Introduction
Materials and Methods
Plant Materials and Light Acclimatization
Artificial Light Sources
Data Collection and Analysis
Results and Discussion
Effects of Artificial Light Sources on Growth Responses
Effects of Artificial Light Sources on Flowering Responses
Introduction
Various plant production systems using artificial light sources, including plant growth chambers, tissue culture rooms, plant factories, and green interior gardens are used in research and as commercial production systems. These systems utilize fluorescent lamps (FL), high-intensity discharge (HID) lamps including high-pressure sodium (HPS) and metal halide (MH), and light-emitting diodes (LED) to provide light for plant growth and development (Tazawa, 1999). White LED has recently been used as a substitute for FL and incandescent lamp (INC) as a general light source. The selection of these artificial lamps for plant production is mainly based on the intended purpose of the lighting and the amount of space available for plant growth, as these light sources vary in radiant yield, heat release, maximum light intensity, and light spectra (quality).
Light quality affects plant growth and development, including germination, stem elongation, flowering, pigment biosynthesis in flowers and leaves, and biosynthesis of secondary metabolites. Studies on flowering have mainly examined the influence of light duration, intensity, timing, and quality using night interruption (NI, night break) lighting in greenhouses under natural light. NI lighting is used to create long days from autumn to spring by supplementing plants with low intensity (photosynthetic photon flux density, PPFD) 1-2 μmol·m-2·s-1 light during the middle of the night, such as 4-h NI treatment, i.e., providing light from 10 p.m. to 2 a.m. For some long-day plants (LDPs) such as petunia, the use of bulb-type FL (compact FL) for NI treatment delays flowering compared to INC because the ratio of red light to far-red light (R:FR ratio) in FL (approximately 8.46) is higher than that of INC (approximately 0.60) (Runkle et al., 2012). For Eustoma grandiflorum, an LDP, flowering is inversely proportional to the R:FR ratio of FL under NI (Sato et al., 2009), and the flower bud formation period is reduced under NI using a light source with a low R:FR ratio (Yamada et al., 2009a, 2009b). In addition, the bolting of spinach, another LDP, is promoted under NI treatment with high FR light (Hamamoto et al., 2004), and the use of lights with a low R:FR ratio promotes flowering in Matthiola incana (Yoshimura et al., 2002).
Because the commercial quality of flowering potted plants, bedding plants, seedlings, and liners is mainly influenced by their compact shape (Mass and van Hattum, 1998; Oh and Kim, 2010), a variety of methods to manipulate light quality have been used to suppress stem elongation. Various studies have been conducted using photoselective films that can remove a certain wavelength of light; when FR is removed, the plant height of LDPs such as petunia decreases (Runkle and Heins, 2001, 2002; Tsuchihashi, 2009). However, the removal of FR has a side effect, i.e., suppressed flowering of LDPs. Furthermore, using NI with a low R:FR ratio to grow LDPs such as petunia and M. incana increases internode length (Runkle et al., 2012; Yoshimura et al., 2002).
Most of these studies (Runkle and Heins, 2001, 2002; Runkle et al., 2012; Shin et al., 2010; Tsuchihashi, 2009; Yoshimura et al., 2002) examined the effects of light on flowering and stem extension by manipulating the wavelengths of natural light during the light period or by utilizing NI lighting in a greenhouse, where sunlight was the major light source. Only a few studies have utilized artificial lighting as a sole light source for ornamental plants: Heo et al. (2002), Oh and Kim (2010), and Park et al. (2015) investigated the growth and flowering responses of herbaceous plants to artificial light sources including FL, INC, and LED, and Park et al. (2012) utilized FL, LED, HPS, MH, and halogen lamps to assess the growth of flowering ornamental plants indoors.
Petunia is a light quality-sensitive LDP and a shade-sensitive sun plant that is mainly planted outdoor in flower beds, hanging baskets, and pots due to its high light requirements. However, this plant can be induced to have a low light compensation point (LCP) of approximately 15 μmol·m-2·s-1 through light acclimatization under shade (Albert et al., 2009), suggesting that it can grow and flower indoors, where irradiance is insufficient for flowering herbaceous plants such as petunia and where their colorful flowers are needed to improve the ornamental value of green interior gardens. Therefore, this study was carried out to examine the effects of several artificial light sources with different light qualities on growth and flowering of petunia under simulated indoor conditions without natural light. The results of this study should help growers select the appropriate lamp that would allow them to grow petunias with high ornamental value under low light conditions indoors, such as underground or in large commercial buildings.
Materials and Methods
Plant Materials and Light Acclimatization
Plug seedlings of petunia (Petunia × hybrida Hort.) ‘Madness Rose’ grown in 288 cell plug trays were purchased from ACC KA Seed & Seedling Co., Ltd. (Jincheon, Korea). Uniform seedlings with eight unfolded leaves were individually planted in a plastic pot (11.5-cm height and 12-cm diameter) filled with soilless medium (Sunshine Mix #4, SunGro Inc., USA) and incubated for a week in a growth chamber maintained at 22 ± 2°C and PPFD of 100 μmol·m-2·s-1 provided by tube-type fluorescent lamps (FL; FL20EX-D/18, Kumho Electrics, Korea) for 9 h per day in order to acclimate to low light quantity and to inhibit flower induction.
The plants were moved to a custom-made phytotron equipped with different artificial light sources at Yeungnam University on February 22, 2013 and were arranged in two blocks with 10 individuals per light source. Artificial light source treatment was carried out for 10 weeks (until May 2, 2013) at a temperature of 22 ± 2°C, PPFD of 25 ± 2 μmol·m-2·s-1, photoperiod of 16 h, and relative humidity of 55 ± 10%. To exclude the effect of heat from the light sources on plant growth and development, four cooling fans (7.0 × 7.0 × T 1.5 cm, 3,000 rpm; EC7015L12EP, Evercool Co., Ltd., Seoul, Korea) designed for computers were placed around the lamps in each block. After transfer to the phytotron, the plants were treated with slow-release fertilizer (15N-4.8P-10.8K; Osmocote Plus 15-11-13 + 2MgO + TE, Everris International B.V., Geldermalsen, The Netherlands) at 3 g·L-1 and watered with tap water at 09:00 once per day.
Artificial Light Sources
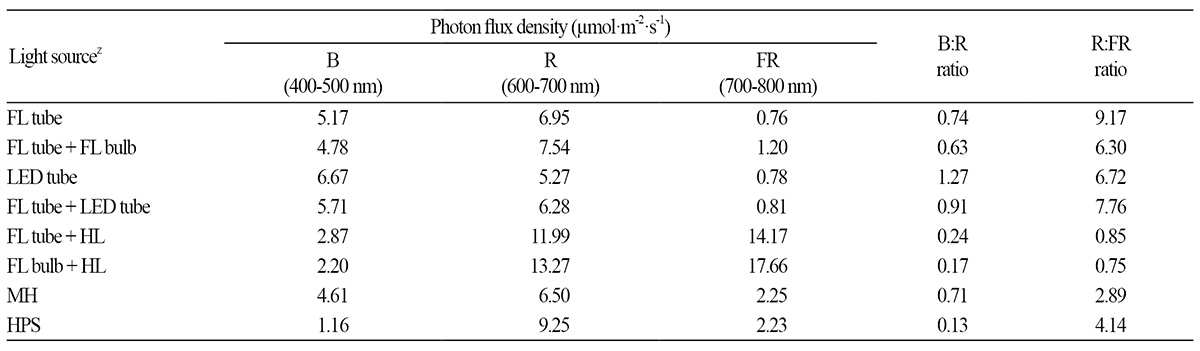
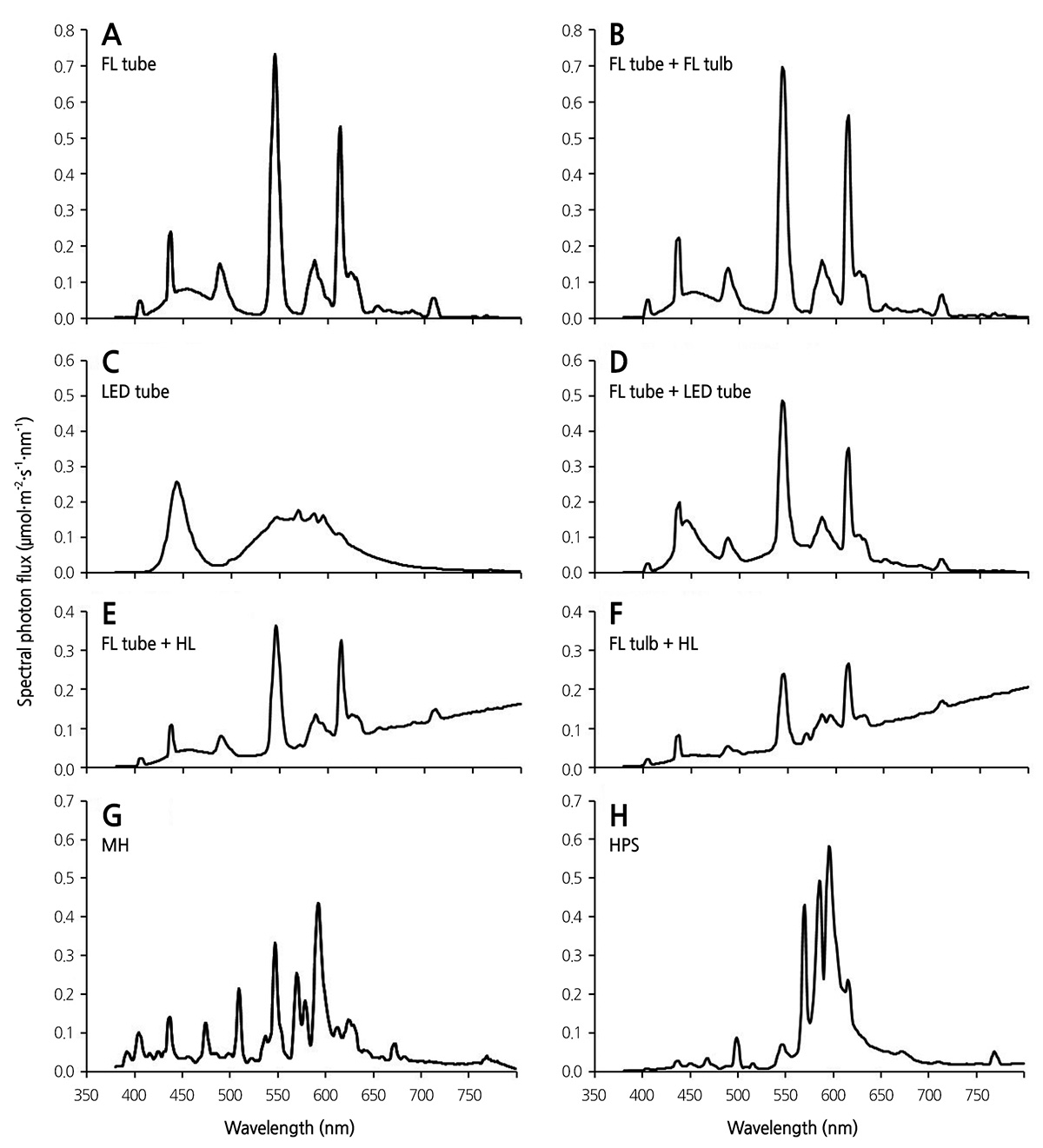
Six types of artificial light sources for use in indoor lighting were selected as follows: tube-type (FL20EX-D/18, Kumho Electrics) and bulb-type (E15EX-D, Osram Korea Co., Ltd., Ansan, Korea) FLs (compact FLs), tube-type cool white LEDs (LED tube; 12 W, Samsung, Korea), halogen lamp (HL; 100W H-PAR30, Ilkwang Co., Ltd., Daegu, Korea), metal halide lamp (MH; GEO-MH 100W-L/P, Geo Lighting, Anseong, Korea), and high pressure sodium lamp (HPS; 100-W B type, Dongsung Electric Co., Ltd., Bucheon, Korea). Plants were grown under eight artificial light conditions provided by solo or mixed sources: FL tube, FL tube + FL bulb, LED tube, FL tube + LED tube, FL tube + HL, FL bulb + HL, MH, and HPS with different light spectra (Table 1 and Fig. 1). FL tube and FL bulb had different light qualities, resulting in different R:FR and B:R ratios.
Using a light meter (LI-250A, Li-Cor Inc., Lincoln, NE, USA), the light intensity of all light sources was adjusted to 25 ± 2 μmol·m-2·s-1 PPFD (approximately 2,000 lux in FL), which is 5-fold the level of standard illumination according to Korean Standards (KS) for working areas (400 lux), corresponding to the mean value of illuminance (1,500-3,000 lux) used in previous studies of indoor plants (Oh et al., 2009).
The light spectrum of each light source was analyzed with a spectroradiometer (PAR-200, J&C Tech Co., Ltd., Korea) (Fig. 1). Spectrum analysis per light source was used to calculate the distribution of blue (B, 400-500 nm), red (R, 600-700 nm), and far-red (FR, 700-800 nm) light and the R:FR and B:R ratios (Table 1).
Data Collection and Analysis
At 10 weeks after treatment, plant height (from medium surface to the apical meristem or tallest flower), number of branches (≥ 2 cm from the primary shoot), stem diameter, internode length, chlorophyll content, leaf size, shoot fresh weight, and dry weight (after 3 d at approximately 70°C) were measured. Leaf size (length and width) and internode length were measured for the largest leaf at the bottom of the first flower and the internode just above the leaf, respectively. Chlorophyll content was determined in the five highest, fully developed leaves using a chlorophyll meter (SPAD-502, Minolta, Japan). Flowering properties such as days to first and second flower, cumulative number of open flowers, and number of visible flower buds (≥ 2 mm) were assessed over 10-week treatment periods. Flowering was recorded when petals were fully unfolded and their color was clearly expressed.
The experiment consisted of two replications with ten plants per replication. Statistical analysis and significance tests were carried out with SAS software (SAS 9.1.3, SAS Inst. Inc., Carry, NC, USA) using Duncan’s multiple range test at 95% confidence interval. Regression analysis was performed using SigmaPlot V10 (Systat Software Inc., Chicago, IL, USA).
Results and Discussion
Effects of Artificial Light Sources on Growth Responses
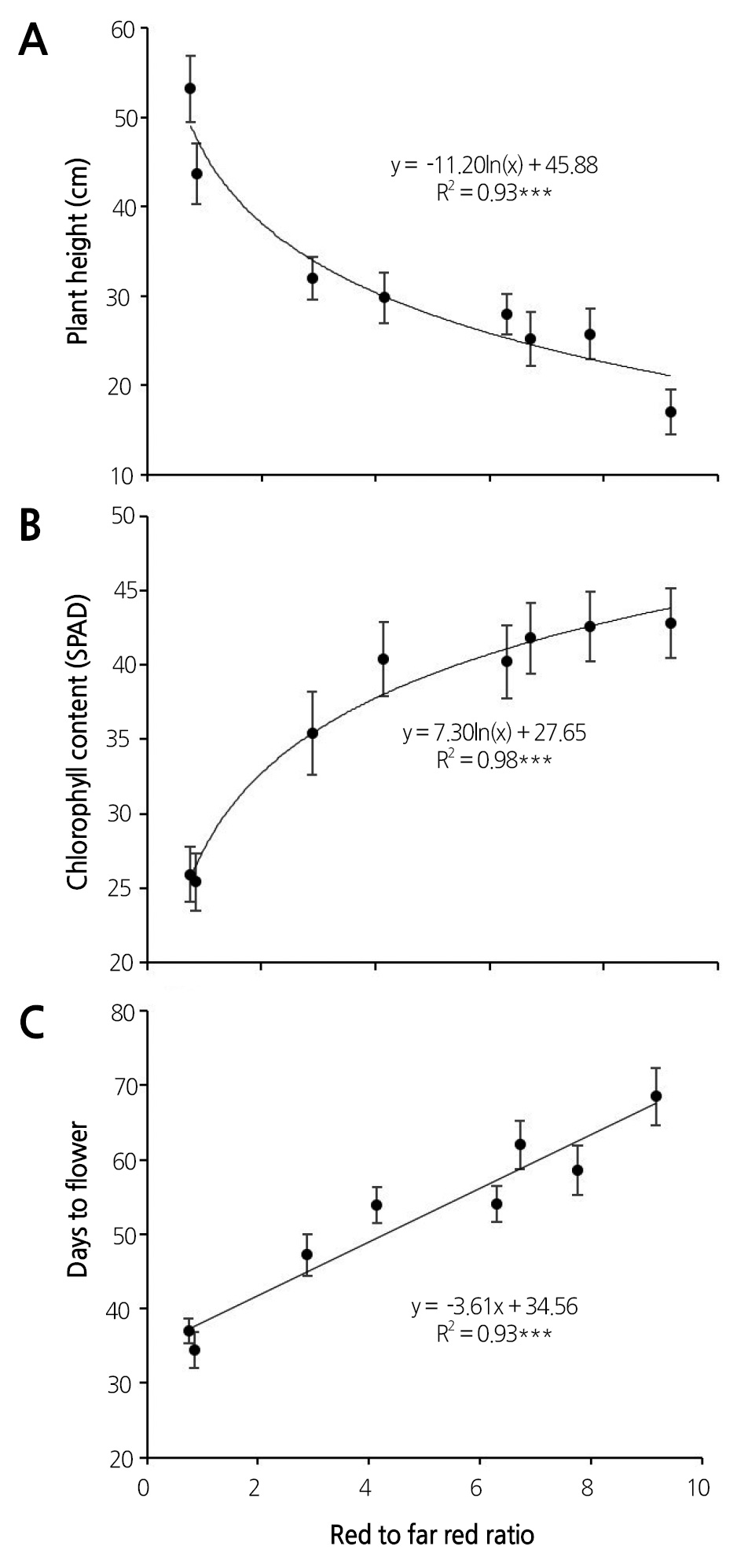
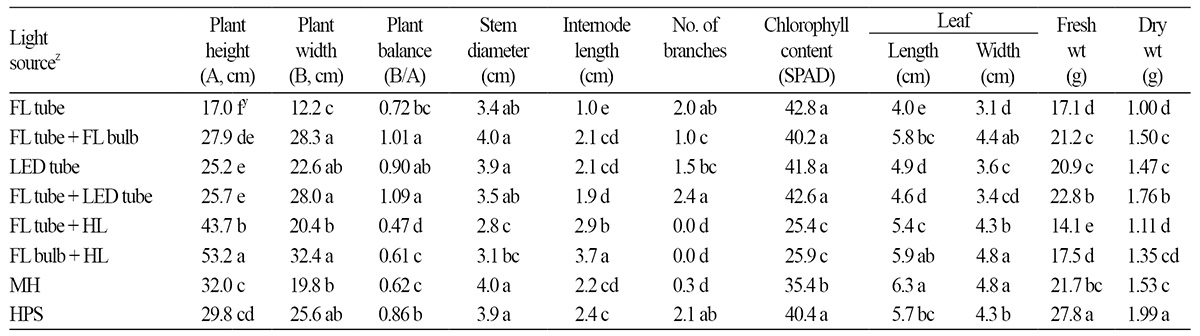
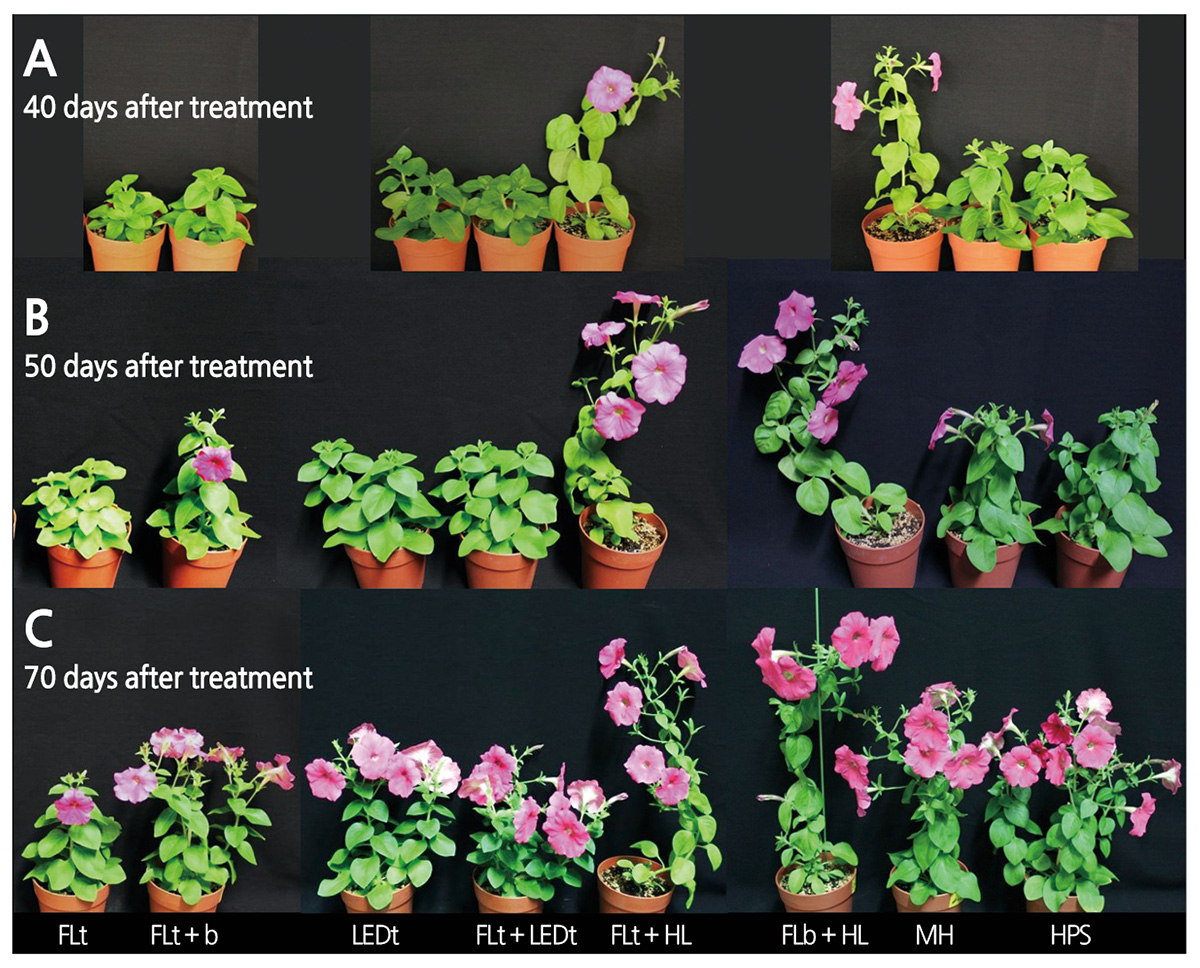
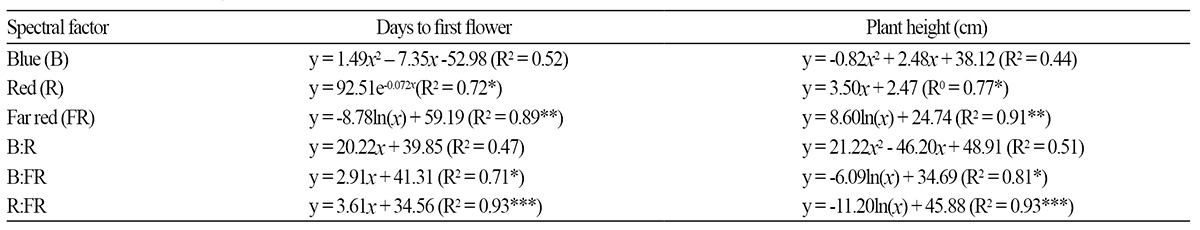
After 10 weeks of light treatments, the growth characteristics of petunia cultivar ‘Madness Rose’ were influenced by artificial light sources. Plant height was significantly suppressed under FL tubes compared to the other light sources (Table 2). On the other hand, stem elongation was stimulated by supplementation with HL with high FR: the R:FR ratio of the light source was inversely correlated with plant height (y = -11.20ln(x) + 45.88, R2 = 0.93, p < 0.001) (Fig. 2A and Table 4). This tendency was also observed in pansy, which exhibited a reduced increase in height in response to the application of FRintercepting film (Tsuchihashi, 2009). The R:FR ratios of FL bulb + HL, FL tube + HL, MH, HPS, FL tube + FL bulb, LED tube, FL tube + LED tube, and FL tube were 0.75, 0.85, 2.89, 4.14, 6.30, 6.72, 7.76, and 9.17, respectively (Table 1). A low R:FR ratio environment generally accelerates stem growth by inducing shade avoidance syndrome (Smith and Whitelam, 1997). Indeed, FR-deficient film reduced stem growth by 34% in petunia (Runkle and Heins, 2002). Based on regression analysis between plant height and spectral factors (Table 4) of light sources, we determined that the R:FR ratio provided a better-fit model to plant height (R2 = 0.93) compared to FR (R2 = 0.91), B:FR (0.81), and R (R2 = 0.77).
The greatest and second greatest internode lengths were observed under FL bulb + HL and FL tube + HL, respectively (Table 2 and Fig. 3), which increased plant height. This trait negatively affects the ornamental value of potted and bedding plants such as petunia. The first-class plant balance index (plant width/height) of flowering potted plants such as poinsettia, kalanchoe, and cyclamen is 1.3-1.8 (NAQS, 2014). Therefore, the higher the plant balance index, the higher the plant quality in petunia cultivar ‘Madness Rose’. The plant balance index was highest under FL tube + LED tube and FL tube + FL bulb treatment and was relatively high under LED tube and HPS treatment (Table 2 and Fig. 3). Meanwhile, the light quality of FL tubes inhibited stem elongation due to the presence of shorter internodes. Similarly, Runkle et al. (2012) reported that the internode length of petunia was shorter under NI lighting with compact FL (CFL) with higher R:FR ratios than under INC or INC + CFL. Also, M. incana and E. grandiflorum exhibited a low internode elongation rate under NI light sources with high R:FR ratios (Yamada et al., 2008; Yoshimura, 2002).
Plants grown under FL tube + HL and FL bulb + HL had relatively small stem diameters (Table 2). These long, thin stems could not support flowers and thus needed support (Fig. 3). Plants under LED tube, FL tube + FL bulb, MH, and HPS had significantly thicker stems than those grown under FL tube + HL and FL bulb + HL (Table 2). Therefore, petunia plants grown under FR-rich light had thinner stems and longer internodes compared to the other light sources emitting relatively FR-poor light; the regression equation of stem diameter to FR light was y = -0.05x + 3.84 (R2 = 0.62, p < 0.05, graph not shown).
Plants grown under FL tube + LED tube produced 2.4 branches, which was no different from those grown under HPS and FL tube but significantly greater than those grown under the other lamps (Table 2). However, branching was not induced under HL-mixed light sources (FL tube + HL and FL bulb + HL) and MH (Table 2), which had relatively low B light and R:FR ratios compared to the others (Table 1). Branching increases volume (massing), which is directly associated with ornamental value in potted and bedding plants (Mass and van Hattum, 1998; NAQS, 2014; Oh and Kim, 2010). In a greenhouse experiment using NI and day extension lighting, the most branching was observed under compact FL and the least was observed under incandescent lamp in petunia (Moe and Heins, 1990; Runkle et al., 2012). Branching of pansy was promoted under a FR-deficient filter (e.g., high R:FR ratio) (Runkle and Heins, 2001). Light conditions in which relatively high levels of FR are provided by HL are highly similar to shade produced by a plant canopy, which increases apical dominance and suppresses branching (Smith and Whitelam, 1997; Tucker, 1975). In the current study, the number of lateral branches also increased under the light source with the highest ratio of R:FR (Table 1 and Table 2), which is similar to the findings of Yamada et al. (2009b), who showed that the number of branches in E. grandiflorum increased with increasing R ratio.
Although light sources with higher R:FR ratios (> 4.0) promoted branching and those with lower R:FR ratios (< 3.0) inhibited lateral bud outgrowth (Table 2), the correlation between R:FR ratio and branch number (y = 0.26x - 0.08, R2 = 0.70, p < 0.05) was relatively weak compared to days to flower and plant height (Fig. 2A, 2C and Table 4). In particular, branching was inhibited under LED tube (R:FR = 6.72) and FL tube + FL bulb (R:FR = 6.30) compared to FL tube + LED tube (R:FR = 7.76). B light generally influences branching, but the results vary depending on species: B stimulated lateral bud outgrowth in Triticum aestivum, Prunus cerasifera, and Rosa but inhibited branching in Solanum tuberosum (Leduc et al., 2014). The response of branching to B can differ among varieties, even within a single species, as observed in tomato (Glowacka, 2006). In petunia cultivar ‘Madness Rose’, the highest B (6.67 μmol·m-2·s-1) and B:R ratio (1.27) of LED tube light inhibited branching, whereas relatively poor branching occurred under FL tube + FL bulb treatment due to its lower R:FR ratio (6.30) and higher FR (1.20 μmol·m-2·s-1) compared to FL tube (R:FR, 9.17; FR, 0.76 μmol·m-2·s-1) and FL tube + LED tube (R:FR, 7.76; FR, 0.81 μmol·m-2·s-1).
The chlorophyll content of ‘Madness Rose’ petunia was lowest under two light sources combined with HL and the second lowest under MH (Table 2). These light sources had more FR light or lower R:FR ratios than the others (Table 1). Chlorophyll contents increased logarithmically with increasing R:FR ratio [y = 7.30ln (x) + 27.65 (R2 = 0.98****, x = R:FR)] (Table 1 and Fig. 2B) and decreased logarithmically with increasing FR light [y = -5.57ln(x) + 41.40 (R2 = 0.95***, x = FR)] (data not shown). This results is very similar to the finding of Casal et al. (1987), who showed that FR-treated Petunia axillaris plants had reduced chlorophyll contents. Another study also showed that the chlorophyll contents decreased under FR-supplemental lighting (Li and Kubota, 2009). In the current study, B light was not correlated with chlorophyll contents (data not shown), although B light was reported to promote chlorophyll biosynthesis in lettuce, spinach, mustard, and wheat (Tibbitts et al., 1983). These results indicate that the chlorophyll content of petunia increases with increasing R:FR ratio or decreasing FR light.
Leaf length and width were greatest under MH and FL bulb + HL, followed by FL tube + FL bulb, HPS, and FL tube + HL (Table 2), whereas leaf size was smallest under FL tube, followed by LED tube and FL tube + LED tube (Fig. 2). A similar effect of FR on leaf growth of lettuce cultivar ‘Red Cross’ was reported by Li and Kubota (2009), who found that FL + FR LED (R:FR = 0.5) increased leaf length and width by 44% and 15%, respectively compared to FL (11.5). A reduced R:FR ratio acts as a signal, promoting shade avoidance responses such as stem (shoot) elongation and leaf expansion, which likely increase the light-interception capacity of the plant (Franklin, 2008).
Fresh and dry weights were heaviest under HPS, whereas there was less dry matter accumulation under the other light sources compared to HPS (Table 2). The optimum B:R ratio for plant growth identified through studies using LEDs is 0.11 to 0.43 (B:R = 1:9 to 3:7) in various plants such as lettuce (Cha et al., 2013; Choi et al., 2014; Lee et al., 2010; Okamoto et al., 1997), sweet potato (Lee and Lee, 2014), and chrysanthemum (Im et al., 2013). In this study, HPS, with a B:R ratio (0.13) in this optimum range (Table 1 and Table 2), increased shoot fresh and dry weights. Similarly, lettuce plants grown under HPS had higher fresh and dry weights compared to those grown under MH and FL (Lee et al., 2013).
Although the B:R ratios of FL tube + HL (0.24) and FL bulb + HL (0.17) were also within the optimum range, this treatment reduced dry matter accumulation, as the accelerated flowering under these light sources might have led to a shorter juvenile phase for vegetative growth. Shoot dry mass has been positively correlated with days to flowering in petunia and pansy (Oh et al., 2010) and in celosia, impatiens, salvia, marigold, and pansy (Pramuk and Runkle, 2005); early flowering is associated with reduced plant dry mass.
Effects of Artificial Light Sources on Flowering Responses
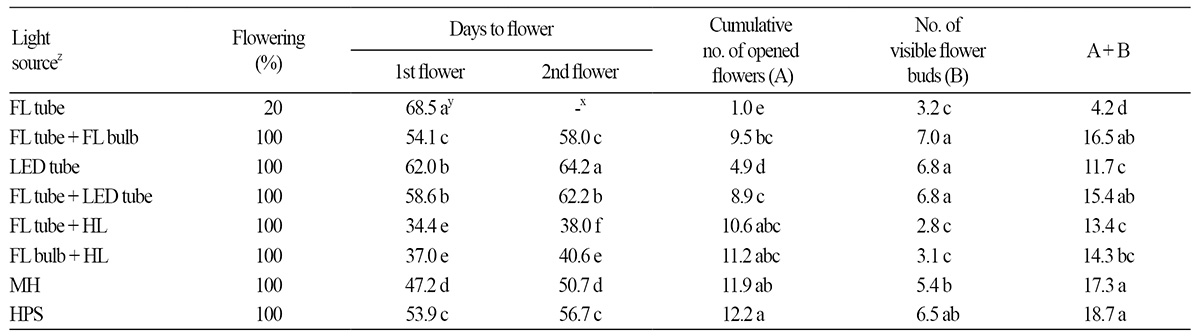
‘Madness Rose’ began to flower on the 34th day of treatment; the earliest flowering occurred under FL tube + HL mixed light source, followed by FL + HL and MH (Table 3). The latest flowering occurred in plants under FL tube, with days to flower twice that of the fastest group. In general, flowering occurred more rapidly with decreasing R:FR ratio (y = 3.61x + 34.56, R2 = 0.93, p < 0.001) (Fig. 2C and Table 4). This observation is consistent with the finding that using INC (with low R:FR ratio) as a light source for NI hastens flowering in petunia and Coreopsis grandiflora (Runkle et al., 2012). Sato et al. (2009) and Yamada et al. (2009a, 2009b) also showed that flowering in Eustoma is promoted when the R:FR ratio of the NI light source is low. In the current study, regression analysis between days to first flower and spectral factors (Table 4) of light sources revealed that the R:FR ratio was more significantly correlated with plant height (R2 = 0.93, p < 0.001) compared to FR (R2 = 0.89, p < 0.01), R (R2 = 0.72, p < 0.05) and B:FR (0.71, p < 0.05).
We observed the flowering responses of petunia to growth under artificial light sources for 10 weeks (Table 3), finding that all plants grown under all light sources flowered, except FL tube, which induced flowering in only 20% of petunia plants. The treatment using FL tubes with the highest R:FR ratio (9.17) was FL tube + LED tube, while the R:FR ratios for FL tube + FL bulb, FL tube + HL, and FL bulb + HL were 7.76, 6.30, 0.85, and 0.75, respectively (Table 1). Reducing the R:FR ratio by 1.41 (from 9.17 to 7.76) accelerated the flowering process by 9.9 d and increased the flowering rate by 80% at 10 weeks after treatment (Table 3).
Table 4. Regression analysis comparing spectral factors of light sources with days to first flower and plant height measured at 10 weeks after treatment in Petunia × hybrida ‘Madness Rose’.
| |
The second flowers in plants grown under FL tube did not open until the end of the experiment, while the flowers of plants in the other treatment groups opened in an orderly manner (Table 3). The cumulative number of opened flowers, which significantly affects the ornamental value of petunia, ranged from 10.6-12.2 under HPS, MH, and two HL-mixed light sources. The high cumulative number of opened flowers under HL-mixed light treatment was similar to the findings of Sato et al. (2009), who showed that the cut flower yield in Eustoma increases when LD lighting is provided by FL bulb with high FR. It appears that HPS and MH, which are widely used as supplemental light sources in the greenhouse, have a higher distribution of wavelengths necessary for photosynthesis than the other light sources, thus increasing the number of flowers. Approximately seven flower buds were generated under the FL tube + FL bulb, LED tube, FL tube + LED tube, and HPS light sources. The cumulative number of flowers was highest under HPS (18.7), followed by MH (17.3), FL tube + FL bulb (16.5), and FL tube + LED tube (15.4).
In this study, ‘Madness Rose’ petunia exhibited somewhat normal growth and flowering under low light conditions of 25 μmol·m-2·s-1 PPFD emitted from FL tube + FL bulb, LED tube, FL tube + LED tube, and HPS (Fig. 3). In petunia cultivar ‘Mitchell’, LCP decreased by 46.4%, from 28 μmol·m-2·s-1 to 15 μmol·m-2·s-1, 1 week after the plants were moved from high light (750 μmol·m-2·s-1 PPFD) to shade conditions (50-350 μmol·m-2·s-1) (Albert et al., 2009). Therefore, it appears that a light acclimatization period of 1 week at 100 μmol·m-2·s-1 PPFD before initiation of treatment induces a reduction in LCP to less than 25 μmol·m-2·s-1.
Plants grown under canopy shade receive light with a lower R:FR ratio or higher FR than ambient light and generally exhibit the shade-avoidance response, i.e., increased internode extension, reduced leaf area, and accelerated flowering (Franklin, 2008; Halliday et al., 1994). In this study, ‘Madness Rose’ petunia plants grown under light conditions with a lower R:FR ratio or higher FR than other light sources exhibited the shade avoidance response. Flowering and stem elongation were more strongly influenced by the R:FR ratio than by B, R, FR, B:R, or B:FR in the light (Table 4), suggesting that phytochrome has a greater effect on flowering and stem elongation than cryptochrome. However, B light promotes flowering in LDPs of the Cruciferae such as Arabidopsis (Thomas, 2006), but it has little effect on flowering in other plant families (Thomas and Vince-Prue, 1997; Runkle and Heins, 2001).
In conclusion, reducing the R:FR ratio using HL with high FR can promote flowering in petunia plants, but their quality as potted or bedding plants may be negatively affected due to excessive stem elongation, weak stems, and poor branching. As the proportion of HL in FL tube + HL and FL bulb + HL treatments used in the current study was approximately 50%, plant quality could likely be improved by reducing the amount of FR in the light source. By contrast, the combined use of FL tube and LED tube, or MH or HPS, increased the number of flowers, promoted branching, and suppressed the growth of stems, thus yielding high-quality plants, although the plant balance index (1.09 and 1.01 under FL tube + LED tube and FL tube + FL bulb, respectively) did not correspond to the desired 1.3-1.8 value of flowering potted plants (poinsettia, kalanchoe, and cyclamen) (NAQS, 2014) due to smaller plant width or greater plant height. However, because it is difficult to install high intensity discharge lamps such as HPS in ordinary households and offices with low ceilings, it would be efficient to use FL mixed with LED fluorescent lamp or FR LED. Further studies are needed to identify the optimum R:FR ratio or PPFD during each developmental stage to produce high-quality plants with accelerated flowering in indoor environments. The results of the current study could be utilized to grow photosensitive flowering plants in growth chambers and closed plant factory systems.