Introduction
Materials and Methods
Plant Materials
Preparation of the Main Plant Extracts
Preservative Conditions for Plant Extracts
Determination of Physical and Chemical Properties
Statistical Analysis
Results
Effects of Plant Extracts on the Apparent Quality of Litchi
Effects of Plant Extracts on the Nutritional Quality of Litchi Pulp
Effects of Plant Extracts on Enzyme Activity and Lipid Peroxides of Litchi Pulp
Discussion
Conclusion
Introduction
Fresh fruits and vegetables have high nutritional and economic value. After picking, they are prone to spoilage, deterioration, and pathogenic microorganisms during processing and transportation (Sharma et al., 2009). Therefore, it is important to reduce postharvest rot and prolong preservation. Up to now, research on the preservation of fruits and vegetables has mainly focused on the use of chemical and natural preservatives. Compared with chemical preservatives, which are harmful to human health, plant extracts are not prone to residues and poisons in various types of food preservation, making them a naturally safe and reliable plant source additive (Li et al., 2019). Plant extracts contain one or more bioactive components, such as alkaloids, organic acids, volatile oils, flavones, polyphenols, polysaccharides, saponins, and tannins, which are obtained by physical or chemical separation and extraction methods and have broad antibacterial and antioxidant effects (Yong et al., 2015; Chen et al., 2018).
Due to their natural edible properties, plant extracts can be used in fruit and vegetable preservation by dipping, fumigating, spraying, or by combining a composite coating with a carrier such as cling paper, and the effect is significant. Studies have shown that tea polyphenols can be used as materials for packaging composite films to keep cherries fresh, which can significantly reduce the rate of rot and quality loss, delay the consumption of soluble solids and Vc, and extend the shelf life (Ye et al., 2018). Isothiocyanate (AITC) is the main active ingredient in the cruciferous plant horseradish, which can effectively reduce the decay of blueberry fruits stored at 10°C (Wang and Chen, 2010). The essential oil of Cyperus rotundus L. has strong antibacterial activity against Staphylococcus aureus (Zhang et al., 2017), and essential oil of lemongrass, thyme, and rosemary reduces the quality loss and delays changes in hardness, acidity, Vc, and respiratory rate of refrigerated nectarines (Abd El Wahab, 2015). The polysaccharides of pomegranate peel have excellent reducing ability and scavenging free radicals, as well as a strong protective effect on liver injury induced by CCI4 in mice (Zhai et al., 2018). The flavonoids of ginkgo can effectively inhibit the growth of Penicillium expansum in diseased apple and reduce the rate of weight loss and browning (Perez-Vizcaino and Fraga, 2018). The film pack containing citric acid significantly inhibited enzymatic browning, reducing quality loss in apple slices during storage (Azevedo et al., 2018).
Litchi (Litchi chinensis Sonn) is a subtropical fruit with a delicate flavor that is rich in nutrition. It has a high medicinal value, is popular with consumers, and has great commodity value (Qi et al., 2015). However, litchi is also one of the most difficult fruits to store because its skin quickly browns and rots after harvest, reducing its commodity value and seriously affecting its storage, transportation, processing, and sales (Wu et al., 2017). The annual loss of litchi due to decay accounts for more than 25% of the total output (Liang et al., 2015). Enzymatic browning is the main reason of litchi fruit peel browning, mainly caused by polyphenol oxidase (PPO), which is the key enzyme for enzymatic browning. It can catalyze the oxidation of endogenous phenols to quinones and cause peel browning (Jiang et al., 2004). Peroxidase (POD) enzyme is another major factor in enzymatic browning of fruits and vegetables. To help solve the problem of litchi postharvest storage, the preservation effect of essential oil extracted from Lysimachi foenum-graecum Hance, Ginkgo biloba extractwas obtained from the leaves, and anthocyanin obtained from eggplant peel was studied in postharvest litchi fruits, specifically aspects of quality such as appearance, nutritional value, and enzyme activity. The results provide a basis for the development of new natural preservatives that are safe and environmentally friendly as well as information on the packaging and processing of fresh fruits and vegetables.
Materials and Methods
Plant Materials
Lysimachi foenum-graecum Hance was collected from the city of Yongzhou, Hunan Province of China. Eggplant is purple and round and is widely eaten and rich in anthocyanins. The leaves of Ginkgo biloba L. were obtained from the city of Yan'an, Shaanxi Province of China. ‘Feizixiao’ is a litchi variety from the city of Dongguan, Guangdong Province of China. Fresh, ripe litchi with no rot or pests was used as material in this experiment.
Preparation of the Main Plant Extracts
The fresh ginkgo biloba leaves, eggplant peels, and L. foenum-graecum Hance were washed and dried to a constant weight at 80°C, then ground into a fine powder and passed through an 80 mesh sieve for use.
Ginkgo biloba extract. Twenty grams of powder from ginkgo biloba leaves was accurately weighed, and the crude extract was obtained at the extraction conditions of 70% ethanol, solid:liquid ratio of 1:20 (g·mL), ultrasonic power of 400 W, and 60°C for 50 minutes. The crude extract was vacuum-filtered and concentrated by a vacuum-rotary evaporation procedure (60°C, 80 r·min) to obtain the extract of ginkgo biloba leaves for use.
Anthocyanin extract. Twenty grams of powder from eggplant peels was accurately weighed and added into a round-bottom distillation flask containing 70% ethanol and a solid:liquid ratio of 1:25 (g·mL), shaken, and ultrasonic extracted at 40°C for 60 min, and then transferred to a centrifuge tube. The supernatant was taken after centrifugation at 4000 r·min for 20 min, extracted with ethyl acetate, and concentrated by rotary evaporation, and then the anthocyanin extract of eggplant peels was obtained after vacuum drying.
Essential oil extract. Fifty grams of powder from L. foenum-graecum Hance was accurately weighed and added into a round-bottom flask containing 4% sodium chloride solution and solid:liquid ratio of 1:10 (g·mL), then soaked for 1 h. The extract was heated with a thermostatic heating jacket to keep it in a slightly boiling state for 6 h. The water vapor containing the essential oil was collected, cooled, distilled through a duct, and then extracted with petroleum ether and subjected to anhydrous sodium sulfate dehydration treatment. The essential oil extract was obtained after petroleum ether was recovered by rotary volatilization.
Preservative Conditions for Plant Extracts
Based on the pre-experiment results, four treatments used in this experiment: 0.05% potassium sorbate, 0.5% essential oil extract, 0.5% ginkgo biloba extract, and 0.5% anthocyanin extract. Natural room temperature was the control (CK). Litchi was divided into 5 groups of 100 each and weighed. After soaking in the above four treatment liquids for 10 min, the samples were taken out and drained, packaged in plastic wrap, and stored at 25°C for 12 d. Related indicators were observed and measured every 2 d, repeated 3 times, and averaged.
Determination of Physical and Chemical Properties
The weight loss percentage was calculated by the following equation: weight loss (%) = (W0–W1)/W0 × 100. W0 is the average weight before treatment, and W1 is the average weight after treatment. The chroma L* value represents the brightness of fruits and vegetables, measured by the WSC-S colorimeter (Cao et al., 2007). The rotten fruit included mildew and rotten skin resulting from fungus, bacteria, and pathogens, etc. The percentage of the counted rotten litchi in the total number of surveys was used as the decay rate (Scott et al., 1982).
Vc content (mg/100 g. F. W.)was determinedusing the 2,6-dichlorophenol indophenol method (Cao et al., 2007). The treated litchis were ground in an electric juice extractor and filtered to obtain litchi juice. Soluble solids content was measured using the PR32 digital rafractometer. Titratable acid content was measured using the neutralization method (Association of Official Agriculture Chemists (AOAC, 2000).
Polyphenol oxidase (PPO) activity was determined using the catechol method at 420 nm (Cao et al., 2007). Peroxidase (POD) activity was based on the determination of guaiacol oxidation at 470 nm by H2O2 (Cao et al., 2007). Malondialdehyde (MDA) content was determined as 2-thiobarbituric acid (TBA) reactive metabolites (Cao et al., 2007).
Statistical Analysis
Data used for statistical analysis were the mean values of three repetitions in each treatment. Statistical analysis was performed using Microsoft Office Excel 2007, and all results are expressed as the mean ± SE of triplicate values.
Results
Effects of Plant Extracts on the Apparent Quality of Litchi
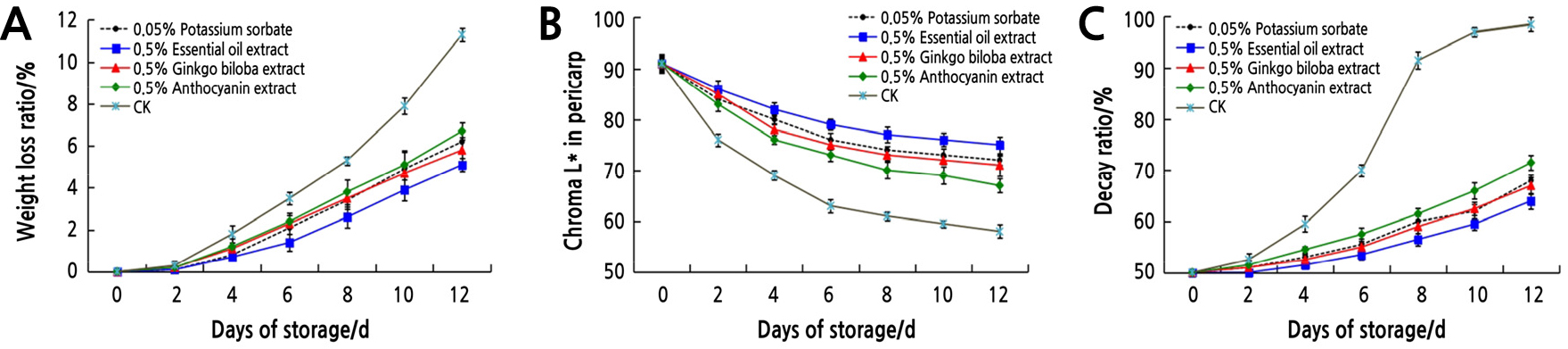
The mean value of the apparent quality of litchi treated with plant extracts is depicted in Fig. 1. The results clearly showed that the weight loss (Fig. 1A) and decay rate (Fig. 1C) of litchi in all treatments increased gradually, while the chroma L* (Fig. 1B) decreased with the extension of storage time. Compared to the control, the four treatments significantly reduced the litchi weight loss and decay rate, while they increased the chroma L* value. When litchi was stored in the control group for 2 days, the chroma L* value decreased rapidly, indicating that the color changed rapidly. Although the chroma L* value decreased gradually with the prolonged storage time, the four treatment groups effectively delayed the litchi browning compared with the control. The rot rate of the control group reached 80% on the 8th day, which was about 4 times that of the other four treatments. From 10 to 12 d, the litchi in the control group were completely spoiled, while the decay rate in the plant extract treatment groups was still below 50%.
It can be seen from the apparent quality of litchi that 0.5% essential oil extract treatment has the best preservation effect on litchi, followed by 0.5% ginkgo biloba extract and 0.05% potassium sorbate treatment, both of which have similar effects and are better than 0.5% anthocyanin extract. The effect of 0.5% anthocyanin extract was inferior to the other three treatments, but it was significantly better than the control. These results showed that these three kinds of plant extracts slowed down the water loss, inhibited browning, reduced the decay rate of litchi to some extent, and had a certain effect on preservation and freshness.
Effects of Plant Extracts on the Nutritional Quality of Litchi Pulp
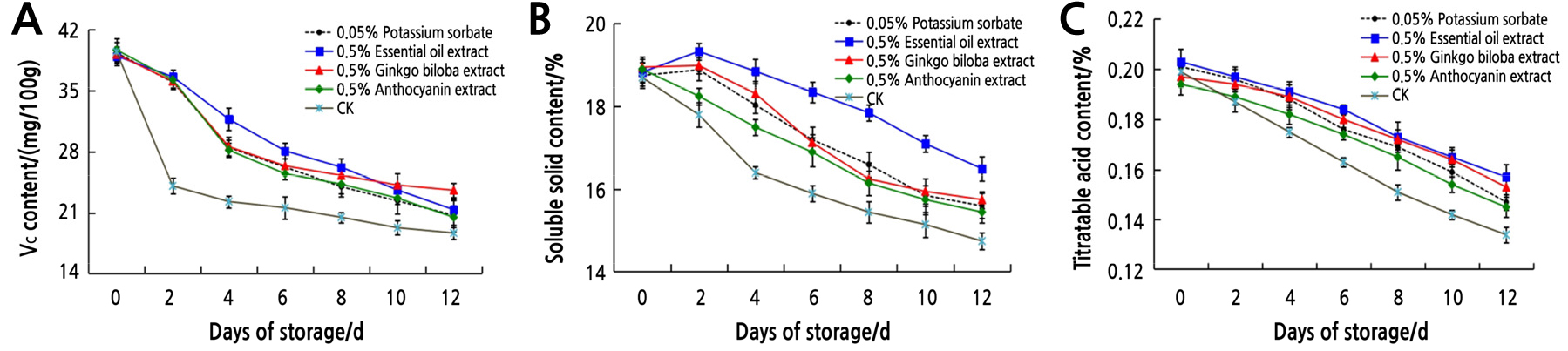
After fresh litchi are picked, respiration and other physiological and biochemical reactions inside the fruit continue. As the substrate for respiration, the content of nutrients usually decreases. The mean value of the nutritional quality of litchi treated with plant extracts is shown in Fig. 2. The content of Vc (Fig. 2A), soluble solids (Fig. 2B), and titratable acid (Fig. 2C) of litchi gradually decreased with the extended storage time in the four plant extracts and control treatments. When stored at natural room temperature, the Vc content decreased rapidly on the 2th day, and the soluble solids content decreased quickly on the 4th day. Compared with the control, all four treatments effectively delayed the decrease of Vc, soluble solids, and titratable acid contents in postharvest litchi. In the early storage period, 0.5% essential oil extract can effectively inhibit the decrease of Vc content, while 0.5% ginkgo biloba extract had a better inhibitory effect in the late storage period, both of which were slightly better than 0.5% anthocyanin extract and 0.05% potassium sorbate treatments. This indicates that plant extracts can delay the decline of Vc content to some extent during a certain storage period. Compared to the other two plant extracts and 0.05% potassium sorbate, 0.5% essential oil extract more effectively inhibited the reduction of soluble solids content. In addition, the effect of 0.5% essential oil extract and 0.5% ginkgo biloba extract in delaying the decrease of titratable acid content was relatively better than 0.05% potassium sorbate. This indicates that plant extracts could reduce the respiration, slow down the reduction of soluble solids and titratable acid contents, delay fruit senescence and the loss of flavor, and finally extend the shelf life of postharvest litchi.
Effects of Plant Extracts on Enzyme Activity and Lipid Peroxides of Litchi Pulp
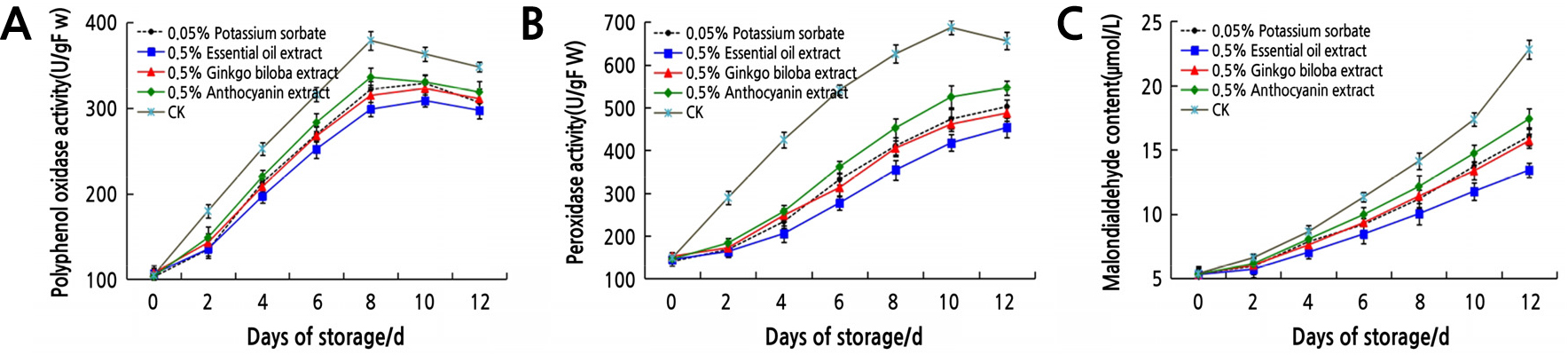
The polyphenol oxidase and peroxidase are closely related to the browning that occurs during the storage and processing of fruits and vegetables. The mean value of enzyme activity and lipid peroxides of litchi treated with plant extracts is showed in Fig. 3. With the extension of storage time, PPO activity (Fig. 3A) of the control and 0.5% anthocyanin extract increased to the maximum value on the 8th day, and that of 0.5% essential oil extract, 0.5% ginkgo biloba extract, and 0.05% potassium sorbate reached the maximum value on the 10th day, and then slowly declined. POD is a major factor in the browning process of fruits and vegetables; enzymatic browning becomes more and more serious with the increase of POD activity. With the extension of treatment time, the POD activity (Fig. 3B) of litchi in the four plant extracts gradually increased, but all were lower than those in the control, which was up to the maximum level on the 10th day and then decreased. At the same storage time, PPO and POD activity of 0.5% essential oil extract treatment was lower than that of other treatments. This indicates that these plant extracts can reduce the activity of PPO and POD and effectively delay the browning of litchi.
MDA is one of the most important products of membrane lipid peroxidation; its production can aggravate membrane damage. MDA content of the four plant extracts and control treatments showed an upward trend with the extension of storage time (Fig. 3C). At the same storage time, the MDA content of the 0.5% essential oil extract treatment was lower than that of the 0.5% ginkgo biloba extract, 0.05% potassium sorbate, and 0.5% anthocyanin extract treatments, which were lower than those of the control. This indicates that plant extracts can effectively reduce membrane lipid peroxidation and membrane loss and prolong the storage period of postharvest litchi.
Discussion
As natural compounds, plant extracts have significant antioxidant, antibacterial, and antitumor activity and scavenge free radicals. The use of plant extracts increases the safety of fruits and vegetables, reduces the disposal of waste, and does not cause harm to the human body and the environment (Yong et al., 2015). The essential oil in this experiment is a volatile oily substance extracted from the traditional medicinal plant, L. foenum-graecum Hance from China. It contains a large amount of aromatic substances, such as esters and organic acid components, and has broad-spectrum bacteriostasis, which is good for food preservation and is an ideal natural preservative (Bajpai et al., 2012). The active ingredients of ginkgo biloba extract are mainly flavonoids, terpene lactones, and ginkgoic acid, etc., which have good antioxidant and antibacterial properties (Tao et al., 2013). Eggplant peel is rich in anthocyanins, a natural food pigment and flavonoids, which has strong antioxidant and anti-inflammatory effects (Fan et al., 2008).
PPO exists in the peel of postharvest litchi, and it oxidizes catechol and other substrates to form quinones to accelerate browning (Jiang et al., 2004). The enzymes of PPO and POD have been shown to promote litchi browning, and their changes in activity may be early signs of browning. This study showed that 0.5% essential oil extract, 0.5% ginkgo biloba extract, and 0.5% anthocyanin extract reduced PPO and POD activity and membrane lipid peroxidation and better protected the color of litchi peel. This indicates that the plant extracts caused changes in the antioxidant activities and free radical scavenging capacities of the fruit tissue, protecting the integrity of the cell membrane, improving the antioxidant defense capacity, and delaying fruit senescence effectively. This finding was consistent with changes in enzyme activity of antibacterial film packaging of fresh-cut lettuce (Deng et al., 2016) and fumigation-treated mango (Perumal et al., 2017) with thyme essential oil. It was also in line with the results of gingko flavonoids (Perez-Vizcaino and Fraga, 2018) and films containing citric acid (Azevedo et al., 2018) used to reduce the browning of apples. Meanwhile, this study also found that these three plant extracts can effectively inhibit rot and the increase in weight loss of postharvest litchi and keep the Vc, soluble solids, and titratable acid contents at a high level. This indicates that the plant extracts had a certain protective effect on the nutrients and flavor quality of litchi pulp and prolonged the shelf life of litchi after harvest. These results were in agreement with the mass loss, acidity, Vc, and soluble solids content in refrigerated nectarines treated with essential oil extracted from lemongrass, thyme, and rosemary (Abd El Wahab, 2015), as well as strawberries steam-treated with tea tree essential oil (Shao et al., 2013).
The essential oil extracted from L. foenum-graecum Hance had the best preservation effect on postharvest litchi in this study. This may be related to the bacteriostatic and antioxidant activities of phenols, organic acids, terpenes, aldehydes, and ketones contained in essential oil (Bajpai et al., 2012). The detection and analysis of the oil extracted from L. foenum-graecum Hance by gas-liquid spectroscopy-mass spectrometry revealed that there were 63 kinds of volatile aroma components in the oil, of which eight such as palmitic acid, heptadecanoic acid, and linoleic acid account for a majority, reaching 28.94% (Wei et al., 2019). The organic acids can pass through the cell membrane of microorganisms and inside the cell change the charge distribution, reduce the water activity, denature and dehydrate the bacterial protein, disrupt cell metabolism, and even cause it to die (Finten et al., 2017). Terpenoids can damage the lipid structure of the cell wall of microorganisms, thereby destroying the structure of cell membranes, causing cytoplasm to leak, and cell lysis leading to its death (Bajpai et al., 2012). The hydroxyl and hydrogen bonds contained in phenolic substances can be embedded in the hydrophobic structure of the microbial cell membrane, destroying the cell membrane structure and causing damage to the cell membrane function, leakage of intracellular substances, and ultimately leading to the death of microorganisms (Xing et al., 2018). These substances in essential oils work together to provide anti-inflammatory and antibacterial effects beneficial for food preservation. Cinnamon and palmarosa essential oils can effectively inhibit the growth of Escherichia coli (Millezi et al., 2016), and L. foenum-graecum Hance essential oil can significantly inhibit the spoilage caused by Escherichia coli, and the effect is better than chemical preservatives (Yan et al., 2016). Meanwhile, the phenolic substances and flavonoids in plant essential oils can act as antioxidants by scavenging free radicals, chelating metal ions, regulating antioxidant enzymes, and inhibiting cell lipid peroxidation. Studies have found that the essential oil extracted from E. asclepium (L.) has a strong antioxidant capacity, which is close to 4 times that of BHA (Bouchekrit et al., 2016), and the scavenging rate of essential oil extracted from perilla leaf on DPPH free radicals is 69.10%, and that of perilla seed oil is 79.11% (Wang et al., 2013). An amount of 6 to 8 µL·L-1 of cinnamon essential oil can effectively maintain the activity of CAT, SOD, and POD of cabbage and preserve the storage quality (Yang et al., 2019). In addition, lemon essential oil can increase the activity of SOD, CAT, and POD of ponkan mandarin orange and inhibit the increase in relative conductivity of the peel and the accumulation of MDA content in the pulp (Zhang et al., 2020). Citric acid can be used as a substitute for sodium hypochlorite used to sterilize spinach leaves and reduce deterioration (Finten et al., 2017). In this study, 0.5% essential oil extract effectively maintained the activities of PPO and POD and reduced the degree of membrane lipid peroxidation. Therefore, we inferred that the essential oil of L. foenum-graecum Hance can be used for preservation of postharvest litchi as both a bacteriostatic and antioxidant agent.
Conclusion
In this study, treatments of 0.5% essential oil extract, 0.5% ginkgo biloba extract, and 0.5% anthocyanin extract on postharvest litchi decreased decay and weight loss and reduced PPO and POD activity and the degree of membrane lipid peroxidation. They also helped maintain better color in litchi peel and higher Vc, soluble solids, and titratable acid contents of litchi pulp. These three plant extracts delayed fruit browning and senescence and prolonged the shelf life of postharvest litchi. It is important to point out that 0.5% essential oil extract had the best effect on preservation of litchi. However, the reason for the better preservation effect of essential oil from L. foenum-graecum Hance on litchi is not clear and needs to be studied further. The results of this study provide a reference for maintaining high quality and improving storage of postharvest litchi, as well as for developing new natural preservatives that are safe and environmentally friendly.