Introduction
Materials and Methods
Plant Materials
Reagents
Light Treatment
Temperature Treatment
Gas Chromatography-mass Spectrometry (GC-MS) Analysis
High-performance Liquid Chromatography-mass Spectrometry (HPLC-MS) Analysis
Statistical Analysis
Results
Discussion
Introduction
Sweet osmanthus (Osmanthus fragrans Lour.), a well-known ornamental germplasm native to East Asia, is one of the top ten traditional flowers in China with a bright color and pleasant fragrance. More than 160 cultivars of O. fragrans have been divided into four groups according to the flower color and blooming season, including Yingui in white, Jingui in yellow, Dangui that is orange/red and that blooms mainly in autumn for the commercial harvest, and Sijigui in yellow which blooms during most of the year (Chen et al., 2021). Owing to their unique oriental scent, with fresh flowers very rich in floral volatiles, including terpenoids, aromatic compounds, C6 compounds and esters (Zeng et al., 2016), commercial extracts from O. fragrans flowers are in high demand for use in expensive perfumes and cosmetics and as functional food additives and medicines (Fu et al., 2019). Therefore, flower color and aroma are both important ornamental and economic qualities of O. fragrans.
Environmental factors can cause changes in physiological and biochemical processes in plants and can affect important ornamental qualities of plants, such as color and aroma (Adil et al., 2019). In strawberries, light and temperature conditions are the most important factors that regulate postharvest aroma; volatile terpenes and benzenoids are influenced by different light and dark conditions (Fu et al., 2017). The contents of volatiles of strawberry at 4°C have been found to be much lower than those at other temperatures (Van de Poel et al., 2014). In Rosa, volatiles are also affected by continuous light or dark treatments (Picone et al., 2004). In sweet cherries (Tsaniklidis et al., 2017), apples (Gouws and Steyn, 2014), and tomatoes (Khairi et al., 2015), light and temperature conditions have a prescribed effect on pigmentation during plant development or postharvest storage. Therefore, light and temperature are key factors that affect the manifestation of color and aroma. In recent studies of the O. fragrans genome, it was found that the evolution of flower color and aroma have certain geographical characteristics, which are closely related to environmental factors (Yang et al., 2018; Chen et al., 2021); however, there is little research on the effects of environmental factors on the flower color and aroma of O. fragrans (Wang et al., 2022).
To determine the effects of environmental factors on the flower color and aroma of O. fragrans, O. fragrans ‘Houban Yingui’ was selected as the plant material to explore the effects of different light and temperature protocols on changes in flower color and fragrance on the basis of earlier work in which the characteristic color and aroma-active components were identified. This study provides a reference for further research on the regulation of environmental factors of the ornamental quality of O. fragrans.
Materials and Methods
Plant Materials
Petal development and senescence of O. fragrans ‘Houban Yingui’ were divided into five stages, as described in Zou et al. (2014). These are S1, the linggeng stage (tight flower buds); S2, the initial flowering stage (partial opening of whorls); S3, the full flowering stage (fully open flower with whorls unfolded); S4, the late full flowering stage (first appearance of browning senescent symptom on petals, and where a few petals began to abscise); and S5, the wilting stage (totally wilted petals attached to the plant) (Fig. 1). Flower branches about 10 cm long of O. fragrans ‘Houban Yingui’ were harvested at the initial flowering stage (S2) from a mature tree sixty years old grown in the nursery of Huazhong Agricultural University (Wuhan, China) (114°22' W, 30°29' N). Five cut flower branches were then placed in individual dishes containing distilled water, according to the method of Zou et al. (2014) (Fig. 2).

Fig. 1.
Developmental stages of Osmanthus fragrans flowers (Zou et al., 2014). (A) Stage 1 (linggeng stage); (B) stage 2 (initial flowering stage); (C) stage 3 (full flowering stage); (D) stage 4 (late full flowering stage); (E) stage 5 (wilting stage).
Reagents
Methanol and methyl tert-butyl ether (MTBE) are chromatographically pure (Tedia Reagent Co., Ltd.); hydroxide potassium (KOH), 2,6-ditert-butyl-4-methylphenol (BHT), sodium chloride (NaCl), ethanol and acetone were all of analytical grade (Sinopharm Chemical Reagent Co., Ltd); naringenin and β-carotene standard samples were purchased from Sigma-Aldrich (St. Louis, MO, USA), and rutin standard samples were purchased from the National Institutes for Food and Drug Control (Beijing, China).
Light Treatment
To study the effect of light on the color and aroma components of sweet osmanthus, flower branches of O. fragrans in dishes were placed under three different light conditions at a temperature of 25°C in an illuminating incubator. In reference to the light treatments in Rosa (Picone et al., 2004), the first group was kept under cool-white fluorescent lighting at a photosynthetic photon flux density of 40 µmol·m-2·s-1 for 24 h; the second group was kept in the dark for 24 h; and the third group was kept in alternating 12 h light and 12 h dark, which was set as the control. After 24 h of treatment, the color and aroma components of the flowers were analyzed.
Temperature Treatment
To study the effects of temperature on the color and aroma components of sweet osmanthus, flower sprays of O. fragrans in dishes were placed under three different temperature conditions with an alternating 12 h cycle of light with cool-white fluorescent lighting at a photosynthetic photon flux density of 40 µmol·m-2·s-1 and 12 h of dark. In reference to the treatment method of strawberries (Van de Poel et al., 2014), the first group was kept at 45°C (high temperature), which is close to the ground temperature in summer, the second group was kept at 4°C (low temperature) which is close to the temperature in winter, and the third group was kept at 25°C, which was set as the control. After 24 h of treatment, the color and aroma components of the flowers were analyzed.
Gas Chromatography-mass Spectrometry (GC-MS) Analysis
The volatiles of the flowers were analyzed using a GC-MS system as described by Cai et al. (2014). The system consisted of a TRACE GC Ultra GC coupled with a DSQ II mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Solid-phase microextraction (SPME) fibers on a 2-cm StableFlex fiber (Supelco Bellefonte, PA, USA) were used to collect and concentrate the aroma compounds. Each flower sample (2 g) was put in a 20-ml glass vial, capped securely with an aluminum seal and a Teflon septum, with 1-µl methyl laurate (0.87 mg·ml-1 in methanol) added as the internal standard. After a 30 min of equilibration, the fiber was inserted into the capped vial for absorption (15 min) (Cai et al., 2014). The GC was fitted with an HP-5 column (30 m × 0.25 mm × 0.25 µm, Thermo Scientific, Bellefonte, PA, USA). The injector was maintained at 250 °C at a transfer line temperature of 280 °C. The ion energy of electron impact ionization was 70 eV and the scanning range was 40–450 Da, with the ion source temperature set to 230 °C. The flow rate of the helium (99.999%) carrier gas was 1.2 ml/min. Analytes absorbed on the fiber were desorbed for 3 min in the GC injector at 250 °C in splitless mode. The temperature was set to 40 °C for 3 min and then increased from 40 °C to 73 °C at 3 °C/min, held at 73 °C for 3 min, and finally raised to 220 °C at a rate of 5 °C/min and held for 1 min (Cai et al., 2014). A total of 17 aroma-active compounds (Table 1) in the flowers of O. fragrans ‘Houban Yingui’ were identified according to a previous gas chromatography olfactometry (GC-O) analysis (Cai et al., 2014).
Table 1.
Relative contents of aroma-active compounds in flower of Osmanthus fragrans ‘Houban Yingui’ under different light and temperature treatments
High-performance Liquid Chromatography-mass Spectrometry (HPLC-MS) Analysis
The color components of the flowers were analyzed using an HPLC-MS system (Shimadzu Corporation of Japan) with conditions identical to those described by Zou et al. (2017).
Each flower sample (0.5 g) was immersed in a 10 mL of 50% ethanol solution in the dark for 3 h to extract the flavonoid components. The obtained extract was filtered with a microporous membrane (0.45 µm) for the HPLC-MS analysis. Flavonoid components were analyzed using a chromatographic column C18 (150 mm × 4.6 mm) (YMC Co. Ltd, Japan). The injection volume was 30 µL, and the detector wavelength was 320 nm. The HPLC-MS conditions were electrospray ionization (ESI), an ion trap analysis, positive or negative ion detection mode types, and full ion scanning with a scan range of 100~700 Da. The electrospray interface drying gas (N2) flow rate used was 1.5 L min-1. The capillary voltage was 4500 V, and the cone hole voltage was 1600 V, with a drying temperature of 200 °C. The mass spectrometry results were analyzed by the LC/MS Solution 3.41 tool attached to the instrument. The composition search was conducted through the chemical structure database (http://www.chemspider.com/) to obtain possible chemical structures (Zou et al., 2017).
Each flower sample (1.0 g) was immersed in mL of an acetone:ethanol (1:1) solution in the dark for 3 h to extract the carotenoid components. First, to saponify the extract under low light or dark light conditions, the extract was rotated and dissolved in 2 mL MTBE (containing 0.01% BHT). Subsequently, 2 mL 10% KOH-methanol solution (containing 0.01% BHT) filled with nitrogen was added, and this was saponified for 10 h in the dark. Then, 4 mL of saturated NaCl aqueous solution and 2 mL of MTBE (containing 0.01% BHT) were added to enable better stratification. The supernatant was concentrated in vacuo and dissolved in 1 mL of MTBE (containing 0.01% BHT). The extracted solution after a constant-volume step was filtered with a microporous membrane (0.45 µm) for the HPLC-MS analysis. Carotenoid components were analyzed using a chromatographic column C30 (250 mm × 4.6 mm) (YMC Co. Ltd, Japan). The HPLC-MS conditions were identical to those described above (Zou et al., 2017).
Statistical Analysis
The data were analyzed with the means ± standard deviations from three replicates. The content was expressed as grams compound per kilogram plant fresh weight (g·kg-1) in each case. Statistical differences of changes in color and aroma compounds between treatments were evaluated by means of a one-way ANOVA (Tukey’s test) using SPSS (IBM, Armonk, New York, US) at a P-value of ≤ 0.05.
Results
The color components of the flowers were analyzed using the HPLC-MS system. Three types of color components, quercetin-O-glycoside, naringenin-O-glycoside and β-carotene, were identified as the characteristic color compounds according to our previous study (Zou et al., 2017). The continuous light treatment and continuous dark treatment increased the content of quercetin-O-glycoside (Fig. 3). The content of quercetin-O-glycoside was 37.29 g·kg-1 under continuous light and 28.68 g·kg-1 under continuous dark, while the control specimen showed a value of 15.92 g·kg-1. Naringenin-O-glycoside decreased slightly under the continuous light treatments. Under continuous light, the content of β-carotene in O. fragrans ‘Houban Yingui’ flowers was 15.04 g·kg-1, which was much lower than that in the control (18.79 g·kg-1). The content of β-carotene increased to 20.57 g·kg-1 under the continuous dark treatment.

Fig. 3.
Effects of light on the characteristic color compounds of Osmanthus fragrans ‘Houban Yingui’. CK (control): 12 h light/12 h dark; LL: continuous light treatment; DD: continuous dark treatment. Different letters indicate significant differences (p < 0.05) based on an ANOVA one-way variance analysis followed by Tukey’s comparison test.
The quercetin-O-glycoside content was greatly affected by the temperature (Fig. 4). Compared to the control, the content of quercetin-O-glycoside was significantly increased from 15.92 g·kg-1 to 58.98 g·kg-1 at a high temperature and to 70.91 g·kg-1 at a low temperature. On the other hand, the content of naringenin-O-glycoside decreased significantly from 9.36 g·kg-1 to 6.80 g·kg-1 at the high temperature compared to the control and increased significantly to 11.45 g·kg-1 at the low temperature. Under the high- and low-temperature treatments, the contents of β-carotene in the O. fragrans ‘Houban Yingui’ flower were 14.68 g·kg-1 and 15.36 g·kg-1, respectively, which were lower than those in the control (18.79 g·kg-1).
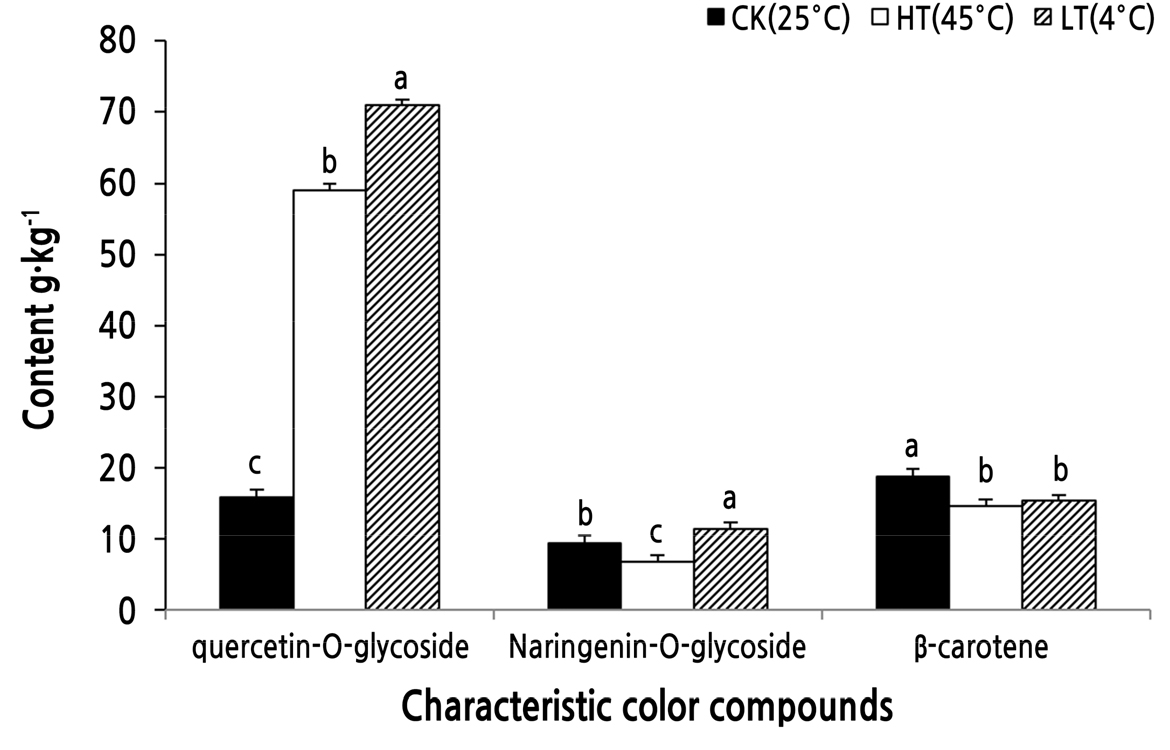
Fig. 4.
Effects of temperature on the characteristic flower color compounds of Osmanthus fragrans ‘Houban Yingui’. CK (control): 25°C; HT: 45°C treatment; LT: 4°C treatment. Different letters indicate significant differences (p < 0.05) based on an ANOVA one-way variance analysis followed by Tukey’s comparison test.
The relative contents of the aroma-active compounds of the flowers were analyzed using the GC-MS system, and the types of aroma-active compounds (Table 1) in the flowers of O. fragrans ‘Houban Yingui’ were identified according to a previous GC-O analysis (Cai et al., 2014). The effects of light and temperature on the relative contents of 17 aroma-active compounds of O. fragrans ‘Houban Yingui’are shown in Table 1. The relative contents of aroma-active compounds were more than 70% in the control, while in the continuous light and dark treatments, the release proportions decreased to 25.16% and 55.35%, respectively. The high-temperature treatment had little effect on the release ratio (74.24%) of aroma-active compounds, but the low-temperature treatment increased the proportion significantly to 78.01%.
It was found that different components of aroma-active substances were affected by the different light conditions. Under the continuous light treatment, the release of both trans-β-ionone and α-ionone was significantly inhibited. The relative contents of trans-β-ionone and α-ionone were 38.98% and 4.64% under the control and continuous dark treatments, respectively, while the relative contents of trans-β-ionone and α-ionone were reduced to less than 5% and 1% under the continuous light treatment, respectively. Under the continuous dark treatment, the relative contents of linalool and its oxidized derivatives cis-linalool oxide, trans-linalool oxide, and cis-pyran oxide linalool were reduced to varying degrees. In particular, the reduction of linalool was the most obvious, with the percentage being higher than 4% in the control and less than 1% under the continuous dark treatment. Under the continuous dark and continuous light treatment, the relative contents of cis-3-hexenyl butanoate, hexyl butanoate, cis-β-ocimene, trans-β-ocimene, γ-terpinene, allo-ocimene, and neo-allo-ocimene were reduced. In contrast, the relative content of 6-ethenyldihydro-2,2,6 -trimethyl-2H-pyran-3(4H)-one was significantly higher under both continuous light and dark conditions compared to that in the control.
Each component of the aroma-active compounds responded differently to the temperature conditions. Trans-β-ionone, α-ionone, linalool, cis-linalool oxide, trans-linalool oxide, cis-linalool oxide (pyranoid), cis-β-ocimene, trans-β-ocimene, γ-terpinene, allo-ocimene, and neo-allo-ocimene were inhibited by the high-temperature treatment, whereas the low-temperature treatment increased the release of these substances to a certain extent in sweet osmanthus. For example, the relative contents of trans-β-ionone and α-ionone in the control were approximately 38.98% and 4.64%, respectively, while the relative content of trans-β-ionone at the high temperature was less than 12% and the relative content of α-ionone was approximately 2%. In contrast, the low-temperature treatment increased the relative contents of trans-β-ionone and α-ionone to 57.65% and 10.48%, respectively. In addition, the aroma-active compound d-limonene, which was not detected in either the control or the high-temperature treatment, was detected at a low temperature, and the relative content of 6-ethenyldihydro-2,2,6-trimethyl-2H-pyran-3(4H)-one was significantly higher at low temperatures than under the control and high-temperature treatments. On the other hand, the relative contents of cis-3-hexenyl butanoate and hexyl butanoate increased to 56% and 3.17% at the high temperature, respectively, which was approximately twice that of the control, and the low-temperature treatment significantly inhibited the release of these two compounds.
Discussion
Light, as an important environmental signal, has a wide range of regulatory effects on the growth and development of plants and has a prescribed effect on the appearance of plant colors. It has been found that the flower color under a weak light treatment is dimmer (Griesbach, 1992) and that the light period extension can gradually increase the content of anthocyanins in the petals (Uddin et al., 2004). In petunias, purple color deepens under strong light, as the darkness may downregulate or inhibit the expression of anthocyanin-related genes, thereby reducing the content of anthocyanins (Albert et al., 2009). In the current study, the O. fragrans‘Houban Yingui’ flower color became pale after a continuous light treatment and deeper after a continuous dark treatment (Fig. 5A). This is most likely due to the different levels of the accumulation of β-carotene, which primarily contributes to the red color (relative to the value of a* according to the Commission International L'Eclairage) of O. fragrans (Zou et al., 2017), whereas flavonoids are assumed to provide the background color (Wang et al., 2018).
Light conditions also had a substantial effect on the volatiles of the flowers. It was found in Gomphrena globosa and Wisteria sinensis that the terpene components of ocimene and linalool were more abundant under light conditions than under dark conditions (Jiang, 2012). Previous research found that the release of terpenoid volatiles in O. fragrans petals is regulated by the circadian rhythm (Zheng et al., 2017), and terpenoid volatiles were found to be activated during the day. In the current study, the volatiles of the flowers decreased significantly under both continuous light and continuous dark treatments, suggesting that the rhythm of the alternating day and night cycle may be more conducive to the synthesis and release of volatiles.
As an important environmental factor affecting plant growth, temperature also has a regulatory effect on the appearance of plant color and fragrances. Increasing the temperature during the flowering period brightens the flower color of some thermophilic plants; however, a much higher temperature during the flowering period would cause most of the plant color to fade. When the temperature is slightly lower, the flower color of most plants can be kept bright for a long time; if the temperature is too low, the color would not be bright and the inherent color would not be displayed (Huang, 1990). In the current study, we found that the flower color of O. fragrans ‘Houban Yingui’became brown under a high temperature, likely due to the oxidation of phenolic compounds; while the flower color was more vivid and lighter at a low temperature (Fig. 5B), most likely due to the decreased level of β-carotene.

Fig. 5.
Effects of light (A) and temperature (B) on the appearance of Osmanthus fragrans ‘Houban Yingui’. CK (control): 25°C, 12 h light/12 h dark; LL: 25°C, continuous light treatment; DD: 25°C, continuous dark treatment; HT: 12 h light/12 h dark, 45°C treatment; LT: 12 h light/12 h dark, 4°C treatment.
In addition to the effect of temperature on the flower color, it also had an effect on the release of floral fragrance in plants. Moderate temperatures accelerate the opening of jasmine flowers and increase the release of certain phenylpropane aroma components. Excessively high temperatures, such as those above 40°C, reduce flower respiration rates and affect the release of aromatic compounds (Gao et al., 2001). In the current study, a high temperature of 45°C significantly promoted the release of cis-3-hexenyl butanoate and hexyl butanoate from sweet osmanthus flowers but inhibited the release of trans-β-ionone, α-ionone, and many monoterpenes and their oxidized derivatives. The release of these aroma-active compounds was similar to the end-flowering period during the natural senescence of O. fragrans flowers, indicating that the effect of a high temperature on aroma release may be due to an accelerated flowering process. On the other hand, the release of aroma-active compounds such as trans-β-ionone, α-ionone, and many monoterpenes and their oxidized derivatives in O. fragrans flowers increased significantly under the low-temperature treatment. This low-temperature (4°C) treatment is close to the outdoor temperature in winter, which may explain why some cultivars of O. fragrans release a stronger aroma in winter (Chen et al., 2021).
In conclusion, this study demonstrates that the environmental factors of light and temperature affect the flower color and scent of O. fragrans ‘Houban Yingui’. Light most likely affects the flower color by regulating the accumulation of β-carotene, while a high temperature in all probability affects the flower color appearance by the oxidation of phenolic compounds in addition to pigments. Compared to the continuous light or continuous dark conditions, the rhythm of alternating day and night is likely to be more conducive to the synthesis and release of volatiles, during which photosynthesis and respiration are not disrupted. Low temperatures are more conducive to the release of aroma-active compounds of O. fragrans. This study provides the first evidence of light and temperature regulation of the color and aroma of O. fragrans; future studies can focus on the related regulatory mechanisms.





