Introduction
Materials and Methods
Fruit Material and Preharvest Treatments
Fruit Physical Quality Parameters
Fruit Chemical Quality Parameters
Statistical Analysis
Results and Discussion
Effect of Preharvest Ca-chitosan Application on Kiwifruit Weight
Effect of Preharvest Ca-chitosan Application on Kiwifruit Firmness During Cold Storage
Effect of Preharvest Ca-chitosan Application on Ethylene Production and Respiration Rates During Cold Storage
Effect of Preharvest Ca-chitosan Application on Delaying of Kiwifruit Maturity and Ripening
Introduction
Kiwifruit (Actinidia sp.) is a climacteric fruit suitable for long-term storage when harvested properly and stored under optimal conditions (Guroo et al., 2017). The loss of postharvest quality during kiwifruit storage mainly results from relatively high metabolic activities (Huang et al., 2017). Cold storage maintains the quality and prolongs the postharvest life of kiwifruit, mostly by retarding the metabolic activity of cells, such as respiration and ethylene production (Shin et al., 2018; Cha et al., 2019). In combination with cold storage, various approaches (e.g., salicylic acid, calcium dips, 1-methylcyclopropene (1-MCP), and modified atmosphere packaging) have been demonstrated to be effective in controlling postharvest life in many crops, including kiwifruit (Fisk et al., 2008; Franco et al., 2008; Kazemi et al., 2011; Guroo et al., 2017; Kwanhong et al., 2017). Recently there has been interest in edible coatings as a cost-effective alternative to the modified atmosphere packaging (Fisk et al., 2008; Huang et al., 2017). As an effective semipermeable barrier, edible coatings reduce qualitative and quantitative losses through modification and control of the fruit’s internal atmosphere (Fisk et al., 2008; Drevinskas et al., 2017; Fortunati et al., 2017; Guroo et al., 2017).
Chitosan (poly β-(1,4) N-acetyl-d-glucosamine) is a natural and safe polymer with a range of applications. It has a high molecular weight that forms a cationic linear polysaccharide derived from the deacetylation of chitin (Drevinskas et al., 2017; Fortunati et al., 2017; Gayed et al., 2017; Huang et al., 2017; Kim et al., 2018; Vivek and Subbarao, 2018). Chitosan is extracted from shellfish exoskeletons or the cell wall of some microorganisms such as fungi (Fortunati et al., 2017; Kim et al., 2018; Vivek and Subbarao, 2018). Chitosan-based coatings are promising as they are edible and can be used to make biologically safe preservative coatings for different types of food (Tezotto-Uliana et al., 2014; Kaya et al., 2016; Fortunati et al., 2017; Gayed et al., 2017; Huang et al., 2017; Kim et al., 2018). In addition, chitosan-based coatings are biocompatible, biodegradable, film-forming, antimicrobial, antioxidative, and nontoxic (Tezotto-Uliana et al., 2014; Kaya et al., 2016; Fortunati et al., 2017; Gayed et al., 2017; Huang et al., 2017; Kim et al., 2018). The advantage of chitosan is that the treatment cost is relatively low and is also currently available for agricultural use as a safe food additive approved by the U.S. Food and Drug Administration (USFDA) (Tezotto-Uliana et al., 2014; Fortunati et al., 2017).
Several studies (Du et al., 1997; Fisk et al., 2008; Kaya et al., 2016; Drevinskas et al., 2017; Fortunati et al., 2017; Huang et al., 2017; Zheng et al., 2017; Vivek and Subbarao, 2018) have shown that the postharvest use of chitosan can affect the quality of kiwifruit and postharvest life. However, the effects of preharvest use of chitosan on postharvest life of kiwifruit have not been reported. In addition, chitosan treatment has been reported to be more effective in postharvest physiology of fruit crops, when combined with other additives, including salicylic acid (Huang et al., 2017), potassium silicate (Mohamed et al., 2017), calcium chloride (Gayed et al., 2017; Mohamed et al., 2017; Kim et al., 2018), gum arabic (Khaliq et al., 2016), lactoperoxidase (Cissé et al., 2015), and antagonistic yeast (Meng and Tian, 2009).
In a previous study, we showed that chitosan combined with calcium chloride (Ca-chitosan) can be used as a coating to increase the shelf life of kiwifruit (Kim et al., 2018). Several studies have demonstrated the benefits of applying pre- and postharvest calcium chloride and calcium chelate on retaining kiwifruit firmness, while slowing down the ripening process during storage (Franco et al., 2008; Kazemi et al., 2011; Shiri et al., 2014, Shiri et al., 2015). Calcium alters the intracellular and extracellular environments, resulting in lower rates of color change, softening, CO2 and ethylene production, an increase in sugar, and a reduction in total acid content that ultimately retard ripening of kiwifruit (Franco et al., 2008). The combination of calcium treatment with other techniques appears to provide an additive or a synergistic effect in kiwifruit maturation and postharvest life. In this study, we investigate the preharvest Ca-chitosan application on the physicochemical properties of ‘Garmrok’ kiwifruit during postharvest cold storage at 0°C.
Materials and Methods
Fruit Material and Preharvest Treatments
This research was conducted from September 28, 2017 to February 10, 2018. Kiwifruit (A. deliciosa ‘Garmrok’) were obtained from a commercial orchard in Sachun, Korea, from 5-year-old vines cultivated on a pergola trellis system and maintained according to the kiwifruit cultivation recommendations. The experimental design was a randomized complete block with three biological replicates (trees) and three treatments (calcium chitosan, Vapor Gard, and control). High molecular weight chitosan (500,000 MW; JS Logistics, Dongkyo-ro, Mapo-gu, Seoul, Republic of Korea) and CaCl2 (77%; Samchun Pure Chemical Co., Ltd., Pyeongtaek, Republic of Korea) were purchased.
For the first treatment, kiwifruit were fully dipped into calcium chitosan (Ca-chitosan) at 100 mg·L-1 in the form of a solution (chitosan 2%, w/v + CaCl2 2%, w/v + 0.2 M acetic acid, 0.02% surfactant). For the second treatment, kiwifruit were fully dipped into 500 times diluted Vapor Gard (Belchim Crop Protection, Ontario, Canada) solution. The Vapor Gard coating solution was prepared following the manufacturer’s instructions. In both treatments, the fruit was treated twice: on September 28, 2017 (140 days after full bloom [DAFB]) and on October 20, 2017 (162 DAFB).
The treated fruit were harvested on November 2, 2017 (175 DAFB) and immediately transferred to the Laboratory of Fruit Science, Gyeongsang National University, Korea. The fruit of each treatment were sorted and those that were uniform in size and free from defects were randomly selected. The selected kiwifruit were directly placed in 10 kg corrugated cardboard boxes laid with a perforated polyethylene film liner and immediately stored at 0°C for 90 days. Three physical and two chemical quality parameters of ‘Garmrok’ kiwifruit that were affected by each treatment were evaluated at 30-day intervals during the 90 days of storage, as described below.
Fruit Physical Quality Parameters
Fruit weight, size, and firmness (core and flesh firmness) were measured for 10 biological replicates (fruits). Fruit weight (g) at harvest was measured using a digital balance. As shown in Fig. 1, the fruit length (mm), longitudinal width (mm), and transverse width (mm) at harvest were measured using a digital Vernier caliper. Firmness was measured using a rheometer (RHEO TEX SD-700, Sun Scientific Inc., Japan) fitted with an 8-mm round flat probe. The fruit was cut through the equator (2-cm radial slice). The firmness of the flesh was measured from the outer pericarp, while core firmness was measured from the core tissue by compressing a depth of 3 mm with a crosshead speed of 120 mm·min−1. The maximum force generated during penetration was recorded as firmness in Newton (N). Ethylene production and the respiration rate were determined by a gas chromatograph (GC-7890B; GC-6890; Agilent Technologies, USA) with three biological replicates.
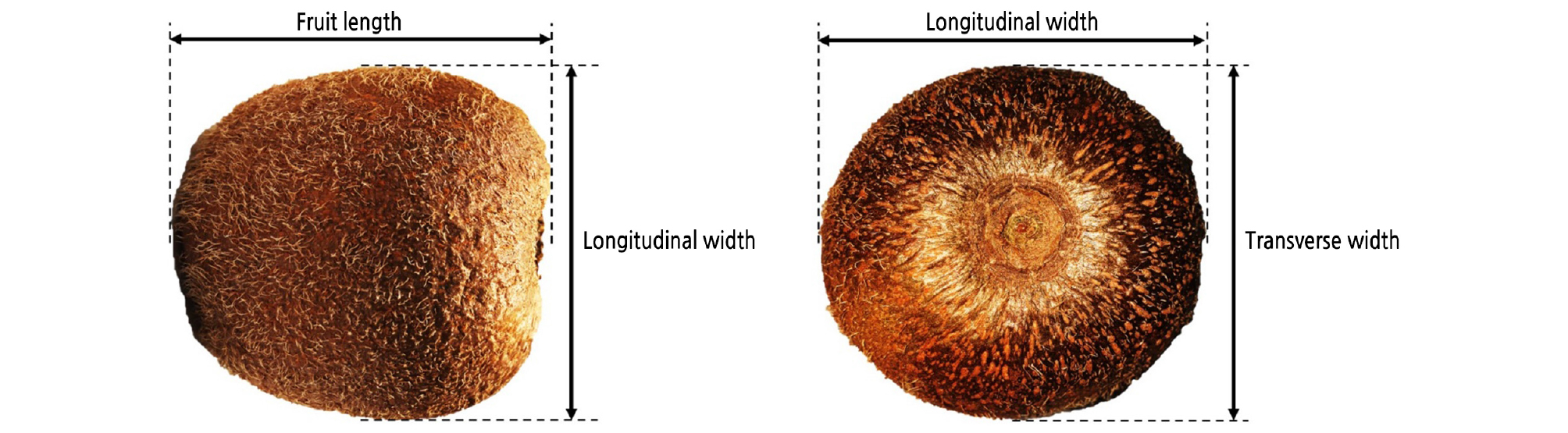
Fig. 1.
‘Garmrok’ kiwifruit size at harvest, including length, longitudinal width, and transverse width [Adapted and modified from Fu et al. (2016)].
Fruit Chemical Quality Parameters
The soluble solids content (SSC) for 10 biological replicates was measured using a hand refractometer (Pocket Refractometer, PAL-1, Atago Co., Ltd., Tokyo, Japan) calibrated in °Brix, expressed as a percentage. TA was measured by titrating with 0.1 mol·L-1 NaOH to an endpoint of pH 8.3, using a professional benchtop BP3001 pH meter (Trans Instruments, Singapore). The results were recalculated and presented as anhydrous citric acid equilibrium and expressed as a percentage. The TA was measured in three replications in which one replicate contained the extract of 10 fruit.
Statistical Analysis
The obtained data were subjected to analysis of variance (ANOVA) using statistical analysis software (SAS, version 9.4, SAS Institute Inc., Cary, North Carolina, USA). To evaluate the significance of differences between the mean values, Duncan’s multiple range test was applied at the significance level of p < 0.05. The graphics of the analyzed results were generated using SigmaPlot v12.0 (Systat Software, Inc., SigmaPlot for Windows, USA).
Results and Discussion
Effect of Preharvest Ca-chitosan Application on Kiwifruit Weight
To understand the effects of preharvest Ca-chitosan treatment, we measured both the weight and size of the ‘Garmrok’ kiwifruit at harvest. These data are presented in Table 1. The greatest fruit weight was observed from the Ca-chitosan- treated kiwifruit (102.2 g, p < 0.05). The average weights of the Vapor Gard-treated kiwifruit (87.3 g) and control kiwifruit (93.6 g) were not significantly different (p > 0.05). When the fruit size at harvest was measured as fruit length, longitudinal width, and transverse width (Fig. 1), there was no difference (p > 0.05) in the size of kiwifruit between the three treatments (Table 1). The use of preharvest Ca-chitosan was highly (p < 0.05) effective in increasing the kiwifruit weight but had no significant effect on kiwifruit size.
Table 1. Effects of preharvest Ca-chitosan application on fruit weight and fruit size at harvest in 'Garmrok' kiwifruit
yThe same letters in each column indicate that the means do not differ significantly at p < 0.05 according to Duncan's multiple range test.
The chitosan concentration used in this study was as previously described (Du et al., 1997; Fisk et al., 2008; Huang et al., 2017; Kim et al., 2018; Vivek and Subbarao, 2018). Mahmood et al. (2017) demonstrated that the application of chitosan (0.5%) increased fruit weight, fruit diameter, and yield in bell pepper (Capsicum annuum cv. Yolo Wonder). They showed that the ability of chitosan as a growth elicitor to increase the weight and diameter can be attributable to cell size enlargement and strengthening of carbohydrate sink. In addition, Abd El-Wahab (2015) showed that the preharvest applications of amino acids and calcium nitrate played a significant role in increasing the weight and yield of apricot (Prunus armeniaca cv. Canino) fruit (kg) per tree. Chitosan has been shown to form a semipermeable film on fruit (Petriccione et al., 2015; Kaya et al., 2016; Drevinskas et al., 2017). The increased weight of the kiwifruit was attributed to the formation of a semipermeable barrier on the fruit surface, which may have reduced the water loss caused by transpiration and respiration. Nevertheless, further experimental investigation is needed to determine whether the effect of preharvest application of Ca-chitosan on the increased kiwifruit weight shares a similar mechanism to the above- mentioned studies.
Effect of Preharvest Ca-chitosan Application on Kiwifruit Firmness During Cold Storage
Loss of firmness is one of the deteriorating factors that limit the postharvest life and quality of kiwifruit. When we examined the effect of preharvest application of Ca-chitosan on the firmness of kiwifruit, the firmness of the flesh and core was reduced in all samples over the storage period (Fig. 2A and 2B). The firmness of the control and Vapor Gard-treated fruit rapidly decreased with a longer storage period, whereas the Ca-chitosan-treated fruit retained greater firmness. In addition, preharvest treatment of Ca-chitosan slowed the loss of firmness in kiwifruit during cold storage, indicating a possible ripening-delaying effect that prolonged the postharvest life. Similar effects of preharvest chitosan, combined with other chemicals, on the firmness of fruit have also been observed in jujube fruit (Ziziphus jujuba Mill. cv. Dongzao; Yan et al., 2012), strawberry (Fragariaananassa cv. Qingxiang; He et al., 2018), pomegranate (Punica granatum cv. Wonderful; Abd El-Wahab et al., 2017), mango (Mangifera indica cv. Zibda; Mohamed et al., 2017), peach (Prunus persica cv. Early Swelling; Gayed et al., 2017), and strawberry (Fragaria ananassa cv. Seascape; Reddy et al., 2000), as they all maintained their firmness during cold storage. Furthermore, several studies have reported that postharvest application of chitosan retained greater firmness in kiwifruit due to the barrier property of chitosan against the diffusion of water through stomata (Du et al., 1997; Kaya et al., 2016; Drevinskas et al., 2017; Huang et al., 2017; Zheng et al., 2017; Vivek and Subbarao, 2018).
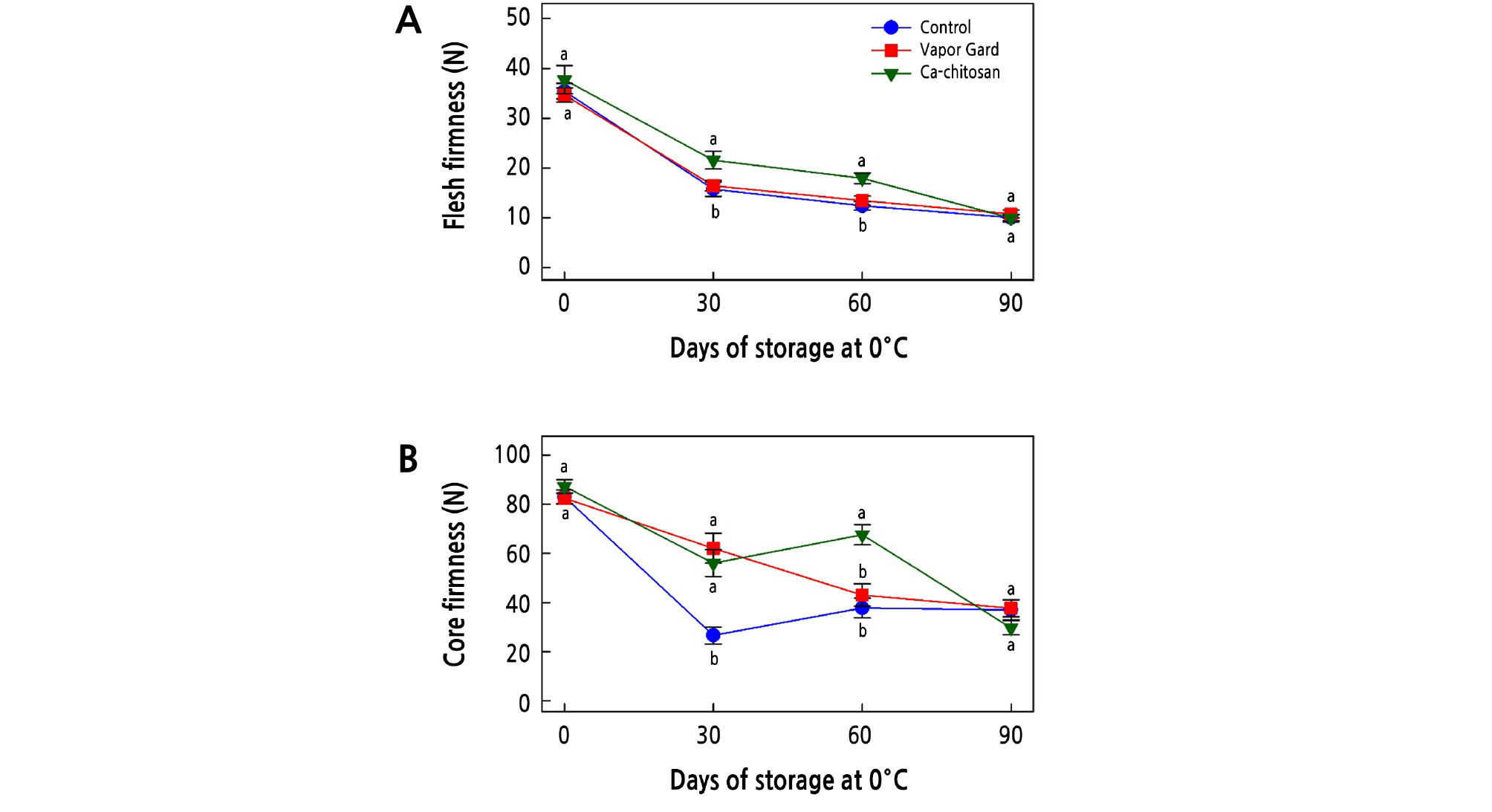
Fig. 2.
Effects of preharvest Ca-chitosan application on flesh firmness (A) and core firmness (B) during cold storage in ‘Garmrok’ kiwifruit. Vertical bars indicate SE with n = 10. The same letters for each treatment indicate that the means do not differ significantly at p < 0.05 according to Duncan’s multiple range test.
Vapor Gard (1-p-menthene) is a terpenic polymer that is a water-emulsifiable organic concentrate used to reduce water transpiration. Vapor Gard is an antitranspirant that forms a coating on the fruit and prevents evaporative water loss, thus retaining fruit turgidity and firmness (Khreba et al., 2014). Curiously, the preharvest treatment of Vapor Gard was less effective in maintaining the kiwifruit firmness than Ca-chitosan. This result is in agreement with that obtained by Khreba et al. (2014) on the postharvest treatment of Vapor Gard and chitosan for strawberry (Fragaria ananassa cv. Sweet Charlie).
In general, water loss is responsible for the decrease in cell turgidity and changes in cell structure and cell wall composition that lead to the loss of fruit firmness. It appears that the barrier property of Ca-chitosan is responsible for the retention of firmness while reducing respiration rate and water loss. It was previously suggested that chitosan might create a modified atmosphere around the fruit surface that deters pectin breakdown and delays the loss of fruit firmness (Reddy et al., 2000; Tezotto-Uliana et al., 2014; Khaliq et al., 2016; He et al., 2018; Vivek and Subbarao, 2018). Furthermore, the delayed reduction in kiwifruit firmness is also explained by the effect of calcium on fruit softening, where calcium is an essential part of the cell wall structure by conferring mechanical strength (Abd El-Wahab, 2015; Abd El-Wahab et al., 2017). As a result, its binding to pectin to form calcium pectate increased the rigidity of the middle lamella of the cell wall that affected membrane organization and function during the postharvest life of fruit (Abd El-Wahab, 2015; Abd El-Wahab et al., 2017).
In this respect, the combination of chitosan with calcium is much more effective in delaying the loss of kiwifruit firmness, and the resulting greater firmness may help to prolong the postharvest life of kiwifruit. In addition to the barrier property of preharvest Ca-chitosan, worthwhile future research should examine the role of preharvest Ca-chitosan treatment in the inhibition of the activity of cell-wall-degrading enzymes and related genes, which is associated with increased kiwifruit firmness during cold storage.
Effect of Preharvest Ca-chitosan Application on Ethylene Production and Respiration Rates During Cold Storage
Ethylene production and respiration are major metabolic changes that determine the postharvest life of kiwifruit. Changes in ethylene production and respiration rates of kiwifruit during storage at 0°C are shown in Fig. 3A and 3B, respectively. In general, ethylene production of all kiwifruit increased at 30 days of storage and then decreased by 60 days of storage, following an increase toward the end of the storage period (Fig. 3A). However, ethylene production was not significantly different (p > 0.05) for any of the three treatments over the storage period, except at 60 days of storage. At harvest, the Ca-chitosan-treated kiwifruit showed a significantly lower (p < 0.05) respiration rate (11.9 mg·kg-1·h-1) than the Vapor Gard-treated (14.2 mg·kg-1·h-1) and control (16.8 mg·kg-1·h-1) samples (Fig. 3B). However, no significant (p > 0.05) differences were detected between the control and the two coated fruit treatments during the storage period.
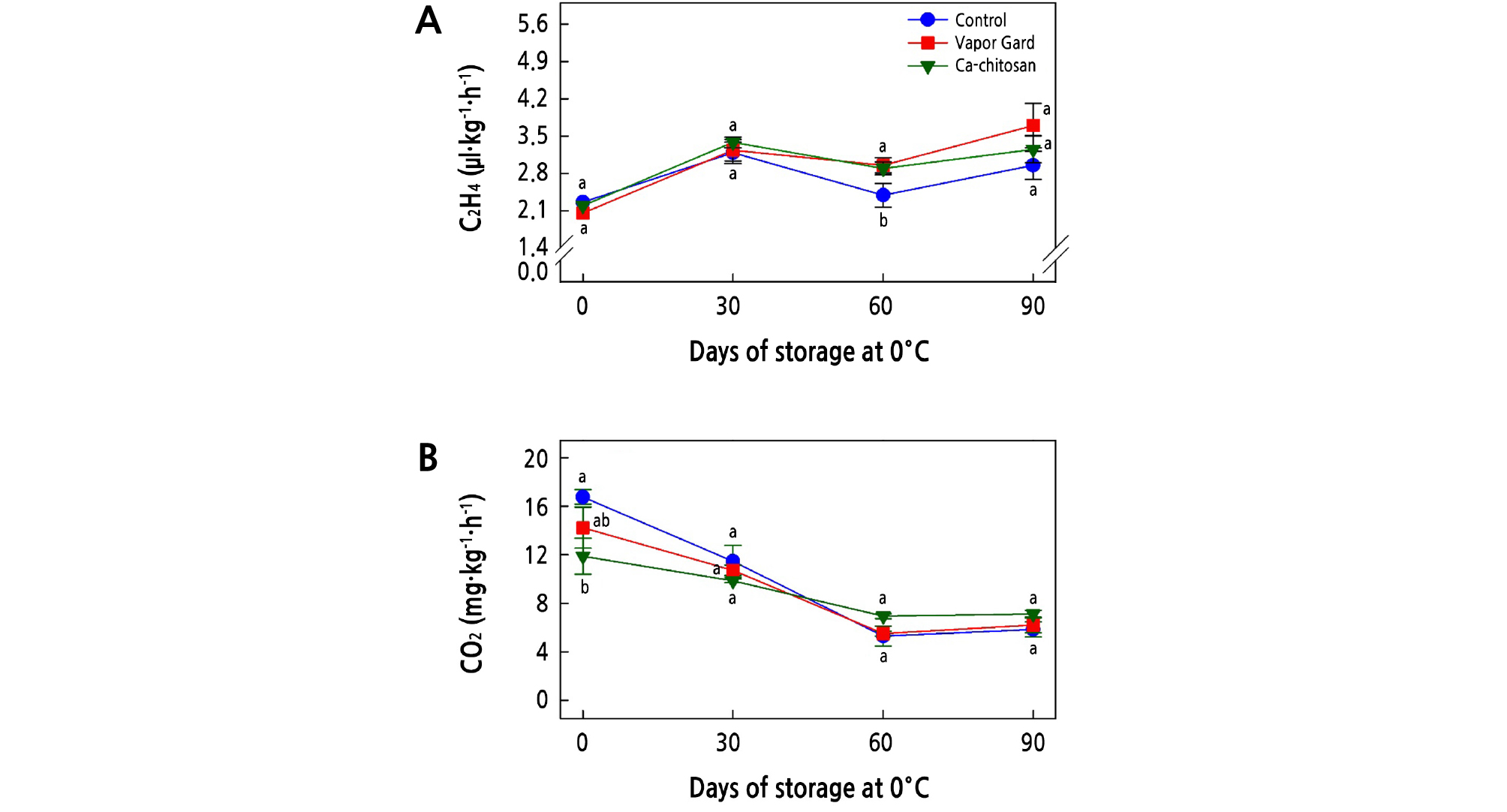
Fig. 3.
Effects of preharvest Ca-chitosan application on ethylene production (A) and respiration rates (B) during cold storage in ‘Garmrok’ kiwifruit. Vertical bars indicate SE with n = 3. The same letters for each treatment indicate that the means do not differ significantly at p < 0.05 according to Duncan’s multiple range test.
Accordingly, the significantly lower (p < 0.05) respiration rate (11.9 mg·kg-1·h-1) in preharvest Ca-chitosan-treated kiwifruit demonstrated the selective permeability of Ca-chitosan to the respiratory gases. We tentatively attribute this reduced respiration rate to the controlled atmosphere created by the chitosan coating over kiwifruit that has a selective permeability to respiratory gases, which in turn decreases the O2 and CO2 exchange of the coated kiwifruit (Vivek and Subbarao, 2018). The coating of fruit with semipermeable films can hinder fruit ripening by modifying the internal CO2, O2, and ethylene production (Reddy et al., 2000). Nevertheless, in this study, the effect of preharvest Ca-chitosan treatment on respiration and ethylene production rates during postharvest cold storage was not significant, which reveals a residual effect of preharvest Ca-chitosan on the postharvest life of kiwifruit.
Effect of Preharvest Ca-chitosan Application on Delaying of Kiwifruit Maturity and Ripening
In an attempt to find the possible chemical changes that might have been affected by preharvest treatment of Ca-chitosan, we measured soluble solids content (SSC) and titratable acidity (TA) over 90 days of cold storage. The SSC of kiwifruit during storage is an important quality index that influences kiwifruit ripening. Overall, SSC increased with longer storage period up to a maximum at the end of the storage period (90 days) (Fig. 4A) (Meng and Tian, 2009; He et al., 2018). The increase in SSC during storage could result from the breakdown of carbohydrates into simple sugars. At harvest, the Vapor Gard treatment resulted in the highest SSC (8.6%; p < 0.05), and SSC did not differ (p > 0.05) between the Ca-chitosan (7.9%) and control (7.4%) treatments at harvest. After 30 days of storage, SSC was not different (p > 0.05) among the three treatments. However, Ca-chitosan-treated fruit had the lowest SSC (13.4 %) (p < 0.05) at 60 days of storage compared to Vapor Gard-treated (14.4%) and control (14.8%) fruit. At the end of storage, the Vapor Gard-treated kiwifruit had the lowest SSC (14.6%, p < 0.05), while the control (15.6%) and Ca-chitosan-treated (15.6%) kiwifruit had higher SSC.
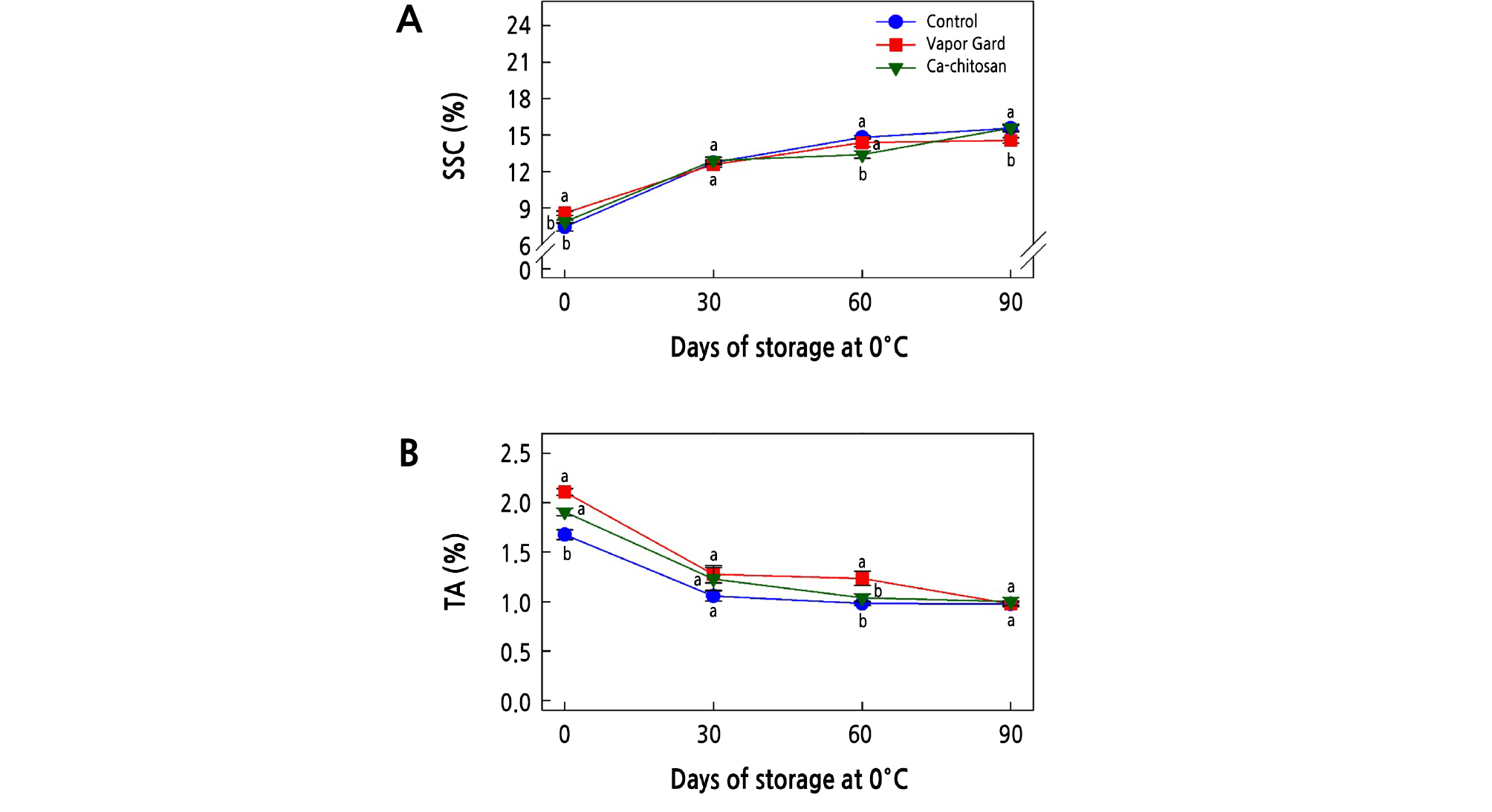
Fig. 4.
Effects of preharvest Ca-chitosan application on soluble solids content (SSC; A) and titratable acidity (TA; B) during cold storage in ‘Garmrok’ kiwifruit. Vertical bars indicate SE with n = 10 (A) and n = 3 (B). The same letters for each treatment indicate that the means do not differ significantly at p < 0.05 according to Duncan’s multiple range test.
As for TA, there was a general tendency of reduction during the cold storage regardless of the preharvest treatments (Fig. 4B). The decrease in TA during storage results from the consumed organic acids used as substrates in the respiratory process. TA that determines the content of organic acid was represented as a percentage of citric acid, as citric acid is the dominant organic acid in kiwifruit (Vivek and Subbarao, 2018; Cha et al., 2019). At harvest, the TA of control kiwifruit was the lowest at 1.7% (p < 0.05), whereas the TA values of kiwifruit treated with Ca-chitosan (1.9%) and Vapor Gard (2.1%) were not significantly different (p > 0.05). Except for at 60 days of storage, however, there was no difference in TA (p > 0.05) among the three treatments throughout the storage period.
Application of Ca-chitosan to kiwifruit may form a thin layer on the fruit surface that can modulate the internal atmosphere. Formation of such a layer aids in the reduction of oxygen, elevation of carbon dioxide, mitigation of respiration, and suppression of ethylene production and metabolic activities, which ultimately lead to high TA and reduced accumulations of SSC (Khaliq et al., 2016; Mohamed et al., 2017). The results shown in Fig. 4A and 4B support the effect of preharvest application of Ca-chitosan with the least significant (p < 0.05) SSC and the highest significant (p < 0.05) TA at harvest, indicating a possible maturity-delaying biostimulant constituent of Ca-chitosan that can prolong the postharvest life. Our results are in line with other studies showing the effects of preharvest application of Ca-chitosan on mango fruit (Mangifera indica cv. Zibda; Mohamed et al., 2017), preharvest chitosan treatment combined with antagonistic yeast on grape (Vitis vinifera cv. Jingxiu; Meng and Tian, 2009), preharvest chitosan oligosaccharide application on strawberry (Fragaria ananassa cv. Qingxiang; He et al., 2018), and preharvest chitosan combined with CaCl2 on peach (Prunus persica cv. Florida Prince; El-Badawy, 2012).
A recent study by Abd El-Wahab et al. (2017) showed somewhat contradictory results in which the control (or untreated) pomegranate fruit (Punica granatum cv. Wonderful) under cold storage displayed the least significant SSC and highest significant TA, while pomegranate fruit treated with preharvest chitosan combined with CaCl2 showed the highest significant SSC and least significant TA. However, the chemical attributes of fruit can be more affected by choice of package and storage conditions than by the coating treatment (Fisk et al., 2008). In our study, preharvest treatment appeared to leave less residual Ca-chitosan on the kiwifruit surface that made it unable to modify the internal atmosphere, which prevented the higher metabolic activities throughout cold storage. Tezotto-Uliana et al. (2014) showed that preharvest treatment of chitosan on raspberry (Rubus idaeus cv. Autumn Bliss) was not sufficiently effective to modify the enzyme activity and anthocyanin synthesis. Nevertheless, the results in this study demonstrate that preharvest application of Ca-chitosan increased the weight of ‘Garmrok’ kiwifruit. Furthermore, preharvest treatment using Ca-chitosan as a biostimulant on ‘Garmrok’ kiwifruit had a possible maturity- and ripening-delaying effect, which may play a positive role in the overall quality during cold storage that creates a new way to extend postharvest life.


