Introduction
Materials and Methods
Experiment I: The Effects of Temperature and Vernalization on Seed Germination Vigor
Experiment II: Effects of Seed Storage Period and Light and Dark Treatments on Germination
Statistical Analysis
Results and Discussion
Characteristics of A. dubia Seeds
Experiment I: Effects of Temperature and Vernalization on Germination Viability
Experiment II: Effects of Seed Storage Period and Light and Dark Treatments on Germination
Introduction
Plants in the genus Artemisia L. within the family Compositae are native to a geographically broad area, extending from northern Asia to the polar regions of the Northern Hemisphere and from tropical and subtropical regions to hot deserts in the Southern Hemisphere, with an estimated 200 to 400 species worldwide, depending on different taxonomic authorities (McArthur, 1981; Mabberley, 1987; Lodari et al., 1989; Ling, 1991, 1992, 1995; Lee et al., 2010). In South Korea, 38 species of Artemisia have been reported to grow on mountains and in fields throughout the country (Ryu et al., 2005).
Research on Korean Artemisia was initiated by Nakai (1911), who recorded three sections of 29 taxa according to floral phenotype, which were subsequently rearranged by Mori (1922) into a simplified list of 40 taxa. However, as a consequence of more recent genetic research based on techniques such as random amplified polymorphic DNA and internal transcribed spacer sequencing, there is currently little consensus regarding the identification and taxonomy of Korean Artemisia species, owing to morphological similarities among the taxa and the diversity of external morphological variations within the same geographical or taxonomic groups (Park et al., 2012). An example in this regard is the true wormwood (chamssuk) native to Korea, which is used as both an edible and medicinal plant and is recorded under the names Artemisia mongolica var. tenuifolia (Park and Lee, 1997) and Artemisia lavandulaefolia (Lee at al., 1999). Thus, further research is required to clarify the taxonomic status of this plant (Lee et al., 2010). We use the name Artemisia dubia Wall. for this wormwood, as recorded in the Korea Biodiversity Information System of the Korea National Arboretum. A. dubia is a perennial herbaceous plant that sprouts in early spring from seeds or rhizome cuttings. It has alternate deeply cleft pinnate leaves, the adaxial surface of which is densely covered with hairs, whereas the abaxial surface is characterized by spiderweb-like hairs, which confer a grayish-white appearance. The light brown flowers, which bloom throughout August and September, are arranged in a conical inflorescence and subsequently give rise to achene fruits (1-2 mm). The average height of A. dubia is between about 1.5 and 2.0 m (KNA, 2016).
Artemisia princeps, commonly referred to as Korean wormwood or mugwort (chamssuk in Korean), is known to have a positive therapeutic effect on abdominal pain, diarrhea, colds, and stroke and has long been used in herbal medicine and folk remedies for its anti-inflammatory effects on bronchitis, stomatitis, cervicitis, and asthma and also has a soothing effect on gynecological disorders (Sim et al., 1992). As a notably rich source of vitamins A, B, C, and D, calcium, proteins, lipids, and carbohydrates, A. dubia is widely consumed as a nutritious vegetable (Park and Lee, 1997), and Koreans have long enjoyed the unique aroma and taste of the young leaves of wild-grown plants, which are used to prepare soups, pancakes, tempura, and tea (Park, 2008).
A. dubia is also commercially cultivated in both open fields and greenhouses. Annually, the rhizomes of this plant are cut into sections and planted in open fields, and although the leaves are harvested three or four times a year, only the young leaves harvested in spring are consumed as a vegetable, whereas those harvested from May onwards are used in the preparation of cosmetics, soaps, beverages, rice cakes, and medicines, owing to their strong bitter taste and aroma, the development of which is attributable to exposure to high temperatures and strong sunlight. However, A. dubia is grown in three regions (Hampyeong, Naju, and Gangjin) and in addition to its seasonal limitation as a vegetable, A. dubia cultivation is extremely labor-intensive throughout the process, including rhizome plantation, pest and weed control, and harvest, which lowers its economic returns. Therefore, this study examined the germination rate, energy, and vigor of A. dubia to determine optimal cultivation conditions in a plant factory system for year-round growth and harvest.
Materials and Methods
Experiment I: The Effects of Temperature and Vernalization on Seed Germination Vigor
Germination Conditions
The experiments were conducted between February and November 2020 for periods of 8 days after sowing in a growth chamber (HB-301S-0, HANBAEK, Gyeonggi-do, Korea), and environmental conditions of light, temperature, and relative humidity (RH) were controlled in the growth chamber with an automatic control system at the Department of Environmental Horticulture, University of Seoul. For all treatments, RH and light exposure were set at 70% and 200 µmol·m2·s-1, respectively, whereas temperature was varied according to the treatment group. The A. dubia seeds used for experiments were gathered at the Hampyeong cultivation site (Hakgyo-myeon Hampyeong-gun, Jeollanam-do) from 2017 to 2019. As a pre-treatment to enhance storage performance, the seeds were sterilized in sunlight for 12 h, dried for 3-4 days in a well-ventilated shady location, and stored under two different temperature conditions: room temperature (25-27°C, RH 50-60%) and low temperature in a refrigerator (Samsung M900 RQ51K92317X, Korea; 3°C ± 0.5, RH 40-45%). Temperature and relative humidity (RH) were measured by a digital thermo-hygrometer (T004, CASS Co. Ltd. China) installed in the seed storage room. To ensure the uniformity of experimental seeds, only those with a diameter of at least 0.6 mm were selected using a standard sieve (TESTING SIEVE 500 µm, Korea). Seed size was measured under a Nikon SMZ18 stereoscopic microscope, and each seed was individually weighed using an HS-S Series precision electronic balance. Prior to sowing, the selected seeds were surface-sterilized with 70% alcohol. The experimental design was a factorial randomized block arrangement with three replications with 200 seeds each. The seeds were placed on Whatman no.1 filter paper in sterilized 900 ´15-mm Petri dishes and immersed in 15 mL of distilled water, ensuring that they did not float. The contents of the Petri dishes were kept moist by spraying with distilled water once or twice daily. Germination was measured as a percentage 8 days after the experiment was initiated.
Effects of Temperature and Vernalization on Seed Germination Vigor
To identify the conditions necessary for breaking dormancy with respect to temperature and length of vernalization, batches of A. dubia seeds, gathered and distributed in November of the 3 years from 2017 to 2019, were divided into two portions, one of which was stored at room temperature (25-27°C) and the other at low temperature (3°C) for 3 months (November 2019 to January 2020). After sowing both groups of each 200 seeds in triplicate for each of the three examined germination temperature conditions (20°C, 25°C, and 3°C ± 0.5) on February 8, 2020, we monitored germination status daily over the subsequent 8-day period. Seeds with a radicle length greater than 2 mm were defined as having germinated.
Experiment II: Effects of Seed Storage Period and Light and Dark Treatments on Germination
Effects of Seed Storage Period on Seed Germination Vigor
The experimental setup used in Experiment II was identical to that used in Experiment I. A. dubia seeds obtained for three consecutive years (2017-2019) and stored at room temperature (25-27°C) were sown in July 2020. Treatment groups were divided by year (2017, 2018, and 2019), and all experiments were conducted in triplicate using a randomized block design. The germination temperature was set to 30°C, the temperature determined to be optimal for germination in Experiment I. Additionally, to investigate the seed germination vigor according to storage temperature based on the experimental results obtained with respect to year, we stored the seeds obtained in 2018 at both low (3°C ± 0.5) and room (25-27°C) temperature for 12 months. In each of the two treatment groups, seeds were prepared as triplicate samples of 200 seeds per treatment group, and germination power was compared by repeating three times.
Effects of Light and Dark Treatments on Germination
To assess the effect of light on germination, seeds were exposed to a continually administered photosynthetic photon flux density (PPFD) of 200 µmol·m2·s-1 (determined using an HD2102.1-HD2102.2 Photo-Radiometer, Italy) for 8 days. In the comparative dark treatment, each treatment group of seeds was wrapped with silver paper, within which they were retained for 8 days. During the 8-day treatment period, the triplicate groups of seeds in each treatment were incubated at the optimal germination temperature of 30°C and inspected daily to assess germination.
Inspection of Germination Characteristics
The numbers of germinated seeds in each of the experimental groups maintained at different temperatures and subjected to differing pre-treatments were counted after sowing, and based on these observations, we calculated the following germination characteristics: percentage germination (GP), mean germination time (MGT), germination rate (GR), and germination performance index (GPI). Percentage germination represents the percentage of germinated seeds relative to the total number of seeds examined, calculated using the equation GP = (N/S) × 100, where N and S denote the total numbers of germinated and examined seeds, respectively. The mean germination time was calculated using the equation MGT = ∑(Ti × Ni)/N, where Ti, Ni, and N denote the number of days after sowing, the number of seeds germinated on the inspection day, and the total (cumulative) number of germinated seeds, respectively. The germination rate was calculated using the equation GR = ∑(Ni/Ti), where Ni and Ti denote the number of seeds germinated on the day of inspection and the number of days after sowing, respectively (Scott et al., 1984). The germination performance index was calculated as GPI = PG/MGT (Sundstrom and Edwards, 1987).
Statistical Analysis
The experimental data were analyzed using the SPSS program PASW Statistics18 (2009-07-30). The standard error of each treatment mean is expressed as an error bar representing the 95% confidence interval (CI). Multiple comparisons were performed using one-way ANOVA at a significance level of 5% for the variation between treatments. Additionally, Duncan’s multiple range test was conducted for inter-treatment comparisons.
Results and Discussion
Characteristics of A. dubia Seeds
Based on measurements obtained from a sample of 50 seeds, we determined that seeds have lengths and widths ranging from 1 to 1.8 mm and 0.5 to 0.7 mm, respectively, and a 1000-seed weight of 128.4 g (Table 1 and Fig. 1). When collecting seeds in the fall (October/November), contaminant material such as dry petals attributable to aberrant shattering made it difficult to sort seeds, whereas after mid-February, significant seed losses occurred due to the natural shattering of mature seeds. Young leaves of A. dubia are used as vegetables and are harvested four to five times a year.
Table 1.
The length, width, and thousand-seed weight of Artemisia dubia harvested from a commercial farm in November of 2019 at Hampyeong-gun
| Length (mm, mean ± SDz) | Width (mm, mean ± SD) | Thousand-seed weight (mg, mean ± SDy) |
| 1.77 ± 0.18 | 0.62 ± 0.03 | 128.4 ± 0.67 |
Experiment I: Effects of Temperature and Vernalization on Germination Viability
Among the different stages of plant development, the germination of seeds, which is influenced by a range of environmental factors, is considered the most sensitive, with temperature tending to have a pronounced effect on the expression and regulation of seed germination (Mangrauthiam et al., 2016). Önen (2006), for example, found that the optimal temperature for germination of mugwort (Artemisia vulgaris L.) seeds is 29-30°C and that the percentage germination is notably reduced if seeds are subjected to temperatures considered to be too low or high, with germination being completely inhibited at 45°C.
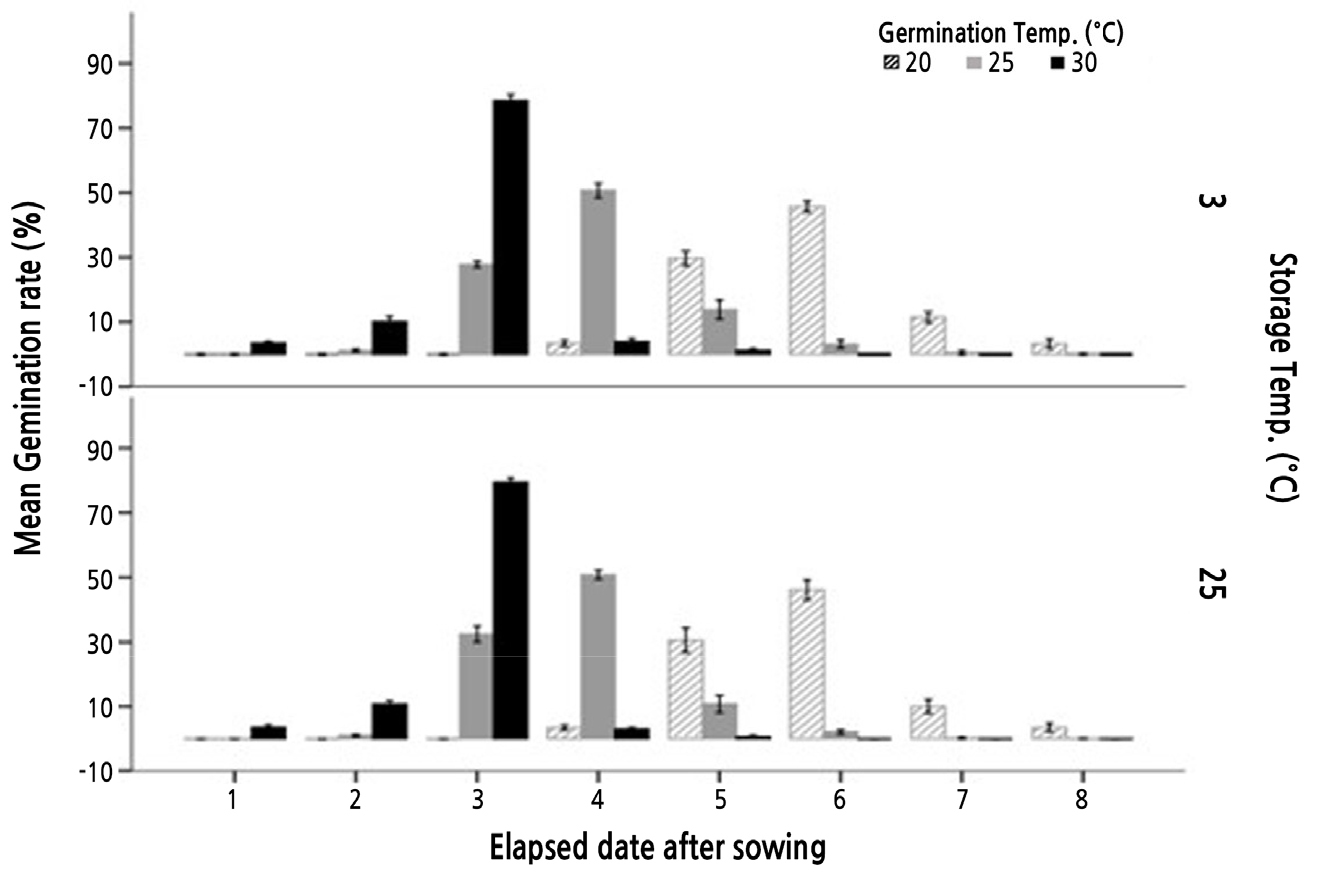
In this study, we detected no significant difference in the final percentage germination (at 8 days after sowing) of A. dubia seeds exposed to temperatures of 25°C and 30°C, whereas germination at both these temperatures was found to be significantly higher than that of seeds subjected to a temperature of 20°C. Therefore, we noted a moderate increase in the total percentage germination of seeds at higher temperatures, with values of 93.5%, 97.4%, and 97.3% being calculated for the temperatures of 20, 25, and 30°C, respectively (Table 2 and Fig. 2). Temperature affects the germination rate at the 3rd day after sowing. At 20°C, germination commenced on day 4 after sowing and peaked at days 5-6, whereas at 25°C, a few seeds began to germinate after 2 days, peaking at 3-4 days, with a germination energy of 33.4% at 3 days. At 30°C, there was a strong germination response after only 2 days, which peaked at 2-3 days, with a germination energy of 93.7% at 3 days (Table 3).
Table 2.
Germination rates of Artemisia dubia seeds treated with a 3-month vernalization (3°C ± 0.5) or storage at room temperature (25-27°C) and subsequent exposure to temperatures of 20°C, 25°C, or 30°C. Seeds were harvested in November of 2019 and sown on the 1st of May, 2020
| Storage temp. (°C) (A) | Germination temp. (°C) (B) | Germination rate (%, mean ± SDz) |
| 3 | 20 | 93.5 by ± 0.93 |
| 3 | 25 | 97.3 a ± 0.25 |
| 3 | 30 | 97.4 a ± 0.10 |
| 25 | 20 | 93.5 b ± 0.58 |
| 25 | 25 | 97.4 a ± 0.35 |
| 25 | 30 | 97.3 a ± 0.35 |
| A | NS | |
| B | *** | |
| A × B | NS |
Table 3.
The germination energy and mean of germination time of Artemisia dubia seeds subjected to different germination temperatures for 3 days after sowing. Seeds were harvested in November of 2019 and sown on the 1st of May, 2020
| Germination Temp. (°C) | Germination energyz(%, mean ± SEy) | Mean of germination timex (mean ± SEy) |
| 25 | 33.4 ± 1.04 | 1.02 ± 0.03 |
| 30 | 93.7 ± 0.30 | 2.71 ± 0.01 |
| t-test | *** | *** |
Additionally, no significant difference was found between a 3-month vernalization treatment (3°C ± 0.5) and storage at room temperature (25-27°C). Percentages of germination rate were 97.3% and 97.4%, respectively, under 3-month vernalization (3°C ± 0.5) and storage at room temperature (25-27°C). In a variety of plants species, low temperature at a certain duration is required for breaking seed dormancy (Hamilton and Carpenter, 1975), although the range and duration of low-temperature treatments differ from one species to the next (Stewart and Whitfield, 1965). Warm or cold moist treatment for 3-4 months is a common method used to break seed dormancy in Magnoliophyta growing in temperate regions, in cases where dormancy is broken by cold stratification or gibberellin treatment (Ryu et al., 2017). For example, the immature seeds of Aristolochia contorta (northern pipevine) are subjected a 12-week cold moist stratification to increase germination percentage by inducing post-development and dormancy breakage (Chi et al., 2013). However, as a result of performing germination experiments in low-temperature treatment of A. dubia seeds, there was no correlation with germination promotion after dormancy (Table 2 and Fig. 2).
The germination data obtained in this study indicate that A. dubia seeds germinate most rapidly and uniformly at 30°C, which is consistent with the results of Önen (2006) for A. vulgaris (29-30°C). In addition, we inferred that A. dubia seeds do not have a specific vernalization requirement with respect to the promotion of post-dormancy germination.
Experiment II: Effects of Seed Storage Period and Light and Dark Treatments on Germination
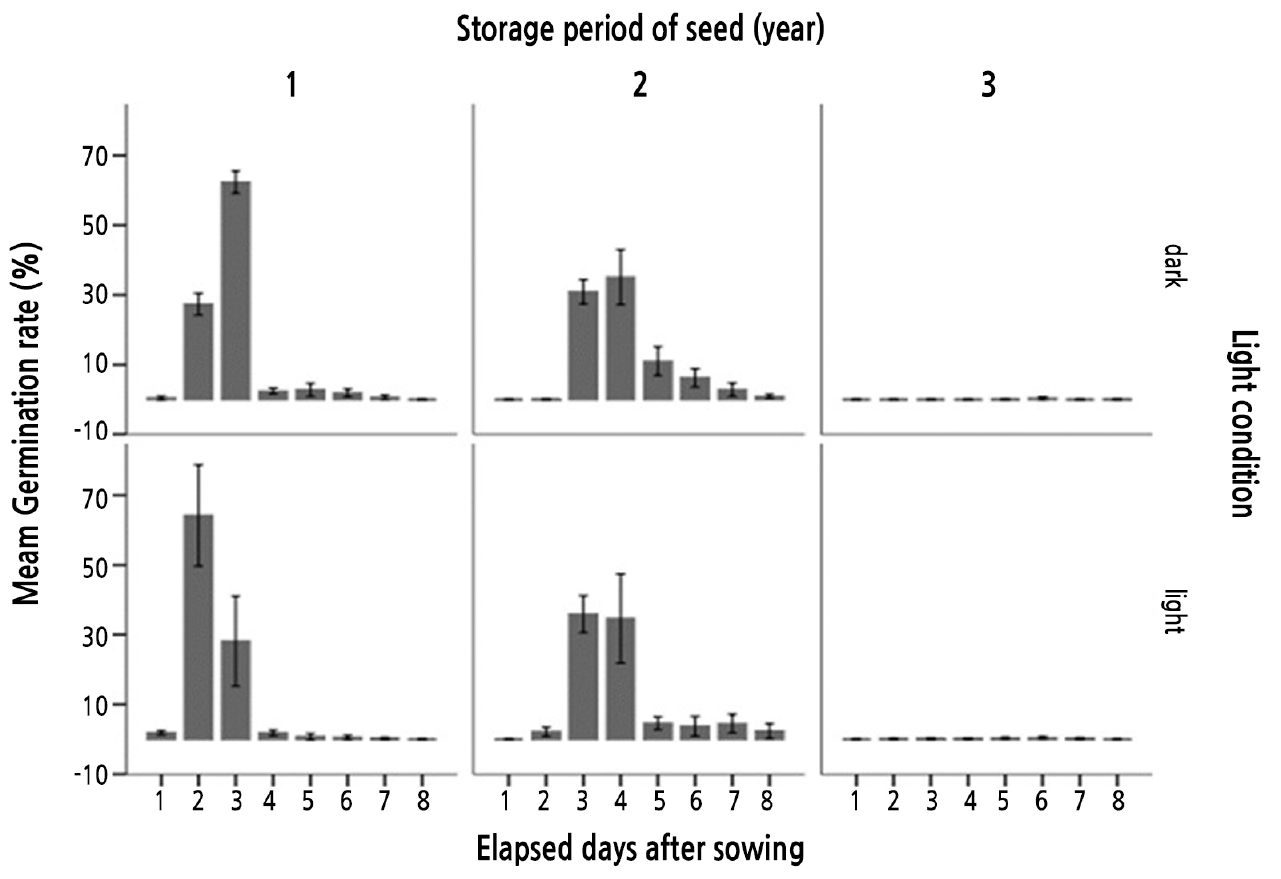
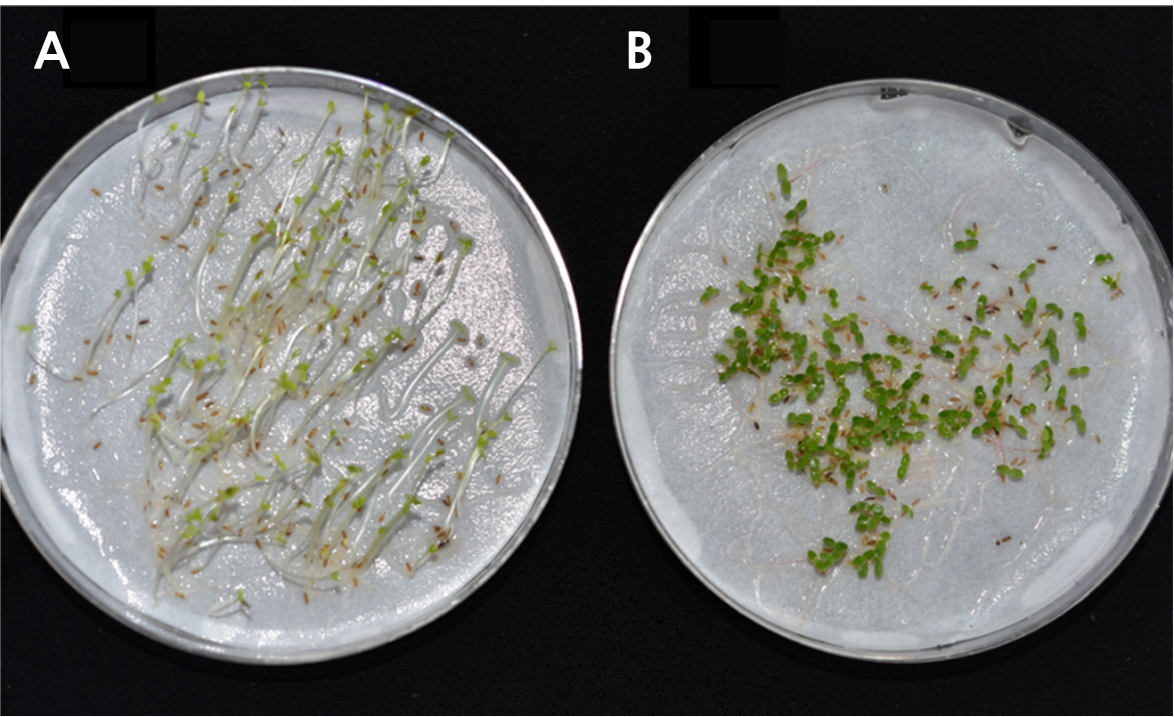
Molecular mobility, which regulates the physiological activities within seeds, is a key factor in the seed aging process, and the properties and range of molecular motion can vary markedly in response to changes in moisture and temperature. The low-temperature, ultra-dry conditions recommended for seed storage contribute to controlling the kinetics of chemical and physical reactions in seeds, thereby retarding molecular mobility (Ballesteros and Walters, 2011). However, although such cool dry storage conditions serve to prolong the period of seed storage, the ensuing gradual deterioration contributes to inevitable seed aging. There is growing evidence that seed deterioration is primarily attributable oxidative processes, as indicated by an accelerated loss of dry seed viability in the presence of oxygen within the storage environment. Consequently, maintenance of dry seeds under oxygen-free conditions is recommended as a measure to extend shelf life during the storage period (Groot et al., 2015). In this context, we performed germination experiments on A. dubia seeds stored at room temperature for 1 (2019), 2 (2018), and 3 (2017) years at 30°C, the temperature verified to promote the earliest onset of germination (i.e., highest germination energy) and the highest total percentage germination. We accordingly found that the seeds stored for 1 year had a high percentage germination of 97%, whereas those stored for 2 years had a somewhat lower percentage of 88%, with the onset of germination being delayed by 1 to 2 days. In marked contrast, however, few of the seeds stored for 3 years remained viable after this period, with a percentage germination as low as 1.4% (Figs. 3 and 4).
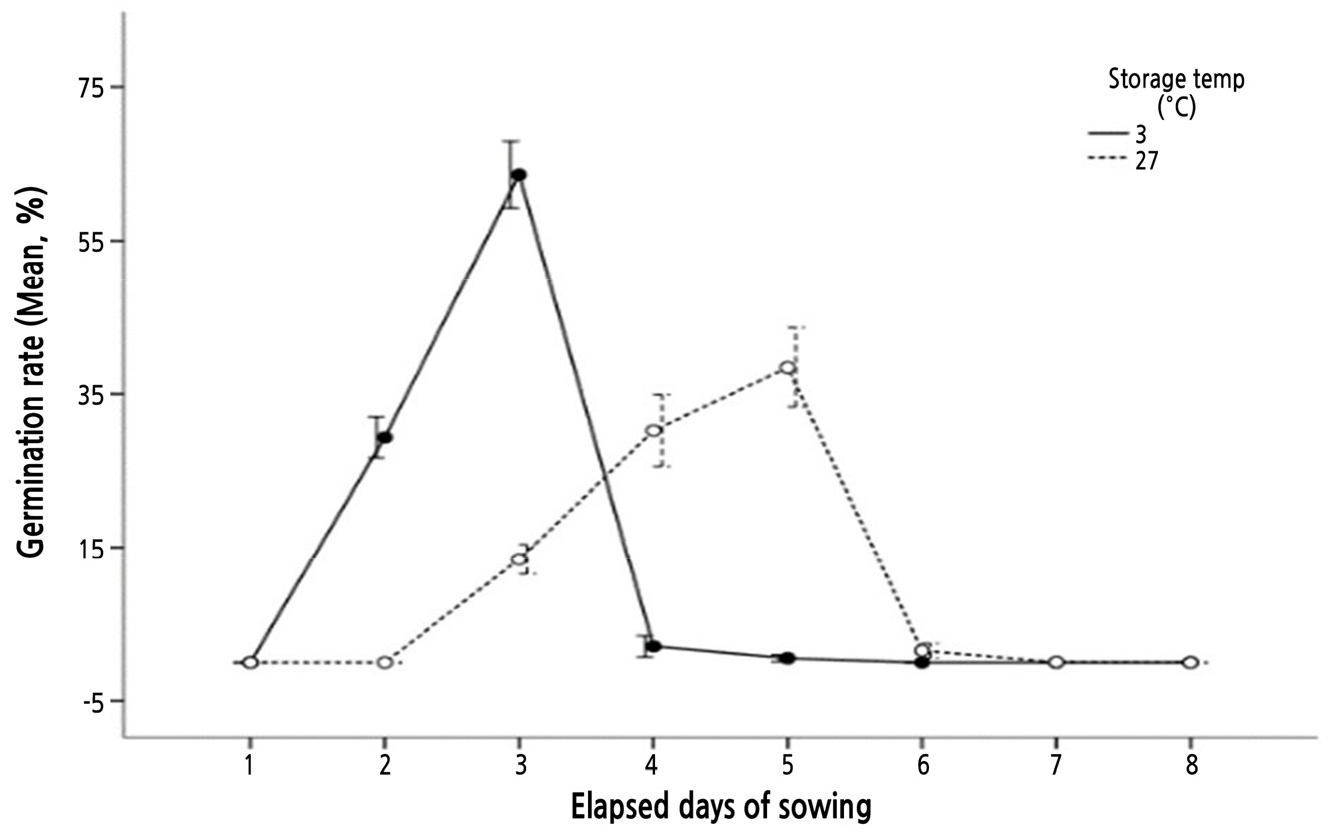
On the basis of these observations, we investigated the percentage germination of seeds harvested in 2018 and stored for 1 year at room (25-27°C) or low (3°C ± 0.5) temperatures to determine seed storage temperature-dependent germination rate. We found that the initial germination of seeds maintained at room and low temperature occurred at 3 and 2 days after sowing, respectively, with 40% germination occurring at 4 and 3 days, respectively (Fig. 5). Moreover, the germination rate affected by the storage temperature was controlled at 3 or 27°C. The germination rate was higher at 3°C (95.6%) than that at 27°C (83.7%). As a result, the difference in percentage germination between the room and low temperature groups was found to be statistically significant (t = 19.104, p = 0.000) (Table 4).
Table 4.
Germination rate of A. dubia seeds for 8 days at 30°C. The seeds were stored for 1 year at 3 or 27°C prior to sowing. Seeds were harvested in November of 2018 and sown in the 8th of May, 2020
| Storage Temp. (°C) | Germination rate (%, mean ± SDz) |
| 3 | 95.55 ± 0.63 |
| 27 | 83.72 ± 1.71 |
| t-test | ** |
In this regard, Li and Pritchard (2009) noted that seed longevity depends on plant species, storage management method, and storage period and that methods for extending seed longevity can make an important contribution to sustaining global agricultural, maintaining food supplies, and preserving thousands of plant species. Moreover, cryopreservation has been experimentally proven to enable the long-term preservation of seeds. Taking this study into account, it will be necessary in future studies to establish a standard storage method for extending the longevity of A. dubia seeds, based on assessments of other temperature conditions and the effects of an anoxic environment.
On the basis of their reception of a combination of environmental signals, such as water availability and light conditions, the seeds of angiosperms make appropriate decisions with regards to the timing of germination. In response to optimal conditions, which involves the phytochrome detection of appropriate light conditions, germination is initiated via the synthesis of gibberellins (GAs). In this regard, Oh et al. (2006) demonstrated that PIL5, a bHLH protein that interacts with phytochromes, inhibits seed germination via a suppression of GA synthesis and that red and far-infrared light signals induce PIL5 protein degradation through the 26S proteasome, thereby negating the inhibitory effect of PIL5 on GA biosynthesis. Accordingly, phytochrome activity can promote seed germination by lowering the expression of PIL5, thereby enhancing GA biosynthesis and reducing GA degradation, and thus highlighting the key regulatory role played by illumination in seed germination. Furthermore, Gu et al. (2017) have shown that the photoreceptor phytochrome B partially inhibits phytochrome interaction factor 1 (PIF1), which plays a major role in promoting seed germination during the early stages following light absorption. These authors also established the role of histone deacetylation as the main mechanism underlying the activity of HDA15-PIF1, the key repression module directing the transcription network of seed germination during light-controlled seed germination. In a study on native weeds, Cho et al. (2011) noted that light treatment does not affect the germination of Vicia tetrasperma (smooth tare), whereas Park et al. (2010) found that Rumex crispus (curled dock) seeds do not germinate under dark conditions.
In this study, we investigated the effects of lighting on the germination status of seeds gathered from 2017 to 2019 under the established optimal germination temperature of 30°C and exposure to 12-h irradiation or 24-h darkness for 8 days. We accordingly found that although the time to reach 40% seed germination was advanced by 1 day under the 12-h photoperiod conditions, the final percentage germination of the two treatments was 97.6% and 97.9%, respectively, the difference between which was non-significant (t =-0.905, p = 0.379). These observations indicate that the germination of A. dubia seeds is not affected by light conditions (Table 5 and Figs. 4, 5, 6). In a study examining the effects of LED illumination on the germination of tarragon (Artemisia dracunculus L), Enache and Livadariu (2016) found that percentage germination (final 90%) in response to illumination with red LEDs was higher than that obtained in response to blue LED treatment, although cotyledon development tended to be superior in seeds exposed to blue LEDs. However, as these authors did not include a dark treatment, only the germination results pertaining to illuminated seeds are consistent with those obtained in this study.
Table 5.
Germination rate affected by light treatments of A. dubia seeds for 8 days at 30°C (sown on the 22nd of August, 2020)
| Light treatmentsz | Germination rate (%, mean ± SDy) |
| Light | 97.61 ± 0.78 |
| dark | 97.88 ± 0.49 |
| t-test | NS |
All integration results suggest that the most uniform and fastest germination rate of A. dubia seeds is at 30°C. In addition, the germination vitality of A. dubia seeds was 2 years, but when the seeds were stored at a low temperature of 3°C, the life of the seeds was extended. Also, there was no significant difference between the germination of seeds subjected to light and dark treatments, indicating illumination does not appear to be an essential factor for the germination of A. dubia seeds.