Introduction
Materials and Methods
Investigation of Bearing Habits
Measurement of Branch Characteristics and Fruiting Performance
Statistical Analysis
Results
Bearing Habits of A. truncatum
Distribution of Parent and Bearing Shoot Characteristics
Correlation Analysis between Parent Shoot and Bearing Shoot Characteristics and Fruiting Performance
Method for Screening and Identifying Parent Shoots with High Fruiting Performance
Discussion
Introduction
Acer truncatum Bunge is a maple tree species in the family Aceraceae (Wu, 2008) and is endemic to China (Li, 2005). The species is drought resistant (Wang et al., 2013), and its leaves turn bright red in autumn (Wang et al., 2015). It is widely used for afforestation in barren mountains and in landscaping. In recent years, the economic value of A. truncatum seeds has gained increased interest. A. truncatum seed protein consists of eight different amino acids that are essential for human health and the total protein content can reach 27%, which is higher than the protein content of soybeans (Glycine max) (Wang et al., 2007). Furthermore, A. truncatum seeds also have high oil content (up to 47%) (Liu et al., 2003; Wei et al., 2018; Liang et al., 2019; Qiao et al., 2019), of which 92% are unsaturated fatty acids (Liu et al., 2003; Wang et al., 2006). Therefore, A. truncatum seed oil was approved as a new resource food in 2011 by the Ministry of Health of China (Ministry of Health of the People's Republic of China, 2011).
A. truncatum seed oil is abundant in nervonic acid, a long-chain monounsaturated fatty acid (Liu et al., 2003; Qiao et al., 2019; Yang et al., 2018; Liang et al., 2019; Liu et al., 2021). Nervonic acid, 24:1 (n-9), is the most important fatty acid in the myelin sheath of nerve cells and plays an important role in maintaining the normal function of the brain and nervous system (Martínez and Mougan, 1998; Amminger et al., 2011; Kageyama et al., 2017; Li et al., 2019). A lack of nervonic acid is associated with demyelinating diseases such as multiple sclerosis and adrenoleukodystrophy (Sargent et al., 1994; Sandhir et al., 1998; Yang et al., 2018). Oral supplementation of nervonic acid could be beneficial patients suffering from these diseases (Bettger et al., 2001; Lewkowicz et al., 2019). In the past, nervonic acid was extracted from shark oil or synthesized chemically, but overfishing of sharks is devastating the ecosystem, and there are many disadvantages of chemical synthesis, such as low yield and the production of by-products, and the process is complicated (Wang et al., 2006). Therefore, extracting nervonic acid from plants is simpler and more sustainable. Only a few plants naturally produce nervonic acid, and A. truncatum is one of only 10 plants with nervonic acid in its seed oil, having a fatty acid composition >4.6% (Ma et al., 2004). Therefore, A. truncatum is potentially a valuable source of nervonic acid.
A. truncatum is mainly cultivated as a landscape tree species, and breeding efforts have focused on its ornamental properties, such as tree shape, leaf color, drought resistance, and stress resistance (Li, 2005; Wooster and Bassuk, 2009). Studies to improve fruit yield are lacking, so the seed yield is relatively low. Previous studies on A. truncatum have mainly focused on the phenotypic diversity of the natural population, stress resistance, and seed content determination (Wu and Duan, 2006), with few studies on the flower-forming and fruit bearing habits. Li (2005) showed that the mixed bud of A. truncatum containing both embryonic leaves and flowers can be located at the top of the annual branch or on the leaf axil. Zhang et al. (2014) showed that there are two types of flowers, male and hermaphrodite, that bloom at different times during the flowering season, and the same A. truncatum individual can display interannual gender alternation. Hu et al. (2009) observed the cell morphology of A. truncatum in the process of sexual differentiation, and suggested that the selective abortion of stamen and pistil occurred in the megaspore stage. Wu and Duan (2007) studied the relationship between ground diameter, plant height, and crown diameter, and the number of new branches, flower number, fruit number, vertical fruit diameter, thickness, and seed weight. However, none of these studies, to our knowledge, have described in detail the bearing habits of A. truncatum and the relationship between its branch characteristics and fruiting performance. Therefore, the objectives of this study were to: (1) investigate the fruiting habits and fruit setting structure of A. truncatum; (2) analyze the influence of bearing branch characteristics on fruiting performance to provide a theoretical basis for its bearing branch group culture and yield improvement; (3) provide an efficient and convenient method for screening and identifying the parent shoots with high fruiting performance.
Materials and Methods
Investigation of Bearing Habits
Investigation into the bearing habits of Acer truncatum was carried out in five different regions using a total of nearly 200 trees from 2014 to 2017. The branch structure, flower-bearing position, and continuous fruiting ability of 2- to 40-year-old A. truncatum individuals were continuously observed and recorded. The location, climate, age, and the number of trees for the five test sites are shown in Table 1.
Table 1.
Overview of test sites and trees
Measurement of Branch Characteristics and Fruiting Performance
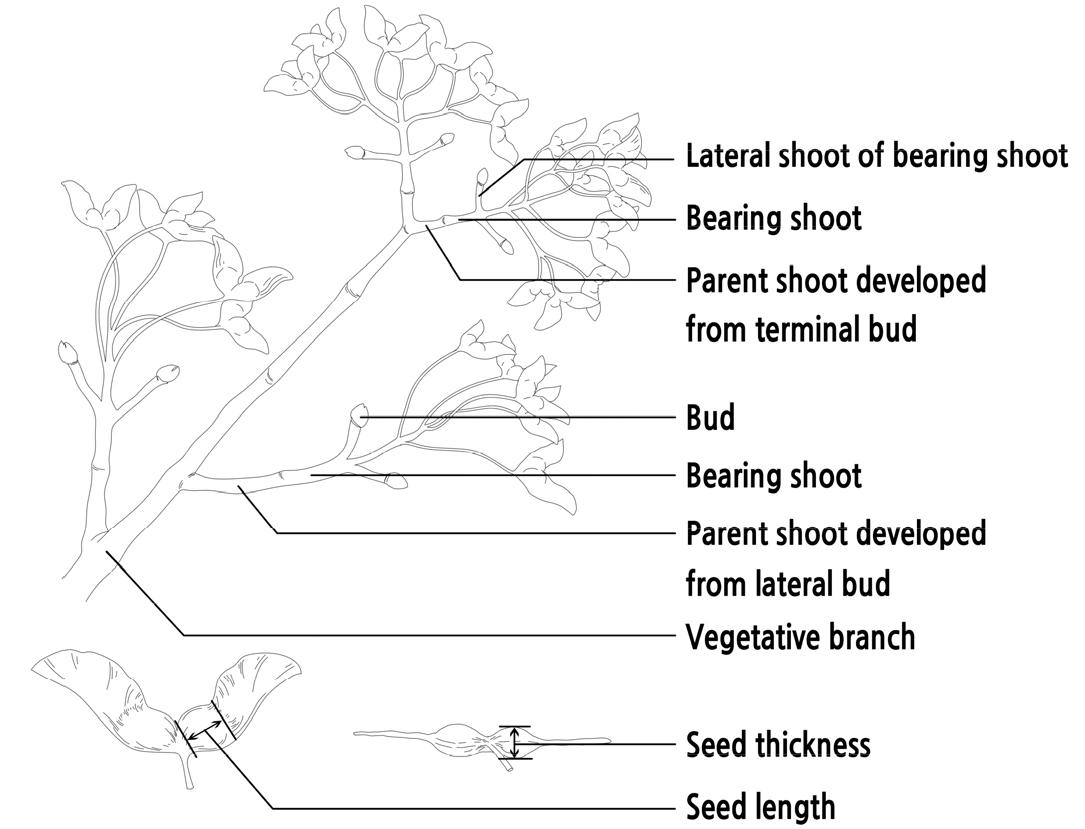
The relationship between branch characteristics and fruit performance was investigated in Dadongliu nursery in the Changping District, Beijing, in October 2017. Three 10-year-old healthy A. truncatum trees were randomly selected for the experiment. A total of 360 bearing shoots were randomly collected from four sides (southeast, southwest, northeast, and northwest) of each tree. The seeds and parent shoots of these bearing shoots were measured after being transported to the laboratory. The position of the parent shoot (developed from the terminal bud or lateral bud) was recorded (as shown in Fig. 1). The lengths of the bearing shoots and their respective parent shoots were measured with a ruler, while the top diameter and basal diameters of bearing shoots and their respective parent shoots were measured with a digital vernier caliper (Forgestar 7D-02150, Shanghai, China). The seeds of each bearing shoot were weighed and counted as the yield of the bearing shoot, and the thickness, length, and weight of the seeds were measured with a digital vernier caliper and 0.1 mg microelectronic scale (Sartorius Quintix 224-1CN, Goettingen, Germany).
The slenderness of the parent and bearing shoots was calculated by the following equation:
Slenderness = (top diameter + basal diameter) / length × 2
Statistical Analysis
The data were analyzed using SPSS Statistics v. 25.0. The Spearman correlation method was used to analyze the correlation of the nine branch characteristic indices (the position, length, top diameter, basal diameter, and slenderness of the parent shoot; and the length, top diameter, basal diameter, and slenderness of the bearing shoot) with the five fruit indexes of A. truncatum (yield of the bearing shoot, number of fruit on the bearing shoot, and seed weight, height, and length). The positions of parent shoots (developed from a lateral or terminal bud) were assigned values of either 1 or 2 for statistical analysis.
To provide a basis for screening and identifying branches with high specific fruiting performance in the actual management of A. truncatum, several methods were proposed by group comparisons. The group comparison analysis began by determining the appropriate parent-shoot characteristics, so the stepwise regression was conducted on the parent-shoot characteristics and specific fruiting performance of A. truncatum to ascertain the standard coefficient of each parent shoot characteristic. It is difficult in practice to measure and calculate slenderness, so we conducted stepwise regression analyses on the four characteristics of the parent shoots (position, length, top diameter, and basal diameter) and the five fruit indexes of A. truncatum. According to the standardization coefficient of each parent shoot characteristic from large to small, the first two characteristics with the greatest impact were used as the basis for grouping. Then each selected parent shoot characteristic was divided into three categories by K-means cluster analysis, and the parent-shoot characteristics were arranged and combined between different groups. Finally, ANOVA was performed to determine the differences in yield of the bearing shoot, number of fruit on the bearing shoot, and seed weight, height, and length among the different groups.
Results
Bearing Habits of A. truncatum
A. truncatum fruit developed from mixed flower buds. On the fruiting tree, the mixed buds were generally borne on the tops of the parent shoots, as shown in Fig. 2. The mixed flowers bloomed from mid-March to mid-April. A bearing shoot was formed at the base of the inflorescence axis and extended forward along the inflorescence axis. After flowering, the bearing shoots became brown and gradually lignified until, by autumn, they were completely lignified, as shown in Fig. 2A and 2C. At the end of the flowering period and the beginning of fruiting, most of the bearing shoots grew two symmetrical shoots lateral to the bearing shoots, as shown in Fig. 2B. The lateral shoots of the bearing shoots could bear terminal buds. When the terminal buds of the lateral shoots are mixed buds, these lateral shoots become the parent shoots in the following year. These parent shoots can produce inflorescences in the following year and may continue to develop new lateral shoots from the bearing shoots. This growth pattern can cause the formation of a Y-shaped fruiting branch group, which is the basis for the continuous fruit setting of A. truncatum, as shown in Fig. 2A. Other than the terminal bud, the parent shoots can also develop from the lateral bud of a vegetative branch on some occasions, as shown in Fig. 2D. On younger trees or branches with strong growth vigor after heavy pruning, the lateral buds of a vegetative branch may be mixed buds and produce inflorescences directly, so these long and vigorous vegetative branches become parent shoots, as shown in Fig. 2E and 2F.

Fig. 2.
Bearing habits of A. truncatum (A) Fruit set of A. truncatum; A1: Parent shoot/the lateral shoot of the previous year’s bearing shoot; A2: Bearing shoot; A3: The lateral shoot of the bearing shoot; A4: The lateral shoot of the bearing shoots of the previous year, of which the terminal buds were not mixed buds and did not develop into bearing shoots; (B) Bearing shoot in fruit growth period; B1: Infructescence; B2: Lateral shoot of the bearing shoot not yet lignified; B3: Bearing shoot not yet lignified; (C) Bearing shoot in fruit growth period; C1: Lignified lateral shoot of bearing shoot; C2: Lignified bearing shoot; (D) Fruit set of A. truncatum developed from the lateral bud of the vegetative branch; D1: Parent shoot developed from the lateral bud of the vegetative branch; D2: Bearing shoot; D3: Lateral shoot of the bearing shoot; (E) Parent shoot on a young A. truncatum plant; E1: Inflorescences developed directly by the lateral buds of the parent shoot with no bearing shoot and lateral shoot of the bearing shoot; E2: Bearing shoot with no lateral shoot of the bearing shoot developed by the lateral bud of the parent shoot; (F) Inflorescences developed directly by the lateral buds of parent shoots developed after heavy pruning; F1: Pruned vegetative branch in the year before last; F2: Inflorescences developed directly by the lateral buds of the parent shoot; F3: Newly developed parent shoot with strong growth vigor in the previous year.
Distribution of Parent and Bearing Shoot Characteristics
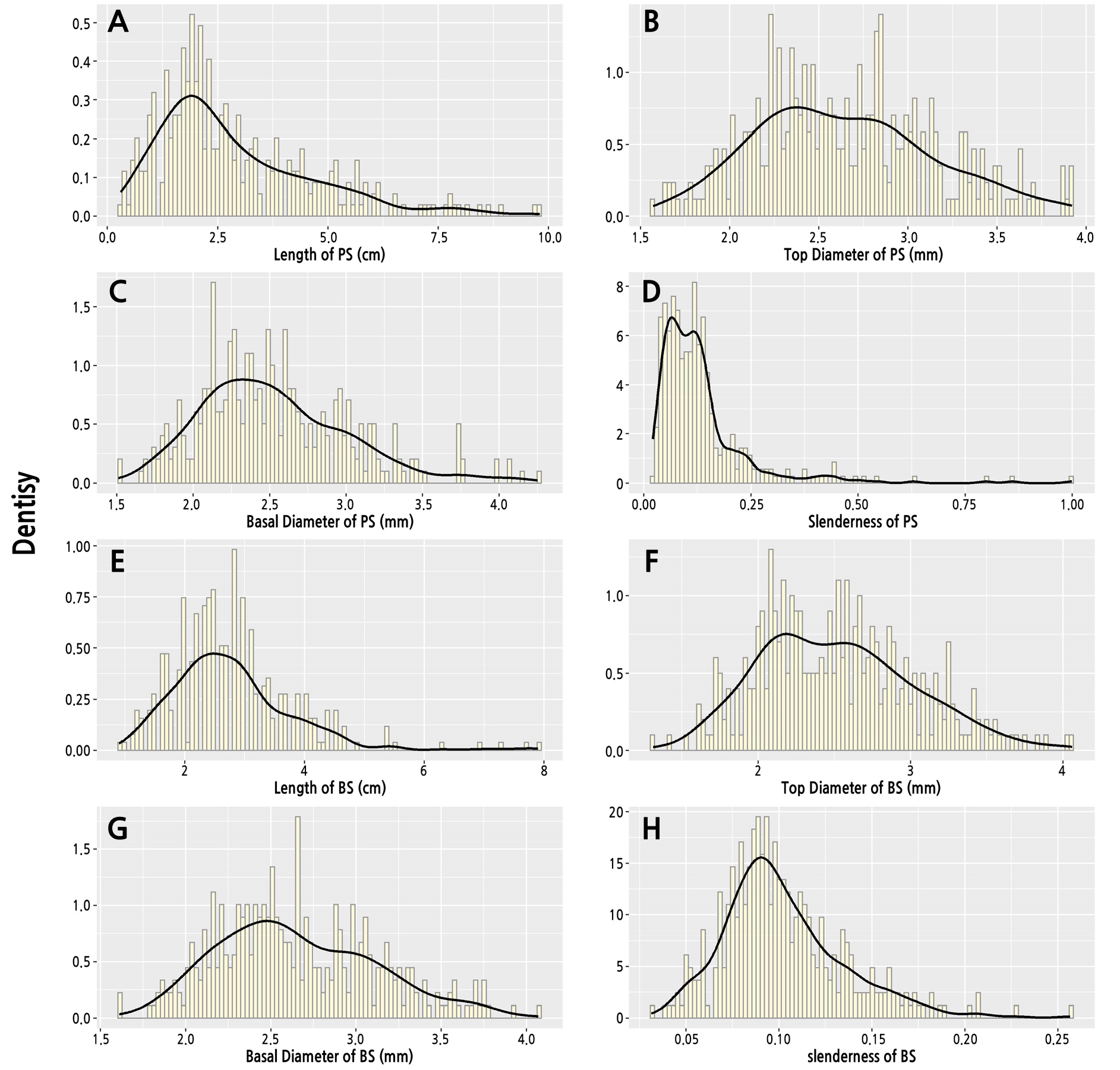
The index distribution and kernel distribution density of A. truncatum parent shoots and bearing shoots are shown in Fig. 3. The length of the parent shoots ranged from 0.3 to 9.2 cm. The highest frequency length observed for the parent shoots was 1.3–2.8 cm. The top diameter of the parent shoots ranged from 1.57 to 3.92 mm. The highest frequency top diameter observed for the parent shoots was 2.21–2.88 mm. The basal diameter of the parent shoots ranged from 1.51 and 4.25 mm, and the highest frequency basal diameter observed for the parent shoots was 2.23–2.69 mm. The slenderness of the parent shoots was between 0.02 and 0.99, and the highest frequency slenderness observed for the parent shoots was 0.05–0.14. Because some parent shoots were short (less than 0.5 cm), the slenderness of these branches was more than 0.25.
The index distribution of the bearing shoots was similar to that of parent shoots. The length of the bearing shoots ranged from 0.9 to 7.9 cm, and the highest frequency length observed for the bearing shoots was 1.9–3.3 cm. The top diameter of the bearing shoots ranged from 1.31 to 4.06 mm, and the highest frequency top diameter observed for the bearing shoots was 2.15–2.86 mm. The basal diameter of the bearing shoots ranged from 1.61 to 4.07 mm, and the highest frequency top diameter observed for the bearing shoots was 2.08–2.77 mm. The slenderness of the bearing shoots was between 0.03 and 0.26, and the highest frequency slenderness observed for the bearing shoots was 0.07–0.13.
Correlation Analysis between Parent Shoot and Bearing Shoot Characteristics and Fruiting Performance
The Spearman correlation analysis results of the nine branch characteristic indexes with the five fruit indexes of A. truncatum are shown in Fig. 4. There was a significant negative correlation between the yield of the bearing shoot and the parent-shoot position with a correlation coefficient of -0.406; the yield of the bearing shoot with a lateral parent shoot was higher than that with a terminal parent shoot. There were significant positive correlations between the yield and the top and basal diameters of the parent and bearing shoots with a correlation coefficient of over 0.3. The parent shoot length had no significant correlation with the yield of the bearing shoot. There was no correlation between the yield and slenderness of the bearing shoot, but there was a significant correlation between the yield of the bearing shoot and the slenderness of the parent shoot, with a correlation coefficient of less than 0.17.
The number of fruit on the bearing shoot showed no significant correlation with either the position or the length of the parent shoot or the slenderness of the parent and bearing shoots. However, there was a significant correlation between the number of fruit on bearing shoot, top and basal diameter of the parent shoot, and top and basal diameter and length of the bearing shoot. The correlation coefficient between the number of fruit on the bearing shoot and the top diameter of the parent shoot, and between the top diameter of the bearing shoot and the basal diameter of the bearing shoot were all greater than 0.44. The correlation coefficient between the number of fruit on bearing shoot and the bearing shoot length was less than 0.2.
There was a significant negative correlation between the seed weight and the parent-shoot position, with a correlation coefficient of –0.469. The top diameter of the parent shoot also showed a significant negative correlation with the seed weight, for which the correlation coefficient was just over –0.1. The length and basal diameter of the parent shoot and the length and slenderness of the bearing shoot had no significant correlation with the seed weight. The seed thickness had a significant negative correlation with the position and length of the parent shoot and the length of the bearing shoot with correlation coefficients of –0.32, –0.23, and –0.16, respectively. There was a significant positive correlation between the seed thickness and the slenderness of the parent and bearing shoots with correlation coefficients greater than 0.18. All other branch characteristics were not correlated with the seed thickness. The seed length had a significant negative correlation with the position and slenderness of the parent shoot, with correlation coefficients of –0.232 and –0.171, respectively. The seed length had a significant positive correlation with the basal diameter and slenderness of the parent shoot and the length of the bearing shoot, but the correlation coefficients were all less than 0.24.
Method for Screening and Identifying Parent Shoots with High Fruiting Performance
Classification of Parent Shoots
Stepwise regression analysis was conducted on the parent-shoot characteristics and fruiting performance of A. truncatum to ascertain the two parent-shoot characteristics with the greatest impact on fruiting performance according to standardized coefficients. The results of this analysis are shown in Table 2. Cluster analysis was carried out on the four parent characteristics ascertained by the regression analysis above (the position, top diameter, basal diameter, and length of the parent shoot), and the results are shown in Table 3. The results of the grouping and classification of parent shoots are also shown in Table 3. Three methods were proposed. Method A is based on the position and top diameter of the parent shoots, method B is based on the position and length of the parent shoots, and method C is based on the top and basal diameter of the parent shoots. The groups represent the parent shoots with different characteristics, under different classifications criteria. In method C, group C-Thin/Thick did not exist, since there was no type with a small top diameter but a large basal diameter in the test bearing shoots. Similarly, group C-Thick/Thin was seldom observed (< 1%). The number of bearing shoots with a thick top diameter but a thin basal diameter and bearing shoots with a medium top diameter but a thick basal diameter were fewer than three, so ANOVA could not be performed for these two groups.
Table 2.
Stepwise regression analysis between parent shoot characteristics and fruiting performance
Table 3.
Grouping and classifications of parent shoots
Group Comparison of Yield of Bearing Shoots Under Different Parent Shoot Classifications
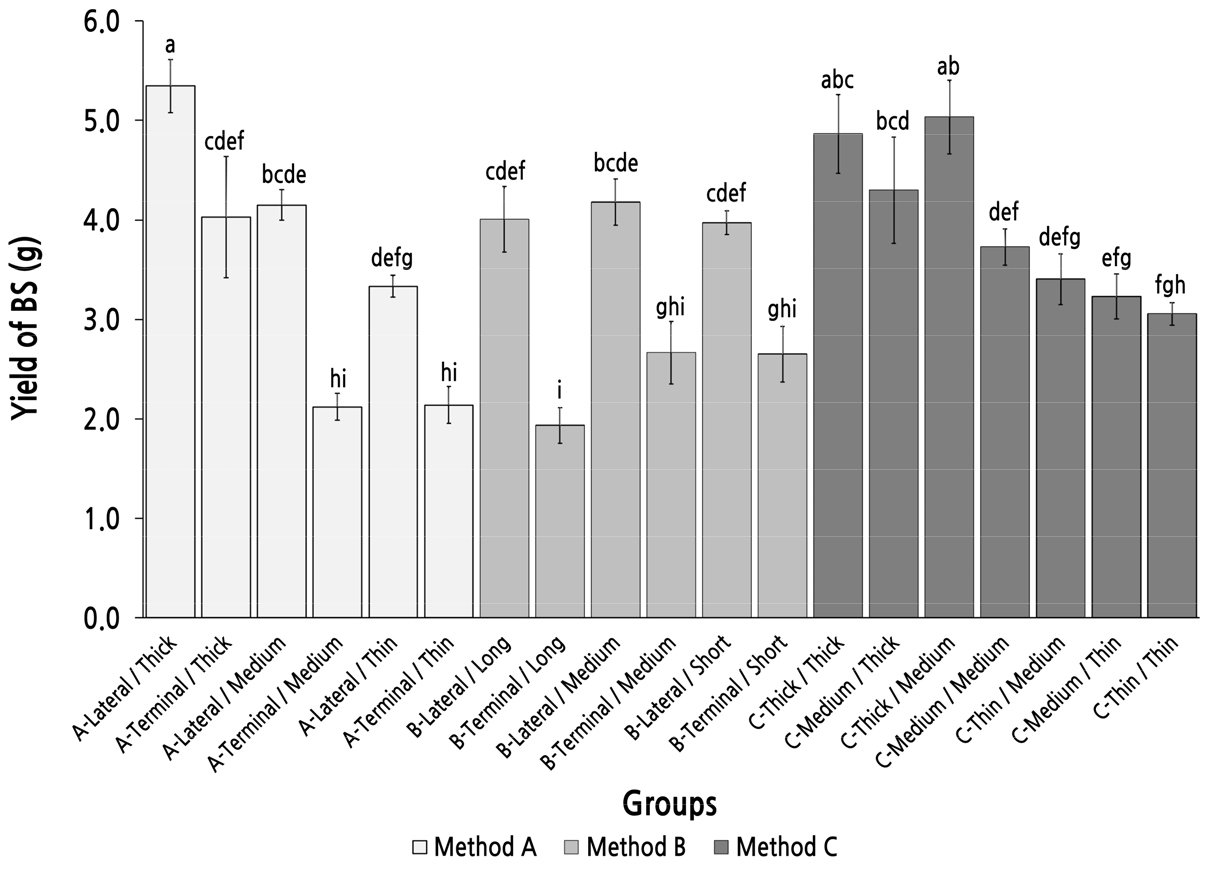
The highest yield (total seed weight) of bearing shoots was found in group A-lateral/Thick, which was the only group for which the bearing shoot yield was over 5 g, and was significantly higher than that of the other groups, except for group C-Thick/Thick and group C-Thick/Medium (Fig. 5). In method A, bearing shoot yield decreased with the diminution of the top diameter of the parent shoot. Among the groups with the same top diameter, the yield of the group with the parent shoot produced from a lateral bud was always higher than that of the group with the parent shoot produced from a terminal bud, and this was consistent in groups for method B. In method B, there were no significant differences between the groups with same length type, which may imply that the length of the parent shoot cannot be used as a basis to judge the yield of the bearing shoot. These results showed that a larger top diameter of the parent shoot is essential for improving the yield of the bearing shoot, and the parent shoot with the highest yield of bearing shoot could not be selected by method B, which was sorted by length and position of the parent shoot.
Group Comparison of the Number of Fruit on the Bearing Shoot Under Different Parent Shoot Classifications
The number of fruit on the bearing shoot was more than 12 in four groups with a thick top diameter (group A-Lateral/Thick, A-Terminal/Thick, C-Thick/Thick, and C-Thick/Medium) and was significantly higher than that in the other 15 groups in which the number of fruit on bearing shoot was fewer than 10 (Fig. 6). In methods A and C, the number of fruit on bearing shoot tended to decrease with the reduction in the top diameter of the parent shoot. The basal diameter of the parent shoot did not strongly affect the number of fruit on the bearing shoot because the groups with the same top diameter type but different basal diameters showed no significant difference in the number of fruit of the bearing shoot. The parent-shoot position seemed to have little influence on the number of fruit of the bearing shoot because the six pairs of groups with the same top diameter but different parent-shoot positions in methods A and B had no significant differences. The parent shoot length also had little influence on the number of fruit on the bearing shoot. In method B, the number of fruit on bearing shoot of all groups was not significantly different, but all were significantly smaller than in group A-Lateral/Thick and group C-Thick/Medium. This result indicated that the parent shoot with the best fruiting performance (highest number of fruit of the bearing shoot) could not be selected by method B, which was sorted by the length and position of the parent shoot.
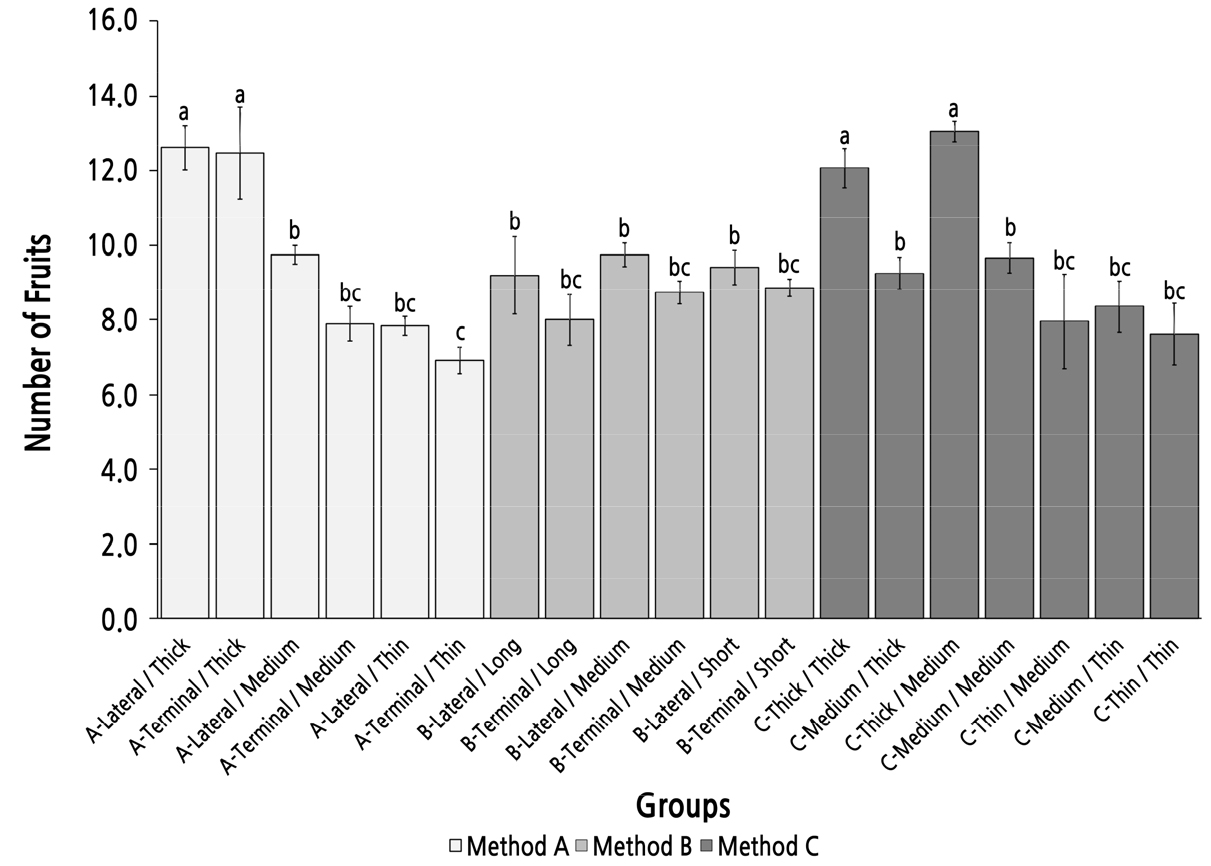
Fig. 6.
Group comparison of the number of fruit on the bearing shoot in different groups under different classifications of parent bearing branches. Error bars represent standard errors. Groups with the same letter are not significantly different (one-way ANOVA, Duncan’s multiple range post hoc test, p < 0.05).
Group Comparison of Seed Weight Under Different Parent Shoot Classifications
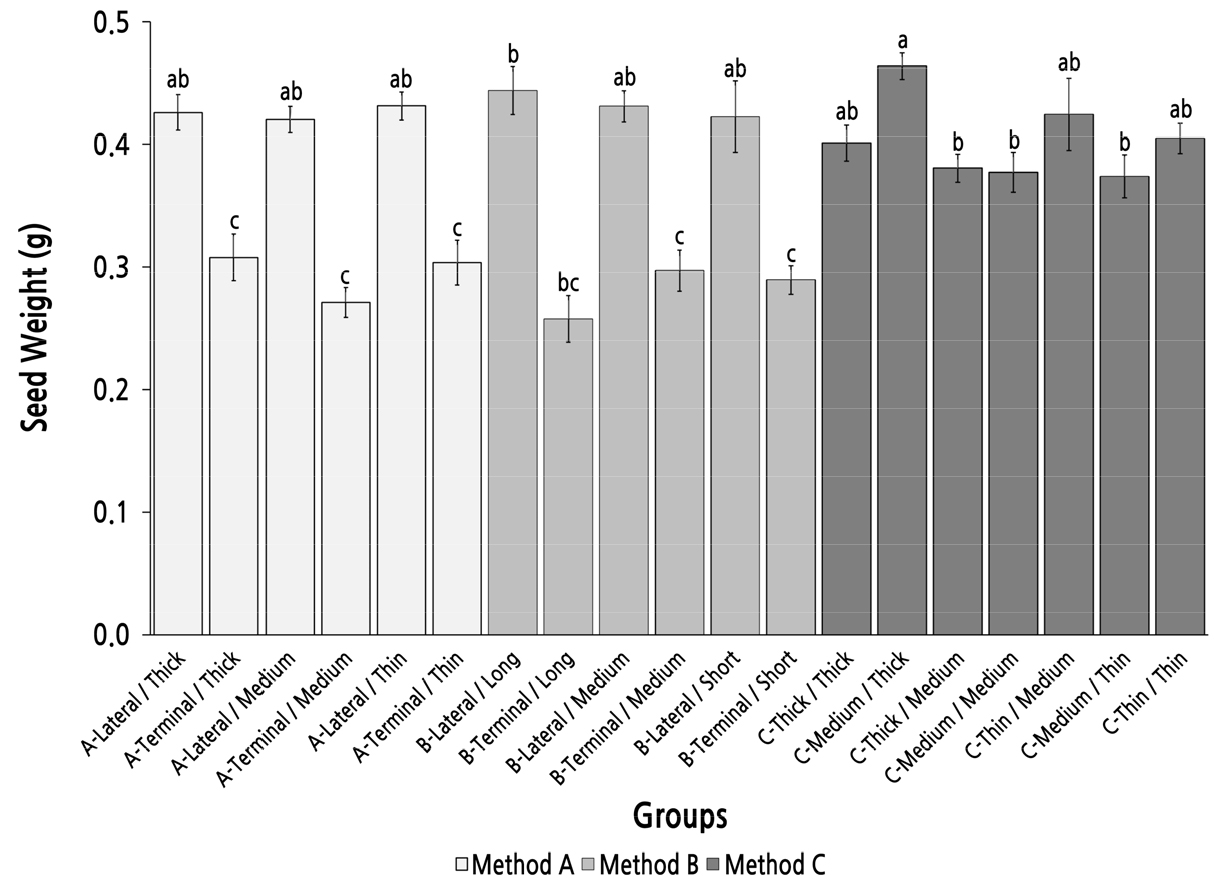
The group C-Medium/Thick had the heaviest seed weight (0.46 g) but showed no significant difference to the six lateral shoot groups in methods A and B (Fig. 7). In methods A and B, there were significant differences between the groups with lateral parent shoots and the groups with terminal parent shoots; the seed weight of the groups with lateral parent shoots was more than 0.41 g and was significantly higher than that of the groups with terminal parent shoots, in which the seed weight was lower than 0.31 g. However, there were no significant differences among the groups with lateral parent shoots or the groups with terminal parent shoots; these results showed that the position of the parent shoot was the main factor that affected the seed weight and the top diameter and length of the parent shoot had little influence on seed weight. In method C, there were no significant differences among the groups, except group C-Medium/Thick. This result indicated that method C was not an efficient strategy to screen and identify parent shoots with high seed weight performance.
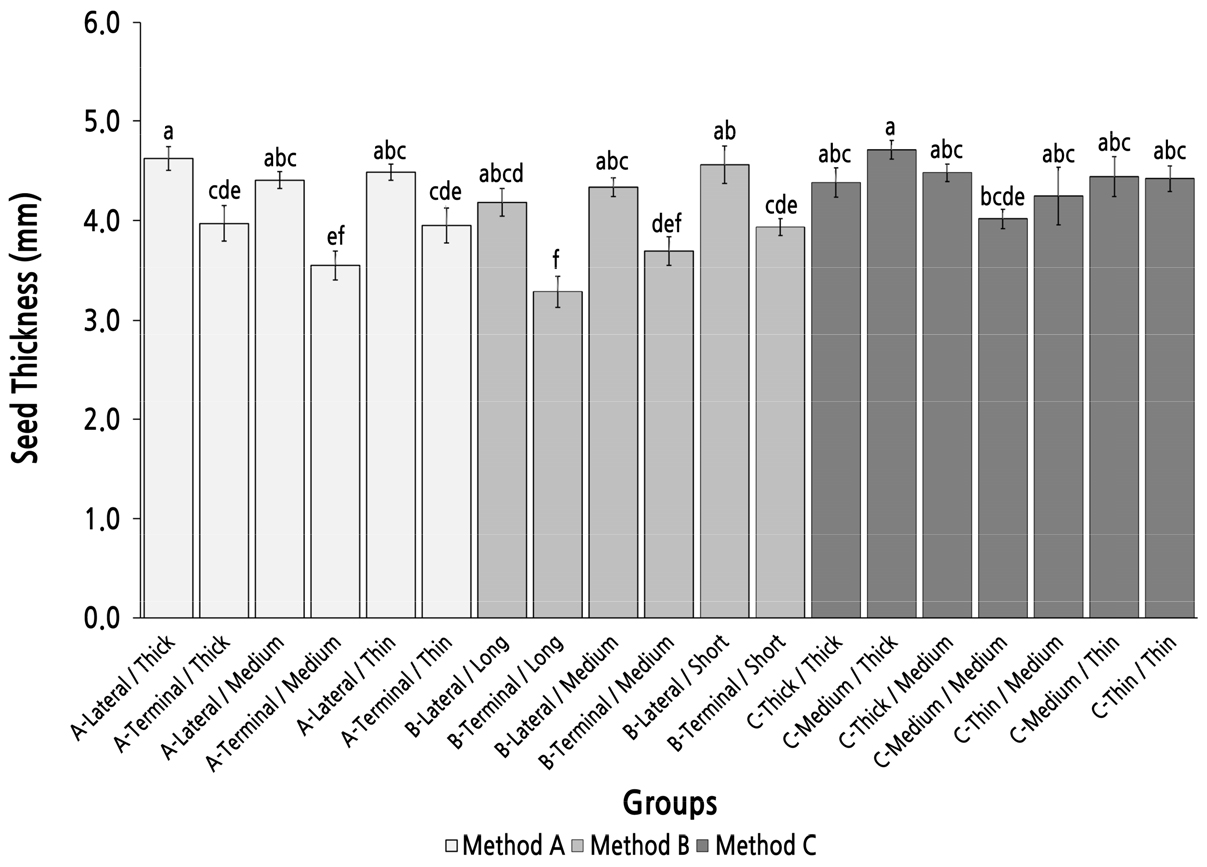
Group Comparison of Seed Thickness Under Different Parent Shoot Classifications
The maximum seed thickness appeared in group C-Medium/Thick. However, there were no statistically significance differences with the other groups in method C except for group C-Medium/Medium and six groups with parent shoots derived from a terminal bud in methods A and B (Fig. 8). The seed thickness in group C-Medium/Thick was also not statistically significant compared with the six groups with parent shoots derived from a lateral bud in methods A and B. There were no significant differences between the six groups with parent shoots derived from a lateral bud in methods A and B. However, there were significant differences between the groups with the same top diameter and length of the parent shoot. These results implied that the parent-shoot position had a considerable influence on the seed thickness, and the top/basal diameter had little influence on seed thickness, and method C was not an efficient method to screen and identify parent shoots with a high seed-thickness performance.
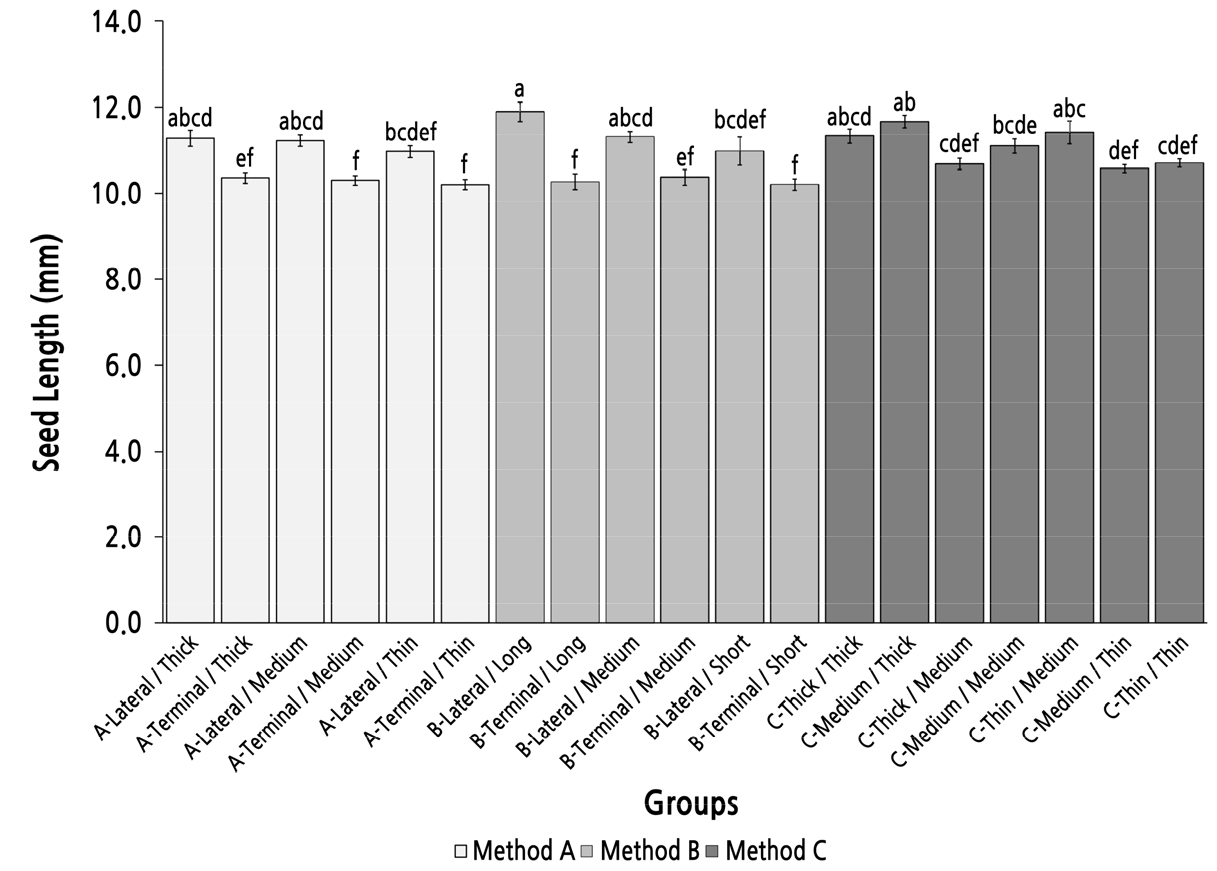
Group Comparison of Seed Length Under Different Parent Shoot Classifications
The maximum seed length was almost 12.00 mm and was observed in group B-Long/Lateral (Fig. 9). The seed length in groups A-Lateral/Thick, A-Lateral/Medium, B-Lateral/Medium, C-Thick/Thick, and C-Medium/Thick was slightly over 11.00 mm; however, there was no significant difference among these six groups. The seed length in groups with parent shoots derived from a terminal bud was slightly longer than 10.00 mm but was still significantly shorter than in the groups in which the seed length was longer than 11.00 mm. In method C, the two groups with a basal diameter thinner than the top diameter (group C-Thick/Medium and group C-Medium/Thin) had significantly shorter seeds than those in group C-Medium/Thick. These results indicated that method A was an efficient method to screen and identify parent shoots with high seed-length performance.
Group comparison results showed that method A (based on the position and top diameter of the parent shoots) was efficient for selecting parent shoots with a high yield (total seed weight), amount of fruit on the bearing shoot, seed thickness, and seed length. Method B (based on the position and length of the parent shoots) was efficient for selecting parent shoots with high seed weight and thickness. Method C (based on the top and basal diameter of the parent shoots) was efficient for selecting parent shoots with high yield (total seed weight), amount of fruit on the bearing shoot, and seed weight. Overall, for practical management of A. truncatum, we suggest that method A is the optimum method for screening and identifying parent shoots with high fruiting performance, among which the parent shoots with a top diameter thicker than 3.08 mm developed by a lateral bud in a vegetative branch had a greater yield (total seed weight), number of fruit, and seed weight. This method could be applied to improve yield in A. truncatum production.
Discussion
The bearing shoots of A. truncatum can bear two symmetrical lateral shoots on which the terminal buds are often mixed buds, resulting in Y-shaped fruiting branches with successive multi-year bearing capacities. This habit also occurs in some cultivars of apples (Malus domestica), pears (Pyrus communis), and walnuts (Juglans sp.) (Sheng et al., 1985; Hissano et al., 1990; Sansavini, 2002; Xi, 2009). The Y-shaped fruiting branches seem like false dichotomous branching, but in fact are not because the lateral shoots of bearing shoots are not developed after the infructescence pedicels fall off. In this way, A. truncatum differs from Paulownia, in which shoots develop from the lateral buds in the following year, after three to four terminal internodes have wilted (Ye and Yang, 2009).
The present study demonstrates that A. truncatum has two fruiting habits—in which the fruit is borne on lateral buds on the long, vigorous parent shoot or on terminal buds on the short parent shoot, similar to some cultivars of peach (Prunus persica), apple, and pear (Saure, 1987; Sansavini, 2002; Xi, 2009; Han, 2016). However, there are few long, vigorous parent shoots with lateral buds that can bear fruit in the natural state of A. truncatum, as young A. truncatum exhibit vigorous vegetative growth prior to fruiting where the shoots are mostly upright and do not form mixed flower buds. During the fruiting period, tree vigor decreases rapidly, the number of short parent shoots increases, and the number of long, vigorous shoots decreases sharply. The short parent shoots of A. truncatum cannot be renewed under natural conditions, resulting in low yield.
These problems can be solved by training and pruning. Optimum training and pruning are used widely for orchard management, as their practice can improve whole-tree light distribution within the canopy (Lordan et al., 2017), balance vegetative and reproductive growth (Grossman et al., 1998), cultivate and induce fruiting set (Lordan et al., 2017), avoid biennial cropping (Olson et al., 1990), and improve fruit quality (Kumar et al., 2010). Bassi et al. (1994) demonstrated that standard and spur-type training systems significantly increase yield in peach. Further, Grossman et al. (1998) indicated that different training systems could allow for higher planting density and orchard yield in peach without negatively affecting fruit quality. The study by Lordan et al. (2017) on pears led to similar conclusions. In addition, Kumar et al. (2010) showed that while moderate pruning did not increase whole-plant yield in peach, it did significantly increase individual fruit weight, size, soluble solids content, and sugar content. Robinson et al. (2016) showed that, compared to minimum pruning, stubbing back (eliminate 1/3 of all fruiting spurs on each branch) did not increase apple fruit yield but did increase fruit size and crop value. A. truncatum needs suitable training systems and pruning methods, whichcould reduce vegetative growth vigor to achieve early fruiting Further, optimum pruning methods—such as stimulating lateral flower buds to induce long, vigorous shoots which could bear mixed bud and then bear fruits in the next year by heavy pruning or promoting the growth of short parent shoots by light pruning—could be utilized to cultivate parent shoots; thus, providing a stable production method for many years. These are important subsequent research areas in A. truncatum production.
The results of the correlation analysis, regression analysis, and group comparison were consistent and showed that the parent-shoot characteristics had a significant impact on the fruit performance of A. truncatum. The parent-shoot position had significant effects on the yield and seed weight, length, and height. Indeed, the parent-shoot positions affected the yield by affecting the seed weight rather than the number of fruit. The yield of the bearing shoots with lateral-derived parent shoots was higher than that of the bearing shoots with terminal-derived parent shoots. The reason for this result may be that two or more terminal-derived parent shoots competed for assimilates from the vegetative branch, and transportation efficiency of assimilates per parent shoot was lower than that of the lateral parent shoots with no competition in the same position. However, further research is needed to confirm this speculation.
Parent shoot length had no significant effect on yield, the number of fruit, or seed weight. This relationship could be explained in part by the findings of Hansen (1969) and Baïram et al. (2019), who reported that the distribution of photosynthetic assimilates between sources and sinks showed few significant differences between branches with different length ranks and could not exclusively be explained by source-sink distance. However, this hypothesis has been controversial (Dengler, 2006; Orians et al., 2015). The top and basal diameters of the parent shoots had little effect on seed thickness and length but had a considerable effect on the yield of the bearing shoot. The parent shoots of A. truncatum affect yield in a way that affects the number of fruit rather than the seed weight, which was consistent with previous research on litchis (Litchi chinensis) (Xu et al., 2010), hazelnuts (Corylus sp.) (Chen et al., 2013), and walnuts (Xia et al., 2018), whereas similar results were not found for tangerines (Citrus reticulata) (Su et al., 2018). The reason the parent shoot diameter affects the yield and number of fruit of the bearing shoot may be that thicker parent shoots have a larger surface area (Kelc, 2007). Hence, they can carry more fruit and have a lower incidence of dropping flowers and fruits. Additionally, the thicker parent shoots may have better nutritional levels and growth vigor and may obtain more nutrition in competition with other branches. The relationship coefficients between the characteristics of the parent shoots of A. truncatum and fruit performance were all less than 0.5. The reason for these results may be that the present study focused only on branch morphology, but fruiting performance is also affected by many other endogenous and exogenous factors such as the distance from the fruit to leaves (Lauri and Kelner, 2011; Belhassine et al., 2019), leaf area of bearing shoots and parent shoots (Sabatier and Barthélémy, 2001; Baïram et al., 2019), and traits of lateral shoots of bearing shoots (Han, 2016), all of which require further research.