Introduction
Materials and Methods
Plant Growth Conditions and Treatments
Morphological Characteristics
Physicochemical Attributes
Statistical Analysis
Results
Morphological Characteristics
Fruit Physicochemical Attributes at the Time of Harvest
Changes in Fruit Physicochemical Attributes During Cold Storage
Discussion
Introduction
Many factors affect plant growth and development, and also regulate or modify plant traits and postharvest quality. Foliar application of certain chemicals is used to enhance growth and yield (Shiri et al., 2014; Shiri et al., 2016a, Shiri et al., 2016b). Among the substances, one of the novel chemicals used in plant nutrition is mammalian sex hormones (MSHs) (Janeczko and Skoczowski, 2005). MSHs are the lipophilic and low-molecular-weight composites that belong to the steroid compounds derived from isoprenoids. They include various types of progesterones, estrones, testosterones, estriols, estradiols, androgens, and androsterones, and have several functions such as regulating growth, development, and reproduction processes, bioregulation for several metabolic pathways, and controlling the mineral and protein metabolism in mammals (Ruiz-Cortés, 2012). Furthermore, there are other utilities like impacts on cell membrane constituents or their conversion to various physiologically and biochemically active components. Previously, it was assumed that MSHs occur in only animals, but more recently MSHs have been extracted and identified from different plant species and their different organs such as roots, leaves, and flowers (Erdal and Dumlupinar, 2010; Erdal and Dumlupinar, 2011a, Erdal and Dumlupinar, 2011b)
Many studies have shown that use of MSHs influences cell division, differentiation, development, and homeostasis, plant vegetative and reproductive growth, as well as plant resistance and defense mechanisms by stimulating the activity of various antioxidant enzymes such as peroxidase (POD), catalase (CAT), superoxide dismutase (SOD), and glutathione reductase (GR) (Janeczko and Skoczowski, 2005; Erdal, 2012 a, Erdal, 2012b; Janeczko et al., 2015; Adeel et al., 2017).
Interest in production and consumption of strawberry (Fragaria × ananassa Duch.) has increased due to the attractive color, good fragrance, pleasant flavour and aroma, and the production of essential nutritional and phytochemical compounds, such as vitamin C, vitamin E, sugars, organic acids, amino acids, anthocyanins, total phenolic compounds, and total antioxidant activity, in the fruit, which have anti-cancer and anti-inflammatory properties (Husaini and Neri, 2016; Ventura-Aguilar et al., 2018). The main purpose of strawberry farming is to produce fruit with an attractive appearance (fruit size, color, and shape) (Roussos et al., 2009). On the other hand, strawberry fruit is very perishable due to its high metabolism and respiration rate, which causes more than 40% losses during storage (Bola, 2016; Horvitz, 2017).
Different strategies have been used to improve strawberry production; among them, the application of plant growth regulators (PGRs) has substantially improved flowering, vegetative reproduction, fruit color development, ripening, senescence, and postharvest quality (Gundogdu et al., 2017; Kumra et al., 2018; Jamwal et al., 2018). But there is no information about the foliar application of MSHs on strawberry. Therefore, the objectives of this study were to: 1) identify the optimal concentration of ethinyl estradiol (EE) and progesterone (Prog) treatments, 2) enhance strawberry productivity and fruit quality at harvest, and also 3) improve the storability of strawberry fruit in cv. Camarosa during cold storage.
Materials and Methods
Two studies were undertaken in 2018. In the first study, we evaluated several morphological characteristics and fruit physicochemical attributes of strawberry (Fragaria × ananassa Duch.) cv. Camarosa in response to exogenous application of ethinyl estradiol (EE) (a synthetic form of estrogen) and progesterone (Prog) during the growing season and at the time of harvest. In the second study, we examined the effects of exogenous application of EE and Prog on several fruit physicochemical attributes and antioxidant enzyme activities of fruit of this cultivar during 4 weeks of cold storage. We selected Camaros due to its extensive use abroad as well as in Iran, and good agronomic performance.
Plant Growth Conditions and Treatments
The study was performed on plants of cultivar Camarosa in a greenhouse at Abrisham city, Isfahan province, Iran (latitude of 32°55'50", longitude of 51°57'31", 1,610 m altitude, 10 ‑ 16°C mean annual temperature and 110 ‑ 160 mm rainfall per year). Green strawberry plants were planted in February 2016 in a glass greenhouse. Plants were arranged in single rows, with 30-cm spacing between the plants and 90-cm spacing between the rows. Before planting, all the dead leaves and runners were removed. Planting beds contained a soil, sand, and peat moss (2:1:1, v/v/v) mixture. Conditions within the greenhouse were as follows: day/night temperatures of 25°C/15°C (± 1°C) and 70% (± 5%) relative humidity with a 14-h photoperiod at a light intensity of approx. 500 ‑ 1,000 µmol·m-2·s-1 PPFD provided by cool-white fluorescent lights, controlled automatically. The greenhouse was equipped with a drip irrigation system and water was supplied according to evaporation demand. During the growing season, Hoagland’s solution was used to provide essential nutrients.
The EE and Prog were dissolved first in a small volume of methanol and then diluted in water in order to obtain the 10-6 and 10-9 concentrations (Erdal and Dumlupinar, 2010). The following exogenous application of 0.0 (untreated control), 0.001, and 1.0 ppm EE and Prog on all parts of strawberry plants (including leaves and fruit) were applied at two stages: 1) when plants reached the 3 ‑ 5 leaves stage and 2) four weeks later. EE, Prog, and other chemicals were purchased from Merck (Merck KGaA, Darmstadt, Germany). Each treatment was comprised of 4 replications and each replication contained 10 strawberry plants, totaling 200 strawberry plants used in this study. Morphological characteristics of the strawberry plants and fruit (discussed below) were determined during the growing season. Fruit were harvested at the same ripening stage (>75% red surface color), then transferred immediately to the laboratory. Within each replication, only fruit having similar size, color, shape, and without any physical injuries or diseased were selected. Physicochemical attributes (discussed below) were assayed at harvest. For this, 60 strawberries from each replication (30 fruit for each evaluated time) were stored in a refrigerator at 4°C and 90 ± 5% relative humidity for 4 weeks. The fruit physicochemical attributes were determined again 2 and 4 weeks after cold storage with 4 replications. At each sampling time, tissue samples consisting of both the achenes and receptacles were selected from the fruit central part. The samples were immediately cut, pooled, frozen in liquid nitrogen, and stored at ‑ 80°C until use for physicochemical attributes.
Morphological Characteristics
Morphological characteristics of the strawberry plants and fruit were measured during the growing season, including number of inflorescences per plant, number of flowers per inflorescence, weight of first fruit, average weight of fruit, length and width of first fruit, and the length/width ratio of first fruit.
Physicochemical Attributes
The pH, total soluble solids (TSS), titratable acidity (TA), and the ratio of TSS to TA (TSS/TA) were assessed with juice obtained using the indelicate Super-Automatic extractor (model A2-104, China). The pH was determined by a pH meter (model 691, Metrohm, AG, Herisau, Switzerland). TSS was determined with a digital refractometer (ATAGO, Tokyo, Japan) at room temperature and expressed as °Brix. TA was determined by titration of 25 mL filtrated juice by 0.1 N NaOH up to a pH of 8.2 and calculated as mg/100 mL (Mazumdar and Majumder, 2003). After the determination of TSS and TA, the TSS/TA ratio was calculated.
The amount of chlorophyll a (Chl-a), chlorophyll b (Chl-b), and total chlorophyll (TChl) in strawberry leaves were determined as described by Arnon (1949). Briefly, 10 mL of 80% acetone (acetone:distilled water 80:20 v:v) was added to 0.5 g of homogenized freeze-dried leaf samples. The homogenate was centrifuged twice at 4°C (10 min, 6,000 g) and the supernatant was used as the crude enzyme preparation. The supernatant was separated and the absorbances were recorded at 663 and 645 nm with a Novaspec II UV/Vis spectrophotometer (Pharmacia, Freiburg, Germany). Chlorophyll content was expressed as mg·g-1 fresh weight (FW). The vitamin C content was measured using a microplate (ELISA) reader (Tecan, Salzburg, Austria) by commercial kits (ZellBio GmbH, Germany) and expressed as mg/100 mL. Anthocyanin content was assayed according to the pH differential method as described by Lee et al. (2005). Briefly, 2.5 g of frozen tissue were ground with a mortar and pestle in liquid nitrogen. Then, samples were extracted with 10 mL of methanol acidified with 0.1% HCl. The samples were then centrifuged at 6,650 g for 15 min. The supernatants were then passed through a 0.45 mm filter. Absorbance was measured at 510 nm and at 700 nm in buffer at pH 1.00 and pH 4.5, using A = (A510 pH1.0 ‑ A700 pH1.0) ‑ (A510 pH4.5 ‑ A700 pH4.5) with a molar extinction coefficient for cyanidin-3-glucoside of 29,600. Results were expressed as micrograms of cyanidin-3-glucoside equivalent per 100 g FW.
The activity of POD, SOD, CAT, and GR were assayed using a microplate (ELISA) reader (Tecan, Salzburg, Austria) by commercial kits (ZellBio GmbH, Germany). All extractions were performed in sodium pyrophosphate buffer. The extractions and measurements were done in a cold room (at 4°C) in darkness. POD activity was expressed as milli Unit (mU)·mL-1, SOD and CAT activities were expressed as U·mL-1, and GR activity was expressed as U·L-1. One international unit is defined as the amount of enzyme which catalyzes the decomposition of one mole of O-2 to H2O2 and O2 per minute at 25°C and pH 7.00.
Statistical Analysis
The first study was performed based on a randomized complete block design with four replications. The second study was carried out according to a two-factor linear design with four replications, where treatments (exogenous application of EE and Prog) and storage times were the factors. Data were analyzed by the PROC ANOVA procedure by SAS software (Ver. 9.1 2002–2003, SAS Institute, Cary, NC). Least significant difference at p ≤ 0.01 and p ≤ 0.05 was calculated to compare differences between means following a significant ANOVA effect.
Results
Morphological Characteristics
Morphological characteristics, such as the number of inflorescences per plant, number of flowers per inflorescence, weight of first fruit, average weight of fruit, and length and width of first fruit, were significantly affected by foliar application of EE and Prog, but the length/width ratio was not significantly affected (Table 1).
Table 1. Effects of foliar application of ethinyl estradiol (EE) and progesterone (Prog) on several vegetative growth characteristics, fruit physicochemical attributes, and antioxidant enzymes activity of strawberry cv. Camarosa at the time of harvest
yData are the mean ± standard error (n = 4).
xMeans within each column followed by the same letter are not significantly different according to the LSD test.
NS, *, **Non-significant or significant at p ≤ 0.05 and p ≤ 0.01, respectively.
All EE and Prog treatments significantly enhanced the number of inflorescences per plant and the number of flowers per inflorescence as compared with control (untreated) plants. The highest weight of first fruit was found in the 1.0 ppm of EE treatment (43.11 g), while other treatments showed no significant differences compared to the control. As shown in Table 1, the average weight of fruit, and the length and width of first fruit significantly increased in response to all EE and Prog treatments, as the lowest amount was obtained in control plants. On the other hand, the length/width ratio of the first fruit was in the range of 1.10 to 1.25, but no significant differences were observed between treated and untreated plants (Table 1).
Fruit Physicochemical Attributes at the Time of Harvest
As shown in Table 1, the Chl-a, Chl-b, and T-Chl contents of strawberry leaves were significantly affected by the foliar application of EE and Prog. The highest chlorophyll content was found in the 0.001 ppm of Prog treatment (0.49, 0.24, and 73.0 mg·g FW-1 for Chl-a, Chl-b, and T-Chl, respectively).
The pH levels of strawberry fruit ranged from 3.47 to 3.63 but the EE and Prog treatments did not significantly affect fruit pH (Table 1). Foliar application of EE and Prog significantly affected TSS, TA, and the TSS/TA ratio at the time of harvest. All EE and Prog treatments significantly reduced the TSS content, as the highest TSS content was obtained in control fruit (9.65°Brix). TA significantly decreased in response to the 0.001 ppm of EE application, while no significant differences were observed between the other treatments. The TSS/TA ratio was significantly reduced in response to EE and Prog treatments, as the highest TSS/TA ratio was obtained in control fruit (1.74).
The 0.001 ppm of EE treatment significantly enhanced the vitamin C content to 765.90 mg·mL-1 as compared with the control (692.86 mg·mL-1), whereas the 1.0 ppm of Prog and of EE treatments significantly reduced the vitamin C content (589.67 and 638.26 mg·mL-1, respectively). Additionally, no significant differences were observed between fruit treated with 0.001 ppm of Prog and control fruit (Table 1). The anthocyanin content of strawberry fruit ranged from 10.32 to 10.92 mg/100 mg but no significant differences were observed between the treatments and the control (Table 1).
Foliar application of EE and Prog significantly affected the antioxidant enzyme activities of strawberry fruit at harvest. As shown in Table 1, the 0.001 ppm of EE and Prog treatments significantly enhanced POD activity as compared with the control, while no significant differences were found between the other treatments and the control. SOD activity significantly decreased to 1.41 U·mL-1 in the 0.001 ppm of EE treatment compared to the control (1.71 U·mL-1), but significantly increased to 2.09 U·mL-1 in the 1.0 ppm of EE treatment. However, no significant differences in SOD activity were observed between the Prog treatments and control fruit. The 0.001 ppm of EE and Prog treatments significantly reduced CAT activity compared to the control, while CAT activity wasn’t significantly affected by the 1.0 ppm of EE and Prog treatments (Table 1). GR activity significantly decreased in response to 0.001 ppm of EE and Prog, whereas 1.0 ppm of Prog significantly enhanced GR activity, and no significant difference was obtained between the 1.0 ppm of EE-treated fruit and control fruit (Table 1).
Changes in Fruit Physicochemical Attributes During Cold Storage
pH was significantly affected by storage time, while the EE and Prog treatments, as well as their interaction with storage time, had no significant effects (Table 2). No significant difference was found between harvest time and 2 weeks of cold storage, but after 4 weeks of cold storage, pH significantly increased to 3.84.
Table 2. Effects of foliar application of ethinyl estradiol (EE) and progesterone (Prog) on several vegetative growth characteristics, fruit physicochemical attributes, and antioxidant enzymes activity of strawberry cv. Camarosa during 4 weeks of cold storage
yMeans within each column followed by the same letter are not significantly different according to the LSD test.
EE: ethinyl estradiol, Prog: progesterone, TSS: total soluble solids, TA: titratable acidity, POD: peroxidase, SOD: superoxide dismutase, CAT: catalase, GR: glutathione reductase.
NS, *, **Non-significant or significant at p ≤ 0.05 and p ≤ 0.01, respectively.
As shown in Table 2, the simple and also the interaction effects of storage time and the treatments significantly affected the TSS and TA content of strawberry fruit. After 4 weeks of cold storage, the TSS content significantly increased as compared with that at harvest time and 2weeks of cold storage (Table 3). At the end of storage, fruit treated with 1.0 ppm of Prog had the highest TSS content (10.03°Brix), while the other treatments showed no significant differences from the control. The TA content significantly increased during the 4-week cold storage compared to that at harvest. At the end of storage, the 1.0 ppm of EE and Prog treatments significantly reduced the TA content, while no significant differences were observed between control fruit and those treated with 0.001 ppm of EE and Prog (Table 3).
Table 3. Effects of foliar application of ethinyl estradiol (EE) and progesterone (Prog) on TSS, TA, the TSS/TA ratio, and vitamin C content of strawberry cv. Camarosa during 4 weeks of cold storage
zMeans within each column followed by the same letter are not significantly different according to the LSD test.
EE: ethinyl estradiol, Prog: progesterone, TSS: total soluble solids, TA: titratable acidity.
The TSS/TA ratio was significantly affected by storage time and the interaction effects of storage time and the treatments, but the effect of the treatments was not significant (Table 2). The TSS/TA ratio significantly decreased from 1.42 to 1.20 after 2 weeks of cold storage, then significantly increased to 1.47 at the end of storage. After 4 weeks of cold storage, fruit treated with 1.0 ppm of Prog had the highest TSS/TA ratio (1.70) as compared with control fruit (1.29) (Table 3).
As shown in Table 2, the simple and also the interaction effects of storage time and the treatments significantly affected the vitamin C content of strawberry fruit. Vitamin C content significantly decreased in response to cold storage, as the lowest content was obtained at the end of the storage time. Moreover, all treated fruit had a higher vitamin C content than the control at 2 and 4 weeks of cold storage (Table 3).
POD activity was significantly (p ≤ 0.01) affected by the simple effects of storage time and treatments, while the interaction effects of storage time and treatments were not significant (Table 2). Without treatment, POD activity significantly decreased from 25.23 at the start of storage to 18.01 mU·mL-1 at 4 weeks of cold storage. However, all EE and Prog treatments significantly enhanced POD activity as compared with control fruit (Table 2).
The simple and the interaction effects of storage time and treatments significantly (p ≤ 0.01) affected SOD activity (Table 2). During cold storage SOD activity significantly increased from 1.77 to 2.36 U·mL-1. At the end of cold storage, fruit treated with 0.001 ppm of Prog had the highest SOD activity (3.25 U·mL-1) (Fig. 1).
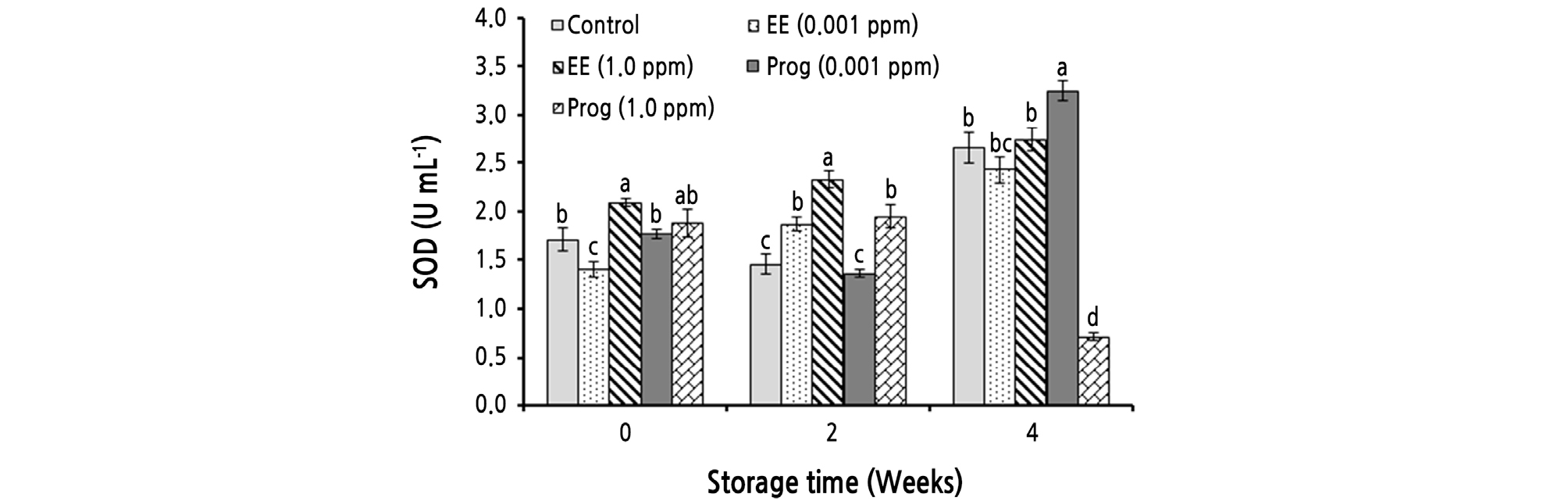
Fig. 1.
Effects of foliar application of ethinyl estradiol (EE) and progesterone (Prog) on superoxide dismutase (SOD) activity of strawberry cv. Camarosa during a 4-week cold storage. Vertical bars indicate standard error (n = 4). Means followed by the same letter are not significantly different according to the LSD test. Slicing was performed based on the storage times.
As shown in Table 2, CAT activity was significantly affected by the simple effect of storage time and the interaction effects of storage time and the treatments, while the simple effect of the treatments was not significant. CAT activity was significantly enhanced during cold storage (Table 2). After 4 weeks of cold storage, the highest CAT activity (29.37 U·mL-1) was observed in fruit treated with 1.0 ppm of EE, which had no significant difference with that of the 1.0 ppm of Prog treatment (28.97 U·mL-1) (Fig. 2).
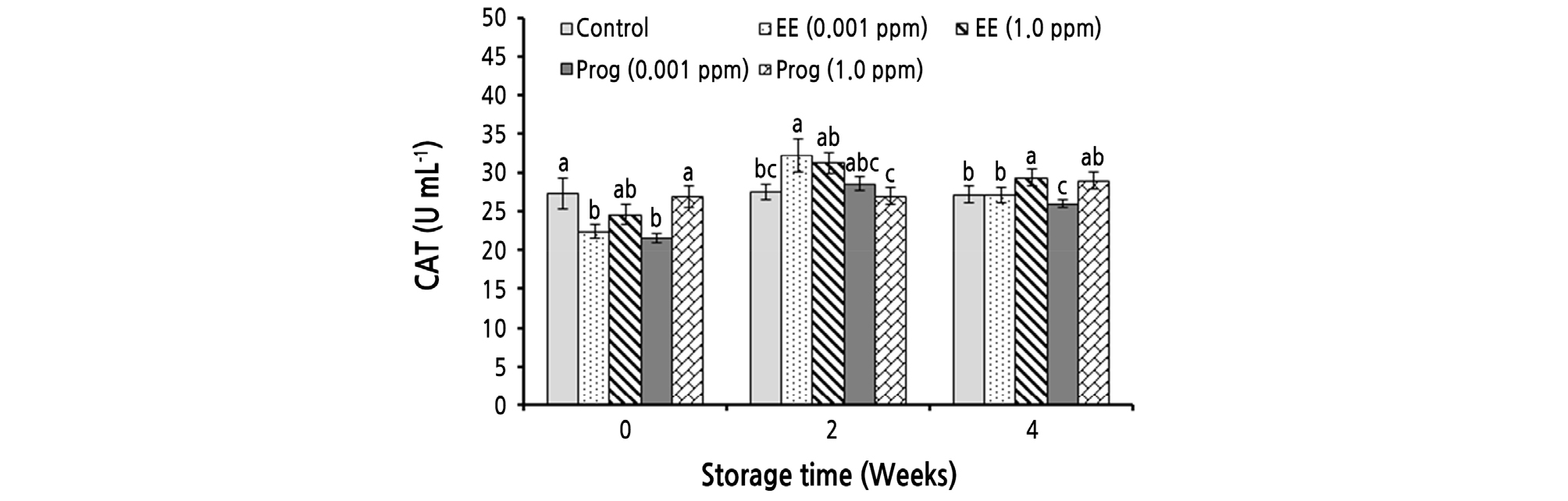
Fig. 2.
Effects of foliar application of ethinyl estradiol (EE) and progesterone (Prog) on catalase (CAT) activity of strawberry cv. Camarosa during a 4-week cold storage. Vertical bars indicate standard error (n = 4). Means followed by the same letter are not significantly different according to the LSD test. Slicing was performed based on the storage times.
The simple effect of the treatments and the interaction effects of storage time and the treatments significantly affected the GR activity of strawberry fruit. Fruit treated with 1.0 ppm of EE and Prog showed the highest GR activity in all evaluated times (Fig. 3).
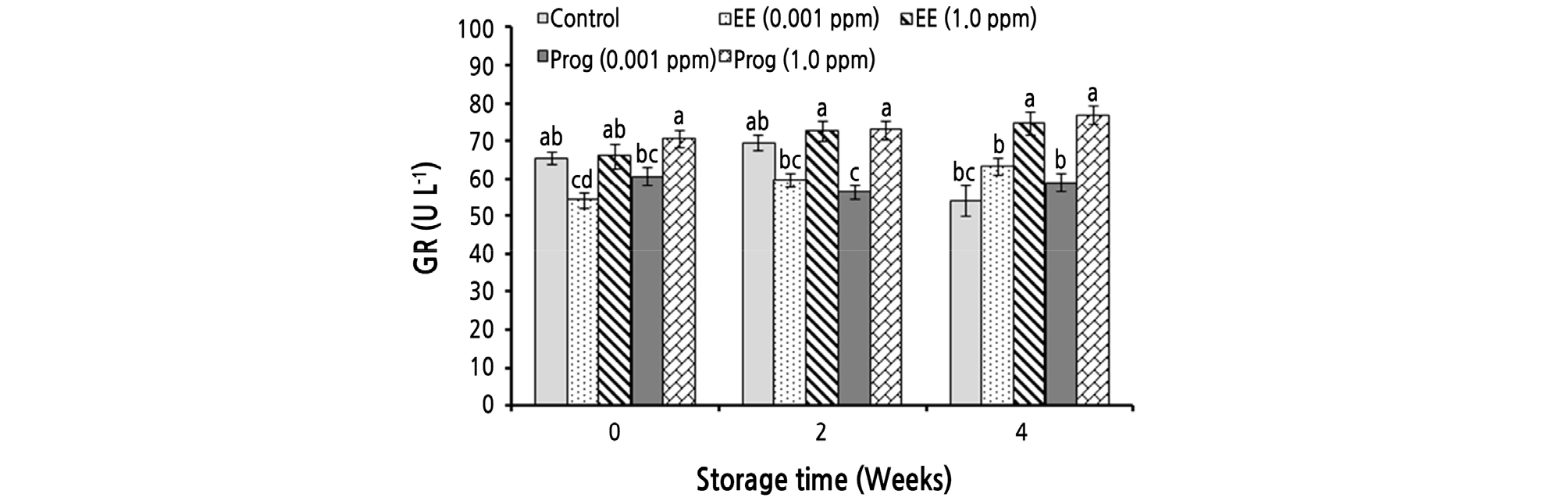
Fig. 3.
Effects of foliar application of ethinyl estradiol (EE) and progesterone (Prog) on glutathione reductase (GR) activity of strawberry cv. Camarosa during a 4-week cold storage. Vertical bars indicate standard error (n = 4). Means followed by the same letter are not significantly different according to the LSD test. Slicing was performed based on the storage times.
Discussion
Our results clearly showed that MSHs influenced the morphological, physiological, and biochemical characteristics of strawberry fruit of cv. Camarosa throughout the growing season, at harvest time, and during cold storage.
According to the previous studies (Janeczko and Skoczowski, 2005; Erdal and Dumlupinar, 2011b), it could be assumed that the effects of EE and Prog treatments on the morphological characteristics and fruit size due to the changes of internal physiology during fruit growth and development stimulated efficient use of resources such as water, nutrients, and other essential compositions. Erdal and Dumlupinar (2011b) reported that enhanced plant growth and morphological characteristics might be related to an increment in cell and plant metabolism which includes the synthesis reactions such as carbohydrates, sugars, proteins and etc. In addition, MSH can influence the metabolism and photosynthesis of plants, enhance cell division and expansion, and subsequently stimulate growth (Janeczko and Skoczowski, 2005).
MSHs have been suggested to be paradoxical hormones exerting either growth stimulatory or inhibitory effects depending on the tissue and treatment regimen. Natural MSHs at physiologic levels inhibited DNA synthesis and decreased cell number in different organism (Lee et al., 2003). The EE and Prog concentration that had the greatest impact on morphological characteristics was 1.0 ppm (10-6 M) as compared with 0.001 ppm (10-9 M). Similarly, some studies mentioned that MSHs positively influenced plant growth at high concentrations, such as 10-6 M. For example, Thukral and Sharma (1992) reported that 10-4 and 10-6 concentrations of estrone enhanced the seedling growth and development in mustard (Brassica campestrist). Additionally, progesterone and b-estradiol even at 10-4 M increased root and shoot elongation in chickpea (Cicer arietinum), bean (Phaseolus vulgaris), and maize (Zea mays) (Erdal, 2009; Erdal and Dumlupinar, 2010; Erdal et al., 2010).
On the other hand, some studies have indicated that these MSHs negatively influenced the growth and development of plants at high concentrations, such as 10-4 M. For example, Janeczko (2000) reported that while estrogen and progesterone at a 10-6 M concentration enhanced the growth of winter wheat (Triticum aestivum) seedlings, they prevented seedling growth when applied at a 10-5 M concentration. Furthermore, Iino et al. (2007) reported that while 10-6 and 10-8 M Prog applications increased seedling growth in Arabidopsis thaliana, higher levels delayed the growth.
Our results confirmed that the strawberry plants treated with EE and Prog had higher chlorophyll contents as compared with the untreated plants. These results are in agreement with Bajguz and Czerpak (1996a), Bajguz and Czerpak (1996b) and Janeczko and Skoczowski (2005), who concluded that exogenous application of MSH enhanced chlorophyll synthesis. Moreover, MSH treatment might have directly or indirectly induced chlorophyll biosynthesis or diminished chlorophyllase activity (Genisel et al., 2013). Chlorophylls have an essential function in the photosynthetic apparatus, so the chlorophyll content in the leaf is an important determinant of the overall photosynthetic performance. The photosynthetic performance affects plant growth, development, and yield (Shinde, 2010). Therefore, the improvement of some morphological characteristics that we observed in the present study might be associated with enhanced chlorophyll contents in response to foliar application of EE and Prog.
According to our results, the foliar application of EE and Prog significantly affected the content of biochemical compounds. This might be due to the effects of MSH on the metabolism of plants. Variations in the endogenous activity and content of different key indicators of nutrients and sugars mobilization, such as hydrolytic activities (protease and a-amylase) and the level of storage substances (carbohydrates and starch) and the final products of their hydrolysis (amino acids and glucose), was observed in response to β-estradiol treatment in lentil (Lens culinaris) tissues (Chaoui and El Ferjani, 2013). These reactions can affect the content of sugars, carbohydrates, and other biochemical and bioactive compounds that we observed in our study. Our results are in accordance with Bajguz and Czerpak (1996a), Bajguz and Czerpak (1996b), Erdal (2012b), Dumlupinar et al. (2011), and Chaoui and El Ferjani (2013), who found that application of MSH significantly influenced the content of bioactive compounds such as sugar, protein, and carotenoid. Furthermore, MSHs may mediate their effects in plants at the level of gene transcription by means of specific receptors as in animal cells, as well as through non-genomic pathways. Yang et al. (2005) characterized a putative membrane steroid binding protein (MSBP1) that binds progesterone with high affinity and functions in regulating different metabolic processes, bioactive compounds, and also growth in Arabidopsis.
In our study, TSS, TA, and vitamin C content significantly increased during the 4-week cold storage. TSS and TA are the main sensory and taste quality parameters in strawberry fruit. The increase in TSS and TA content during storage time might be related to water losses, changes of polysaccharides and pectin materials into various sugars, destruction of starch, changes in juice content, and increases in mono- and disaccharides in response to starch degradation (Shiri et al., 2011; Shiri et al., 2016a). Vitamin C (ascorbic acid) is an important nutrient quality parameter and is very sensitive to degradation due to its oxidation compared to other nutrients during food processing and storage. Ishaq et al. (2009) reported that the change in ascorbic acid content during storage could be due to the oxidation of ascorbic acid to dehydroascorbic acid and then further degradation to 2,3-diketo-gluconic acid by the action of ascorbic acid oxidase (Akhtar et al., 2010; Shiri et al., 2016b).
The EE and Prog treatments significantly affected the antioxidant enzyme activities, such as POD, SOD, CAT, and GR, in the strawberry fruit. The highest enzymes activities were obtained at 1.0 ppm (10-6 M) of EE and Prog treatments, in which the most desirable plant morphological characteristics were observed. Dogra and Kaur (1994) indicated that MSH enhanced CAT and POD activities at 10-4 to 10-8 M concentrations in wheat. Moreover, Erdal and Dumlupinar (2011b) reported that the highest SOD, POD, and CAT activities were observed at a 10-6 M concentration of progesterone, and 10-9 M concentrations of β-estradiol and androsterone in chickpea plants. Foliar application of MSH might improve plant tolerance to adverse environmental conditions by enhancing the activities of antioxidant enzymes (Erdal and Dumlupinar, 2011b). Enhancement in the activities of antioxidant enzymes by MSH treatments was previously reported by Erdal and Dumlupinar (2010) in chickpea, Erdal (2012b) in wheat, and Genisel et al. (2013) in chickpea.
Reactive oxygen species (ROS), have a high correlation with the postharvest senescence of fruits and create oxidative injury to plant cells via membrane lipid peroxidation and protein destruction. Many antioxidant enzymes such as POD, SOD, CAT, and GR detoxify ROS in plants, and the activities of some antioxidant enzymes are enhanced during storage time. However, scavenging of ROS by antioxidant enzymes may decrease their levels during storage (Guo et al., 2018). In the EE and Prog treated plants, the enhanced activity of POD, SOD, and GR may indicate the possible participation of MSH in postponing membrane destruction (Janeczko and Skoczowski, 2005).
Our results revealed that foliar application of ethinyl estradiol (EE) and progesterone (Prog) produced fruit with improved appearance and nutritional quality, as treated plants showed more desirable morphological characteristics such as the number of inflorescences per plant, number of flowers per inflorescence, weight of first fruit, average weight of fruit, length and width of first fruit, and also the contents of chlorophyll and vitamin C content, and the activities of POD, SOD, and GR at the time of harvest. On the other hand, the length/width ratio of first fruit and anthocyanin content was not significantly affected by EE and Prog application. Moreover, quality was improved in treated fruit during 4 weeks of cold storage as compared with untreated fruit. Finally, the foliar application of EE and Prog at 1.0 ppm (10-6 M) may be most appropriate in order to produce strawberry fruit of cv. Camarosa with better fruit quality and storability.




