Introduction
Materials and Methods
Plant Materials
Free-hand Sectioning
Preparation for Fixation Methods
Leaf Morpho-anatomical Observations
Statistical and Cluster Analysis
Results
Light Microscope Observations
Scanning Electron Microscope Observations
Discussion
Conclusion
Introduction
Leaf morphological characteristics have been evaluated by means of microscopic analysis to investigate taxonomic classifications related to environmental changes, phylogenetic relationships, and confirmation of polyploidization (Moreira et al., 2012; Karwowska et al., 2015; Khan et al., 2017; Hussain et al., 2018; Sandoval-Zapotitla et al., 2019; Khan et al., 2023). However, leaf anatomical studies in some succulent plants, such as Echeveria, are challenging due to their thick cuticles (Von Willert et al., 1992), foldable cell walls (Fradera-Soler et al., 2022), high water content (Griffiths and Males, 2017), film-thin layers, the presence of epicuticular waxes and tannins, and other unique anatomical characteristics that cause technical problems, resulting in limited anatomical studies of the genus (Rost, 1969).
Several studies have used a microtome to prepare sample slides and observe them under a microscope to investigate anatomical structures, such as in the cases of Crassula (Karwowska et al., 2015), and Kalanchoe (Moreira et al., 2012). To obtain a cross-section, a microtome is commonly preferred owing to its efficient production of thicknesses up to 500 nm without damage to the inner structures (Mohammed et al., 2012). Despite its advantages, the microtome requires multistep sample preparation, such as embedding, involving a considerable amount of time and the use of toxic reagents (Johansen, 1940). Furthermore, microtomes are not always accessible to all laboratories (Ribeiro and Leitão, 2020). In contrast, the use of a razor blade, also called freehand sectioning, is a manual and age-old method that does not require any equipment but only skill and patience. This method is rarely used for plants such as succulents but has been successful in several other studies (Jones, 2011; Silva et al., 2014; Ribeiro and Leitão, 2020).
In general, to observe samples under a microscope, a fixation method is used to preserve the structure of the plant. Common fixation chemicals such as formaldehyde - acetic acid - ethanol (FAA), ethanol, and methanol are generally used and vary depending on the type of plant being sampled. Formaldehyde is commonly used to preserve cellular and plant structures in anatomical studies (Johansen, 1940); it is an ethanol-based fixative and considered a “standard preservative” that has been applied to woody and tough herbaceous stems and old roots given its rapid penetration and effectiveness (Johansen, 1940; Huang and Yeung, 2015; Stasolla and Yeung, 2015; Yeung et al., 2015). However, FAA is a toxic chemical that can cause plant cells to plasmolyze (Stasolla and Yeung, 2015). In contrast, methanol has been reported as an alternative to standard FAA protocols (Kim, 2020). In addition, ethanol is relatively nontoxic and is widely used as a fixative to remove water and coagulate proteins from tissues (Eltoum et al., 2001). However, the effects of methanol and ethanol vary depending on the species. Talbot and White (2013b) reported that methanol fixation was preferable for preserving the tissues of A. thaliana, whereas ethanol fixation at 70% and 100% was best for cotton and barley, respectively. Therefore, these three common fixative chemicals were tested in this study.
Light microscopy (LM) and scanning electron microscopy (SEM) are the predominant techniques used to visualize leaf micros tructures (Kwak et al., 2020), evaluate stomata characteristics of mutated plants (Cabahug-Braza et al., 2023), study inflorescence development (Hwang et al., 2014), analyze pollen morphologies (Cho and Ding, 2021), and elucidate structural and histochemical of in vitro-plantlets (Manokari et al., 2022). Succulent leaf blades are thick and contain a large amount of chlorophyll, making it difficult to distinguish cell outlines under a microscope (Yuan et al., 2020). Therefore, observations of leaf morpho-anatomical traits under LM require practical and suitable clearing agents compared to SEM (Yuan et al., 2020). Light microscopy is easier to handle; however, the samples must be cut thinly and made translucent using a series of clearing agents (Yeung, 1998). Compared with LM, SEM requires a series of chemical fixations, critical point drying (CPD), and coating of the samples with metal, which is both time-consuming and leaves the epidermal cells prone to shrinkage and collapse (Yuan et al., 2020). Both types of microscopies require optimized fixation methods before the samples are observed, though there is no universal method for processing plant tissues because suitable fixation depends on specific plant tissue characteristics (Chieco et al., 2012). Based on the needs of the researchers, the choice of LM or SEM depends on the desired imaging quality, ease of handling for sample illumination, skills with and knowledge of microscopy equipment, and the preparation of slides using suitable fixation methods.
To provide vital information about the leaf morpho-anatomy of Echeveria species, the use of freehand sectioning on 15 Echeveria cultivars with the use of three types of fixation agents (specifically, methanol, ethanol, and FAA) observed under both LM and SEM was investigated in this study. This study aimed to provide a practical and straightforward method that is both efficient and effective for preserving tissue morphologies in succulent plants and other related species.
Materials and Methods
Plant Materials
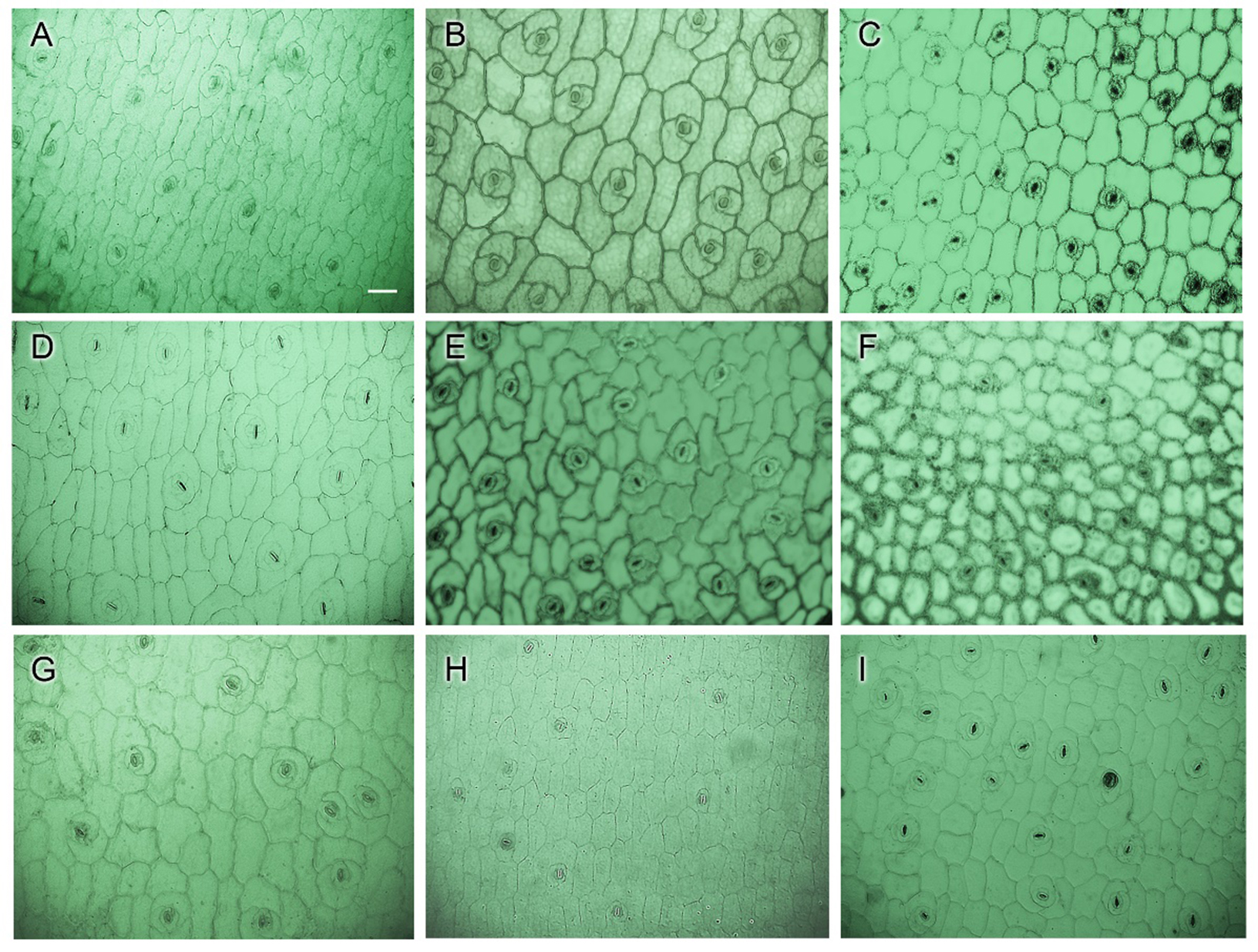
Fifteen cultivars of Echeveria were used in this study. These were E. ‘Benbadis’, E. ‘Brave’, E. colorata, E. ‘Cubic Frost’, E. ‘Dark Ice’, E. ‘Doterang’, E. ‘Glam Pink’, E. ‘Loy’, E. ‘Milk Rose’, E. ‘Fire Pillar’, E. ‘Peerless’, E. ‘Silhouette’, E. ‘Snow Bunny’, E. ‘Tippy’, and E. ‘Viyant’ (Fig. 1). These fifteen Echeveria cultivars exhibit eye-catching morphologies and various color displays and are considered as popular cultivars in succulent ornamental plant markets. The succulents were obtained from a succulent nursery in Goyang City, Gyeonggi-do, Republic of Korea. Leaves from the three lower whorls (location of the mature leaves) were collected from the mother plants of each plant to serve as experimental propagules.

Fig. 1.
Fifteen (15) Echeveria cultivars subjected to a leaf anatomical evaluation: A. E. ‘Benbadis’; B. E. ‘Brave’; C. E. colorata; D. E. ‘Cubic Frost’; E. E. ‘Dark Ice’; F. E. ‘Doterang’; G. E. ‘Fire Pillar’; H. E. ‘Glam Pink’; I. E. ‘Loy’; J. E. ‘Milk Rose’; K. E. ‘Peerless’; L. E. ‘Silhouette’; M. E. ‘Snow Bunny’; N. E. ‘Tippy’; and O. E. ‘Viyant’.
Free-hand Sectioning
Whole leaves from each cultivar were collected from the basal portions, and cross-sections were obtained using a double-edged razor blade after fixation in the first step of each fixative. The leaves of Echeveria plants are rigid; thus, there is no need for additional support such as polystyrene layers (Ribeiro and Leitão, 2020). Owing to the presence of abundant tannins and mucilage in succulent plants, the blade was replaced every two to three sections. Subsequently, free-hand sectioning proceeded with the subsequent steps of each fixative.
Preparation for Fixation Methods
The leaves were immersed in FAA fixative for 3 h at room temperature and then rinsed with distilled water. The fresh FAA solution was made by mixing 10 ml 95% ethyl alcohol, 5 ml acetic acid, 50 ml 10% formaldehyde and 35 ml distilled water. Leaves were sectioned using a sharp blade. The sectioned leaves were then cleared thrice in a xylene series (ethanol and xylene solution at three concentrations: 3:1, 1:1, and 1:3) for 5 min at each concentration; however, this step was skipped for those observed under SEM. The samples were then dehydrated in an ethanol series (50%, 70%, and 100%) three times for 30 min each.
For ethanol fixation, whole leaves were fixed in 70% ethanol for 1 h, cut using a razor, and dehydrated twice in 100% ethanol for 30 min.
In the methanol fixation method, the whole leaf tissues were immersed in methanol overnight. On the following day, the leaves were sectioned and then subjected to an ethanol series for 30 min at each concentration (50%, 70%, and 100%) prior to scanning.
Leaf Morpho-anatomical Observations
For light microscope observations, the samples from freehand sections of different fixation methods were washed with distilled water before they were stained with 0.5% toluidine blue (TBO) at a pH of 4.0. Afterward, the samples were rinsed in distilled water (three times for about 15–20 mins) and mounted onto slides. Mounted samples were observed under a light microscope (BX53F; Olympus, Japan). The upper epidermis was peeled off from the leaf blades using tweezers, spread flat in water, and pressed slightly into slides with the assistance of coverslips. The sections were observed under a light microscope (BX53F; Olympus, Japan).
For SEM, samples from different fixatives were directly transferred to a critical point drier and then coated with gold using a coating device (NeoCoater, MP-19020NCTR). All samples were observed under an accelerating voltage of 10–15 Kv using SEM (JSM-6510 with standard accessories).
In addition, the presence of leaf epicuticular wax (EW) on the surfaces of the Echeveria cultivars was noted. These were then graded based on the abundance of the EW as abundant, moderate, or minimal, as indicated in work by Cabahug et al. (2020).
Statistical and Cluster Analysis
Numerical data obtained from the morpho-anatomical evaluation were subjected to an analysis of variance (ANOVA). Significant differences between the mean values were analyzed using Duncan’s multiple range test (DMRT) at a significance level of 5%.
Results
Light Microscope Observations
In general, the effects of the three fixatives observed under a light microscope were maintained in the cross-sections. However, the samples fixed in methanol exhibited better contrast than those fixed in FAA, displaying a clearer appearance owing to the loss of cellular content and some cells shrinking in size. Additionally, the use of 70% ethanol yielded poor results, and several fixed samples exhibited signs of swelling or shrinkage (Figs. 2.1B, 2.2B, 2.3B).
The morphologies of the essential components of the succulent leaf, including the epidermis and hypodermis, were also observed and analyzed. The upper epidermal cells showed no significant changes despite the application of fixatives. The mesophyll cells and underlying epidermal and hypodermal layers were relatively well preserved in most examined plants. However, the shape of the hypodermal cells was varied owing to the fixatives. Among them, samples fixed in ethanol led to damaged, hard-to-distinguish cell outlines and swelling of the cells in E. ‘Brave’ (Fig. 2.1–B2), E. colorata (Fig. 2.1–B3), E. ‘Cubic Frost’ (Fig. 2.1–B.4), E. ‘Dark Ice’ (Fig. 2.1–B5), E. ‘Doterang’ (Fig. 2.2–B1), E. ‘Peerless’ (Fig. 2.3–B1), E. ‘Silhouette’ (Fig. 2.3–B2), E. ‘Snow Bunny’ (Fig. 2.3–B3), and E. ‘Tippy’ (Fig. 2.3–B4). Tissues fixed in FAA showed a loss of cellular content, presenting translucent cell walls due to the use of a clearing reagent (Fig. 2.1–A3). The results of samples fixed in FAA, in this case E. ‘Loy’ (Fig. 2.2–A4) and E. ‘Viyant’ (Fig. 2.3–A5), exhibited vague and homogeneous adjacent cells compared to those fixed in ethanol and methanol. The methanol fixative preserved the morphology of the tissues better than FAA and ethanol did, which presented a visualized hypodermis layer. It was observed that some cultivars, specifically E. ‘Cubic Frost’, E. ‘Doterang’, E. ‘Peerless’ E. ‘Silhouette’, and E. ‘Viyant’, presented challenges regardless of the fixation method. These species have hypodermic structures that are either rounded or oblong but are difficult to observe using FAA or ethanol fixation methods (Figs. 2.1, 2.2, 2.3-A and 1.3-B).
Epidermal cells observed under LM showed epidermal cell boundaries with clearer outlines in the majority of cultivar images (Figs. 3.1, 3.2). Epidermal cells were observed to be polygonal or irregular in shape with different levels of thickness. The periclinal walls were non-reticulate to reticulate and the anticlinal walls had nearly straight, straight, and sinuous lines (Table 1). Some green patches are visible in the mesophyll cells of E. ‘Silhouette,’ E. ‘Snow Bunny,’ and E. ‘Tippy’, which are mesophyll cells that are tightly attached to the epidermal layer (Fig. 3.2).
Table 1.
Leaf Micro-Morphology Observed under LM in Echeveria Cultivars
Scanning Electron Microscope Observations
Generally, the epidermal cell morphology showed wrinkles, cracks, or collapsed cell surfaces, which varied among the fixation methods and leaf characteristics when observed using SEM (Figs. 4.1, 4.2, Fig. 4.3.4.3). The amount of epicuticular wax (EW) present on the leaf surface affected the fixatives differently. Plants with minimal epicuticular wax had defined cells with clearer outlines and smoother surfaces than those with prominent epicuticular wax (Table 2). However, different fixation methods were found to be effective in particular species. For instance, E. ‘Brave’ is described as having an average amount of epicuticular wax; the use of FAA provided a recognizable epidermis shape with no surface cracks compared to fixing cross-sections in ethanol and methanol (Fig. 4.1-A2). While E. ‘Glam Pink’, E. ‘Silhouette’, and E. ‘Tippy’ are also in the same group as E. ‘Brave’, their micromorphology was maintained when fixed with methanol compared to those treated with ethanol or FAA. Among them, E. colorata was stable with a typical epidermis cell shape appearance when fixed with FAA and ethanol (Figs. 4.1-A3, B3), while methanol fixation made the epidermis surface rough, but the epidermis boundaries were still visible (Fig. 4.1-C3). Compared to FAA and methanol fixation, ethanol consistently produced poor images, with shrinkage observed in a majority of the succulent cultivars, regardless of the abundance of epicuticular wax.

Fig. 4.1.
Leaf micro-morphology of Echeveria cultivars observed using scanning electron microscopy (SEM): 1. E. ‘Benbadis’; 2. E. ‘Brave’; 3. E. colorata; 4. E. ‘Cubic Frost’; 5. E. ‘Dark Ice’ using various fixation methods: 1. FAA; 2. ethanol; and 3. methanol (200× magnification, scale bar = 50 µm).
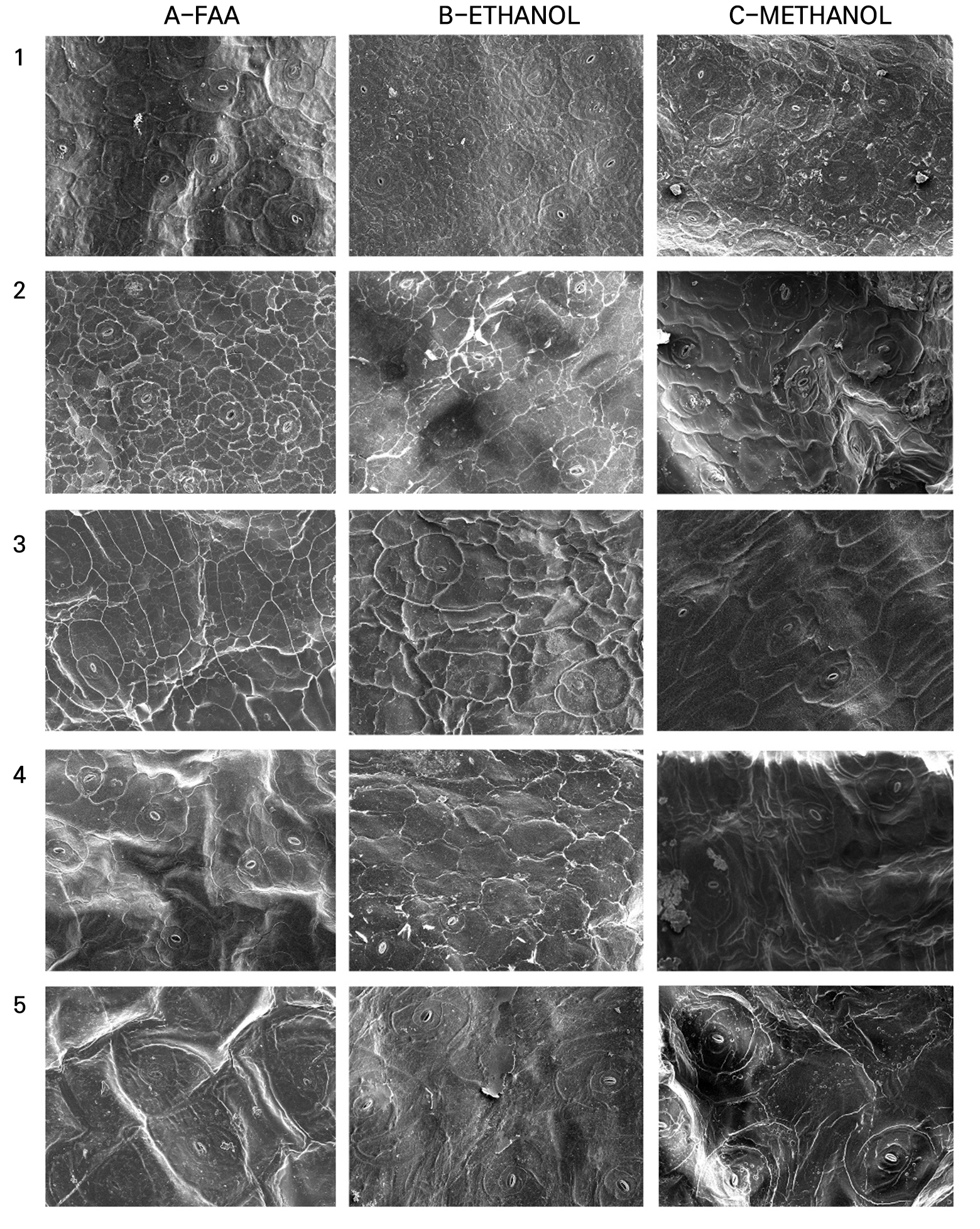
Fig. 4.2.
Leaf micro-morphology of Echeveria cultivars observed using scanning electron microscopy (SEM): 1. E. ‘Doterang’; 2. E. ‘Fire Pillar’; 3. E. ‘Glam Pink’; 4. E. ‘Loy’; 5. E. ‘Milk Rose’ using various fixation methods: 1. FAA; 2. ethanol; and 3. methanol (200× magnification, Scale Bar = 50 µm).

Fig. 4.3.
Leaf micro-morphology of Echeveria cultivars observed using scanning electron microscopy (SEM): 1. E. ‘Peerless’; 2. E. ‘Silhouette’; 3. E. ‘Snow Bunny’; 4. E. ‘Tippy’; and 5. E. ‘Viyant’ using various fixation methods: 1. FAA; 2. ethanol; and 3. methanol (200× magnification, scale bar = 50 µm).
Table 2.
Amount of Epicuticular Wax of Echeveria Cultivars
| Cultivar Name | Epicuticular Wax (EW) | EW Amountz |
| E. ‘Benbadis’ | + | 1 |
| E. ‘Brave’ | + | 1 |
| E. colorata | + | 1 |
| E. ‘Cubic Frost’ | + | 2 |
| E. ‘Dark Ice’ | + | 1 |
| E. ‘Fire Pillar’ | + | 1 |
| E. ‘Doterang’ | + | 2 |
| E. ‘Glam Pink’ | + | 1 |
| E. ‘Loy’ | + | 1 |
| E. ‘Milk Rose’ | + | 2 |
| E. ‘Peerless’ | + | 2 |
| E. ‘Snow Bunny’ | + | 3 |
| E. ‘Silhouette’ | + | 1 |
| E. ‘Tippy’ | + | 1 |
| E. ‘Viyant’ | + | 3 |
When the amount of epicuticular wax is abundant, cell deformation is affected, limiting the effectiveness of fixation methods. Among the cultivars that have abundant waxy leaves, the epidermal surfaces of E. ‘Cubic Frost’ and E. ‘Snow Bunny’ showed cell deformation and indistinguishable cell boundaries regardless of the fixation method used. On the other hand, E. ‘Viyant’, which also contains abundant EW, was found to have clear boundaries of cells and a smooth leaf surface when fixed in FAA, whereas relatively low-quality cells treated with ethanol and methanol retained their shape but tended to show wrinkling. This trend was also observed in E. ‘Doterang’ and E. ‘Milk Rose’, which contain an average amount of EW. In contrast, the shape and appearance of the epidermal cells of E. ‘Benbadis’ and E. ‘Dark Ice’ had favorable results when fixed in methanol; the leaf surfaces were not smooth, but the boundaries of the cells were distinguishable. FAA fixation also resulted in an epidermal cell morphology that was relatively maintained regardless of wrinkling. However, the ethanol treatment induced cracking of the leaf surfaces.
Discussion
Different fixative and imaging methods can affect the quality of the leaf sample images produced for analysis (Kawai et al., 2013; Urban et al., 2018). In addition, different responses of fixative methods arise from variations of the structure of the leaf epidermal surface coating or wall composition (Talbot and White, 2013b). Echeveria succulent plants are well known to have high homoplasy in their morphology (Gontcharova and Gontcharov, 2007), and their anatomical organs are diverse in terms of intermediate stages between all-cell succulence and storage-succulence and the performance of hydrenchyma and chlorenchyma (Fradera-Soler et al., 2022). Even when plants look very similar, their water content varies widely between species due to the amount of mucilage (Griffiths and Males, 2017). Therefore, selecting a suitable fixation treatment for each species is a challenge.
In this study, the morphoanatomical characteristics of Echeveria cultivars were evaluated by comparing three fixative treatments using LM and SEM. In general, the preparation method for LM is fast and straightforward, whereas that for SEM is time-consuming. Unlike images captured using basic LM, those captured under SEM have a higher resolution.
Ideally, fixation chemicals should preserve plant tissues and be nontoxic or minimally toxic. Alcohol-based tissue fixatives, such as ethanol and methanol, have been reported as alternatives to standard protocols (FAA).
Methanol was revealed to be effective through freehand sectioning, in which the cell morphology was preserved better than with the conventional LM method. Samples fixed with methanol showed more intense metachromasy when stained with TBO than those fixed with FAA, which caused a loss of the cellular content with only the cell walls remaining (Alwahaibi et al., 2018; Shah et al., 2017). Methanol has a simple structure and is a highly polar solvent that is closer to water than ethanol in terms of the structure, allowing it to penetrate the tissues rapidly and replace free water throughout the tissues, meaning also that samples can be left uncut in the solvent (Talbot and White, 2013b). Methanol has been shown to be efficient in producing a smooth epidermis and preserving the trichomes of Salvinia auriculata (Neinhuis and Edelmann, 1996) as well as lessening the damage to plant waxes compared to fixation with ethanol or acetone (Pathan et al., 2010). Talbot and White (2013b) also reported that methanol fixation provided the most consistent preservation of the surface morphology after CPD. However, the results of this study showed that the effects of a methanol treatment varied depending on the plant characteristics. Methanol fixation was also recommended for samples intended for SEM; however, the thickness of the sample and the incubation time in methanol must be optimized prior to use (Neinhuis and Edelmann, 1996).
A challenge in observing tissue surfaces under SEM is overcoming the shrinkage of tissue during a lengthy preparation process. Previous studies have compared the surface morphology change when using different protocols such as combining FAA with glutaraldehyde, dehydration with ethanol followed by CPD, but they all caused both animal and plant tissues to shrink to approximately 67–75% of their original sizes (Moncur, 1979; Boyde and Boyde, 1980; Beckett et al., 1984). Several investigations have reported that samples were not preserved well after long processing; particularly, chemical fixation causes poor preservation (Parsons et al., 1974; Boyde and Boyde, 1980), while methanol fixation exhibits a more preserved morphology (Neinhuis and Edelmann, 1996). Samples fixed in methanol followed by ethanol dehydration showed less cell wall wrinkling or folding than FAA according to SEM findings, similar to results obtained for Arabidopsis thaliana (Talbot and White, 2013b). Variations in methanol fixation were also tested with bean leaves to focus on the drying procedure after methanol fixation in work by Pathan et al. (2010), who indicated that bean leaves fixed in methanol followed by freeze-drying provided better preservation quality than air-drying after methanol fixation.
Generally, the common choice in histopathology is FAA because it is easy to use, inexpensive, preserves the tissue morphology well, and has rapid penetration (Johansen, 1940; Stasolla and Yeung, 2015; Huang and Yeung, 2015). This fixative has been applied to most tissues, such as woody stems, old roots, and tough herbaceous stems for anatomical and morphological studies (Yeung et al., 2015). Despite its advantages, formaldehyde causes plant cells to become plasmolyzed (Stasolla and Yeung, 2015), damages leaf epicuticular wax structures after ethanol series dehydration (Juniper and Jeffree, 1983), and is toxic to those who work with it, primarily causing eye and upper respiratory tract irritation (Kulle et al., 1987; Lang et al., 2008).
The use of FAA and ethanol was not favorable for LM; however, they partially retained the mesophyll structures according to SEM imagery, which varied between the samples. A well-known difficulty in preparing tissues for SEM is preventing tissue shrinkage during the fixation, dehydration, and CPD steps (Talbot and White, 2013a, 2013b). Shrinkage can result from water or ethanol retention in tissues after drying (Boyde and Maconnachie, 1979), which particularly causes challenge when working with succulent plants given that the water content of their organs may reach 90 ~ 95% (Griffiths and Males, 2017). Boyde and Boyde (1980) reported that unfixed potato tuber tissues, following dehydration and CPD, exhibited less shrinkage (15%) than those fixed in glutaraldehyde, indicating that chemical fixation is not critical for quality preservation (Parsons et al., 1974). A FAA fixation treatment caused partial cells of the epidermis to shrink, damage, or fold in A. thaliana, which also occurred in barley and cotton but was generally less significant (Talbot and White, 2013b). For Echeveria cultivars, FAA preserved the epidermis morphology fairly well for E. ‘Dark Ice’, E. ‘Loy’, E. ‘Peerless’, and E. ‘Viyant’.
Conclusion
Numerous studies have investigated suitable methods for studying leaf anatomy. However, there is no standard technique for all plants; the appropriate technique will differ based on the characteristics of the plant in question. In this study, the effects of common fixatives were found to vary according to the plant characteristics, even when the plants belonged to the same genus. The use and selection of appropriate methods and types of microscopy, with respect to the skill of the researcher and the availability of equipment, must be considered to ensure an efficient and accurate investigation. This study indicates and uses an alternative protocol that is both cost- and time-effective, which will be helpful in future studies examining the anatomy of other succulent genera.