreover, oryzalin is comparatively more effective in inducing stable polyploids in plants with, an increased survival rate, and is less toxic to animals due to a greater affinity to bind with plant tubulin (Morejohn et al., 1987). Lower concentrations of oryzalin (1/100) compared to colchicine strongly bind to tubulin during cell division (Morejohn et al., 1987; Ramulu et al., 1991). Tetraploid watermelon induced by oryzalin had larger leaves, and flowers, and a thicker rind as compared to diploids (Jaskani et al., 2004; Contreras et al., 2007). Therefore, oryzalin is preferred over colchicine, and has been shown to be more effective to induce polyploidy than other substances in lily (Lilium sp.) (Chung et al., 2004), chrysanthemum (Chrysanthemum morifolium) (Viehmannovã et al., 2009), and cymbidium (Cymbidium sp.) (Hwang et al., 2015).
The objective of this study was to examine the effects of different concentrations and treatment methods of oryzalin in the polyploidization of watermelons.
Materials and Methods
Application to Shoot Apexes
Diploid watermelon seeds (‘Nuneichan’, The Dream Co., Ltd.) were sown in a tray. Oryzalin was dissolved in 0.1% dimethyl sulfoxide (DMSO) and diluted with distilled water to prepare a 1% stock solution. The treatment concentrations were 0, 5, 10, 15, 20, and 25 mg·L-1, and 10 µL of each concentration was added to shoot apexes of the watermelon seedlings when cotyledons were developed using pipettes. To increase the absorption of oryzalin (Fig. 1), cotyledons were fixed with a clip for 7 days, after which the clip was removed. After oryzalin treatment, the second true leaf was collected and ploidy was analyzed by flow cytometry (Partec PA, Ploidy Analylzer, Germany). The leaf tissue (0.5 cm2) was chopped with a sharp razor blade in a 50 × 12 mm plastic Petri dish with 500 µL of nucleus isolation buffer. The standard peak was programmed to appear at about channel 50 of relative fluorescent intensity. This setting was kept constant and was readjusted for every measurement.
Application to Shoot Apexes with Oryzalin + Tween20
To enhance the effectiveness, the surfactant Tween 20 was added to the oryzalin solution. Oryzalin treatment concentrations for this method were 0, 25, and 30 mg·L-1, and 30 µL Tween 20 was added to 50 mL of each concentration of oryzalin. The solutions were applied in the same manner and polyploidy was analyzed by flow cytometry as described above.
Seed Soaking
A total of 50 seeds were sown per Petri dish on top of absorbent cotton measuring 9 cm in diameter. Following this, 0, 30, and 35 mg·L-1 solutions of oryzalin were added to soak the cotton and submerge the seeds. The Petri-dishes were placed in a 25°C incubator for 3 days. The seeds were germinated, and managed in a greenhouse with 50 implanted trays. Polyploidy was analyzed using flow cytometry using the second true leaf.
Stomata Observation
To observe the morphological changes of oryzalin-treated seedlings, the epidermis was separated using forceps. The separated epidermis was placed on a glass slide and covered with cover glass. The slides were observed under an optical microscope (BX61, Olympus, Japan) at a magnification of 400×.
Chromosome Observations
For chromosome observations, watermelon root tips were collected at 8:00 a.m. from the greenhouse. Following this, the root tips were prefixed with 8-hydroxyquinoline at 20°C for 3 h and then fixed overnight in ethanol: acetic acid solution (3:1, v/v) at room temperature. The root tips were then placed in an ethanol solution (70%) and stored in a refrigerator (Kwon et al., 2017; Cuyacot et al., 2017). The root tips were then washed with double-distilled water and treated with an enzyme mixture containing 0.3% cytohelicase, 0.3% pectolyase, and 0.3% cellulase at 37°C for 40 min. Following this, the root tips were squashed with 17 µL of acetic acid (70%), and air-dried. The chromosomes were counterstained with 2 µL·mL-1 of 4',6-diamino-2-phynylindole (DAPI) in VECTASHIELDmedium and observed under a fluorescent microscope (BX61, Olympus, Japan).
Results and Discussion
Application to Shoot Apexes
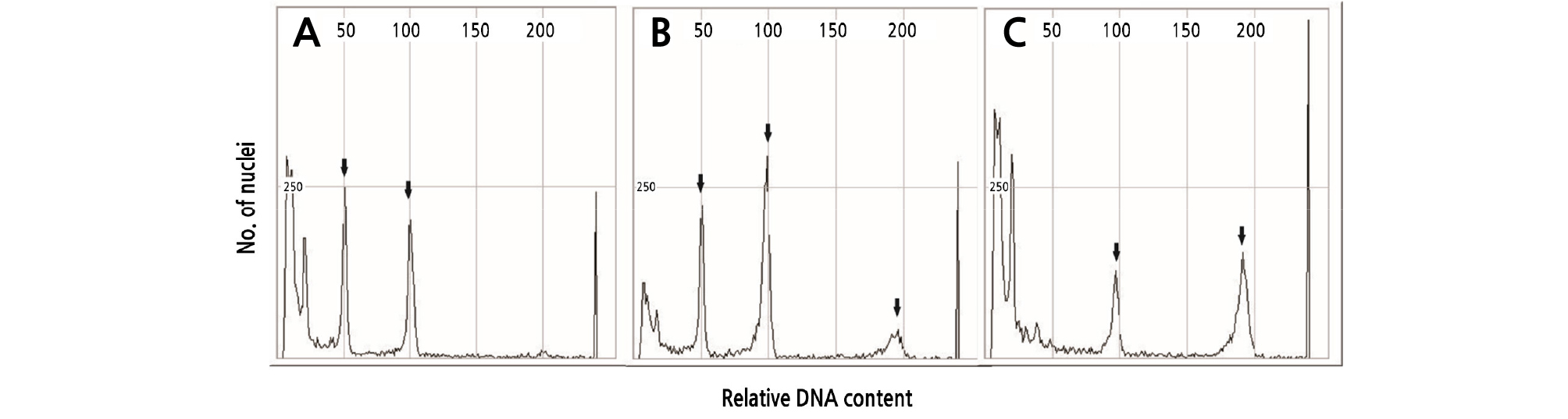
DNA contents of diploid, mixoploid, and tetraploid plants were analyzed by flow cytometry. For diploid plants, peaks were observed at 50 and 100 (Fig. 2A). In the mixoploid plants, peaks were observed at 50, 100, and 200 (Fig. 2B). However, the tetraploid peaks were observed at 100 and 200 (Fig. 2C). The results of watermelon polyploidy depending on the concentration of oryzalin treatment are shown in Table 1. Oryzalin treatments with 5 and 10 mg·L-1 concentrations did not result in polyploidy. In case the of the 15 mg·L-1 treatment, 48 seedlings were diploids and the remaining 2 were mixoploids. Further, the 20 mg·L-1 treatment resulted in 35 diploids and 15 mixoploids. Thus, treatments with 20 mg·L-1 or lower concentrations did not result in tetraploids. In the case of the 25 mg·L-1 treatment, 8 mixoploids, and 42 tetraploids were observed, with an 84% polyploidy rate. In a study by Raza et al. (2003), in watermelon, the highest number of tetraploids was found with 0.01% colchicine. The highest survival rate and ploidy level of Watsonia lepida was observed in plants treated with 60 µM of oryzalin for 24h (Gao et al., 1996; Ganga and Chezhiyan, 2002).
Table 1.
Effect of oryzalin drop treatmentz on ploidy level in watermelon| Oryzalin concentration (mg·L-1) | No. of seedlings | No. of plants | Efficiencyy (%) | ||
| Diploid | Mixoploid | Tetraploid | |||
| 0 | 50 | 50 | 0 | 0 | 0 |
| 5 | 50 | 50 | 0 | 0 | 0 |
| 10 | 50 | 50 | 0 | 0 | 0 |
| 15 | 50 | 48 | 2 | 0 | 0 |
| 20 | 50 | 35 | 15 | 0 | 0 |
| 25 | 50 | 0 | 8 | 42 | 84 |
yNo. of tetraploid plants/50 × 100.
Oh et al. (2015) found that a 1% colchicine treatment in watermelon had the same efficacy as 1% oryzalin with a tetraploidy rate of 86%. The oryzalin concentration used in our experiment was 25 mg·L-1, which was 400 times lower than the colchicine concentration in the study mentioned above. Thus, oryzalin is considered to be an effective substitute for colchicine considering that it is less harmful to humans and more affordable than colchicine. In Watsonia lepida, 0.25 µM colchicine was effective at inducing polyploidization, while 2.5 µM oryzalin resulted in the highest polyploidization rate (Ganga and Chezhiyan, 2002; Zlesak et al., 2005). Similarly, a higher survival rate of explants was found with 100 to 1,000 times lower oryzalin concentration than colchicine (Yemets and Blume, 2008; Miguel and Leonhardt, 2011).
Application to Shoot Apexes with Oryzalin + Tween20
The results of adding the surfactant Tween 20 to oryzalin are as shown in Table 2. For the treatment with o 25 mg·L-1 oryzalin + Tween 20, 30 out of 50 plants were mixoploid, while 10 plants were tetraploid, with a tetraploidy rate of 20%. The treatment with 30 mg·L-1 oryzalin + Tween 20, resulted in 35 mixoploids and 15 tetraploids, with a 40% tetraploidy rate. Thus, the addition of Tween 20 resulted in a lower percentage of tetraploids than the 25 mg·L-1 treatment of oryzalin alone.
Table 1.
Effect of oryzalin + tween 20 drop treatmentz on ploidy level in watermelon| Oryzalin concentration (mg·L-1) | No. of seedlings | No. of plants | Efficiencyy (%) | ||
| Diploid | Mixoploid | Tetraploid | |||
| 0 + Tween 20 | 50 | 50 | 0 | 0 | 0 |
| 25 + Tween 20 | 50 | 10 | 30 | 10 | 20 |
| 30 + Tween 20 | 50 | - | 35 | 15 | 40 |
yNo. of tetraploid plants/50 × 100.
Seed Soaking
Table 3 shows the results of soaking seeds in oryzalin. In the case of the 30 mg·L-1 treatment, all seeds remained diploid. For the 35 mg·L-1 treatment, 5 out of 20 seeds were tetraploids with a treatment efficiency of 25% (Table 3). Kwack and Lee (1999) reported, the highest efficiency of 22.5% when watermelon seeds were treated with a colchicine concentration of 0.1%, although there was a difference in the tetraploidy rate among cultivars. According to Contreras et al. (2010), oryzalin-treated tetraploid Cryptomeria japonica exhibited thickened and twisted leaves with an otherwise wild-type phenotype. In our study, soaking the seeds in oryzalin resultrd in lower polyploidization than that observed with the treatment where oryzalin was placed on the shoot apex (84%).
Table 1.
Effect of oryzalin seed soaking treatment on ploidy level in watermelon| Oryzalin concentration (mg·L-1) | No. of seeds | No. of plants | Efficiencyz (%) | ||
| Diploid | Mixoploid | Tetraploid | |||
| 0 | 20 | 20 | 0 | 0 | 0 |
| 30 | 20 | 20 | 0 | 0 | 0 |
| 35 | 20 | 15 | 0 | 5 | 25 |
Morphological Changes in Leaves
The morphological changes observed in leaves after oryzalin treatment are shown in Fig. 3. Oryzalin treatment caused the leaves to become small, thick, and crumpled. In general, with regard to polyploidy, the stem becomes thicker and leaves and flowers enlarged because of tissue expansion (Cockerham and Galletta, 1976; Kim et al., 2003). In watermelons, treated with colchicine, the tetraploid leaf is not deeper green and wider than the diploid leaf (Koh, 2003). In the case of tetraploids, leaf size have been reported to increase (Koh, 2003). In the case of our treatment with 35 mg·L-1 oryzalin, the same effect was observed at a concentration 25 times lower than that of colchicine at the efficiency of 25%. In our experiment, the tetraploid leaves were small and malformed. This difference was observed in the second true leaf after the first treatment, and the leaf was judged to be weakened by oryzalin treatment. Thus, we believe that whether or not polyploidization has occurred after oryzalin treatment can be judged based on the weakness of the true leaf.
Characteristics of Stomata
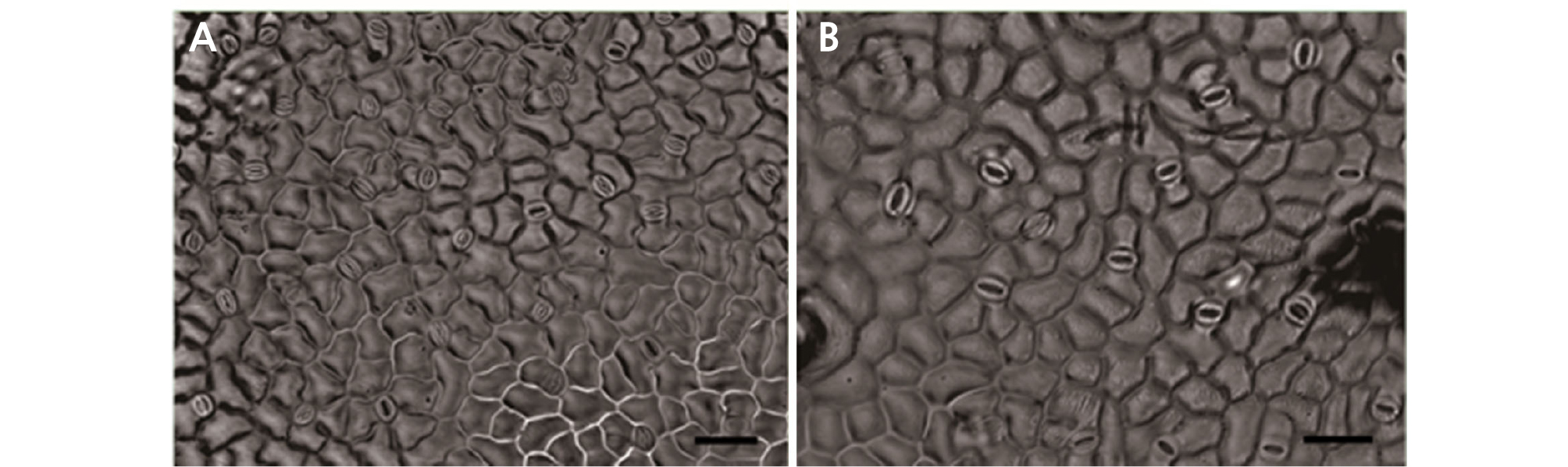
Polyploidy can be easily assessed via visualizing guard cells because the stomata in tetraploids become larger than those of diploids (Kadota and Niimi, 2002; Zlesak et al., 2005; Chen, 2010). Morphological changes of the stomata of diploid and tetraploid leaves are shown in Table 4 and Fig. 4. In the case of diploid leaves, the number of stomata per unit area was 34.67, whereas the number of stomata per unit area for tetraploid leaves was reduced to 18. The length of the diploid stomata was 3.91 µm, but that of the tetraploid stomata was 6.15 µm, which was twice as long as the length of the diploid stomata. The width of the diploid stomata was 2.93 µm and that of the tetraploid stomata was 3.46 µm (Fig. 4). Use of white manganese also results larger stomatal size of tetraploids than that of diploids (Yang et al., 2006; Kwon et al., 2014), and stomata decreased in density, similar to the results observed in our experiment.
Table 4.
Comparison of stomata distribution and size in diploid and tetraploid watermelon| Ploidy level | Stomata | ||
| Density (cell/mm2) | Length (𝜇m) | Width (𝜇m) | |
| Diploid | 34.67 ± 0.57z | 3.91 ± 0.07 | 2.93 ± 0.05 |
| Tetraploid | 18.00 ± 1.00 | 6.15 ± 0.05 | 3.46 ± 0.05 |
Chromosome Observations
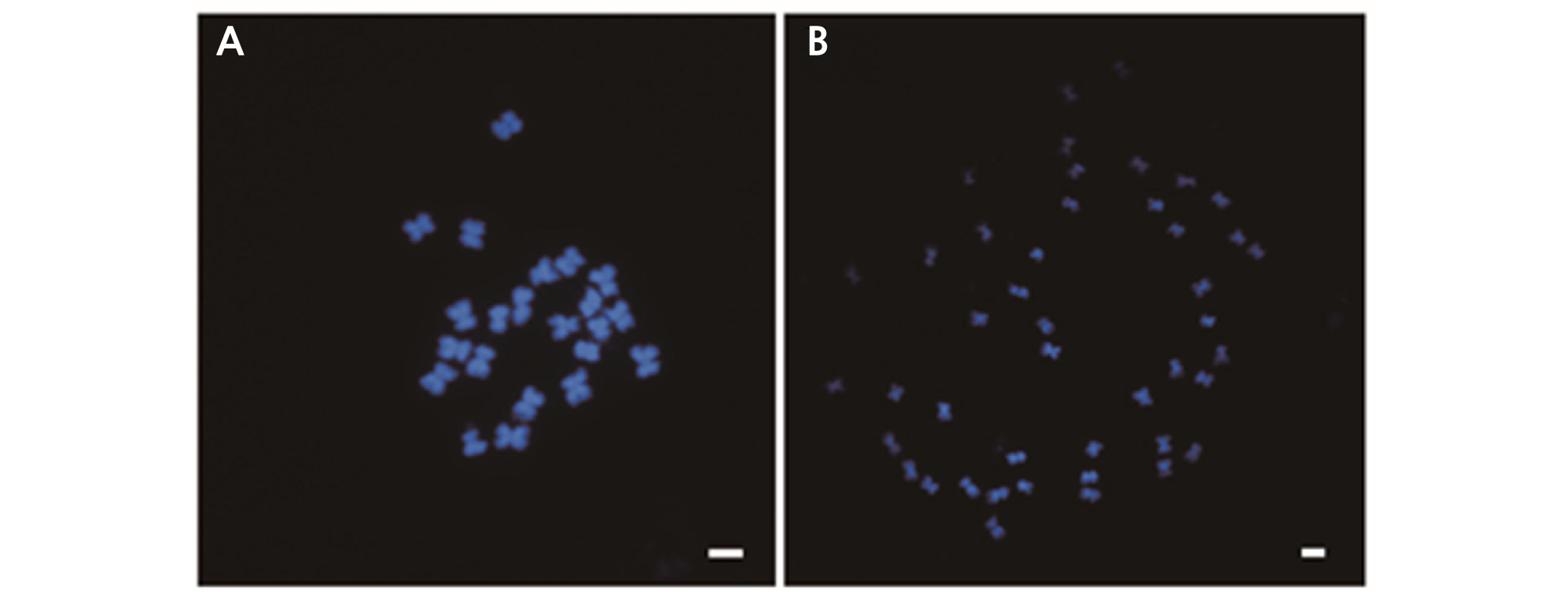
Observing the number of chromosomes is another method to determine ploidy (Sari et al., 1999). The number of chromosomes in the root tip cells were investigated by flow cytometric analysis to confirm the ploidy level. The chromosomes were examined and we found 2n = 2x = 22 chromosomes in diploid plants and 2n = 4x = 44 chromosomes in tetraploid plants (Fig. 5). Moreover, our chromosomal observations were consistent with flow cytometric analysis results. We found that it was more effective to apply 25 mg·L-1 oryzalin directly to the shot apex of watermelon seedlings than to immerse the seeds in oryzalin solution. Thus, oryzalin was found to be effective in polyploidization of watermelon and can be used as a substitute for colchicine.
We predict, that this treatment method and concentration of oryzalin can be effective to induce polyploidy in other plants. Therefore, we intend to repeat this experiment in other species to induce stable polyploids.